Abstract
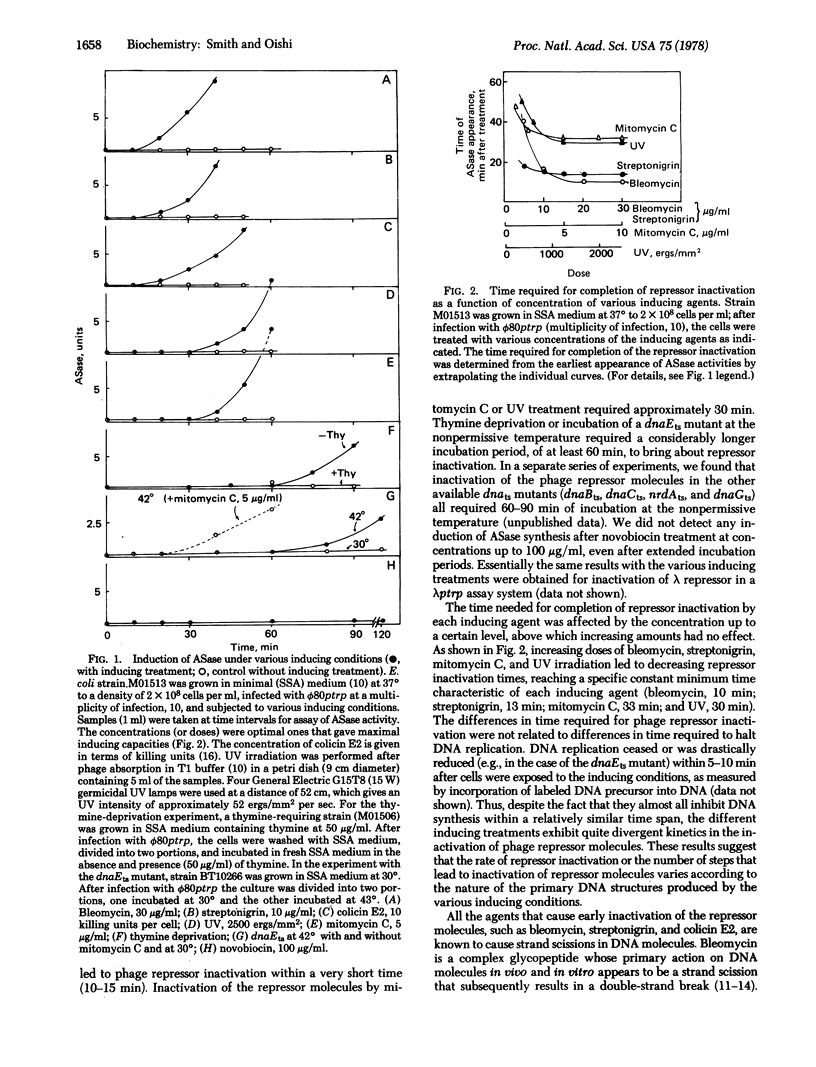
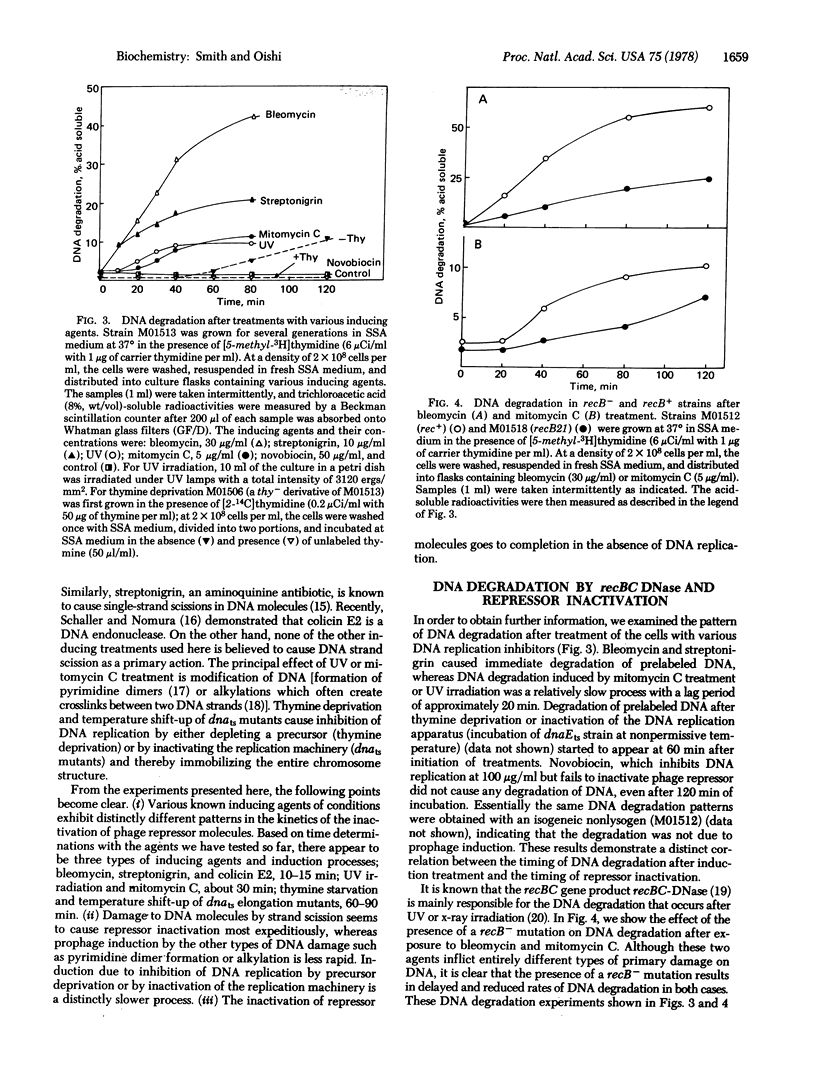
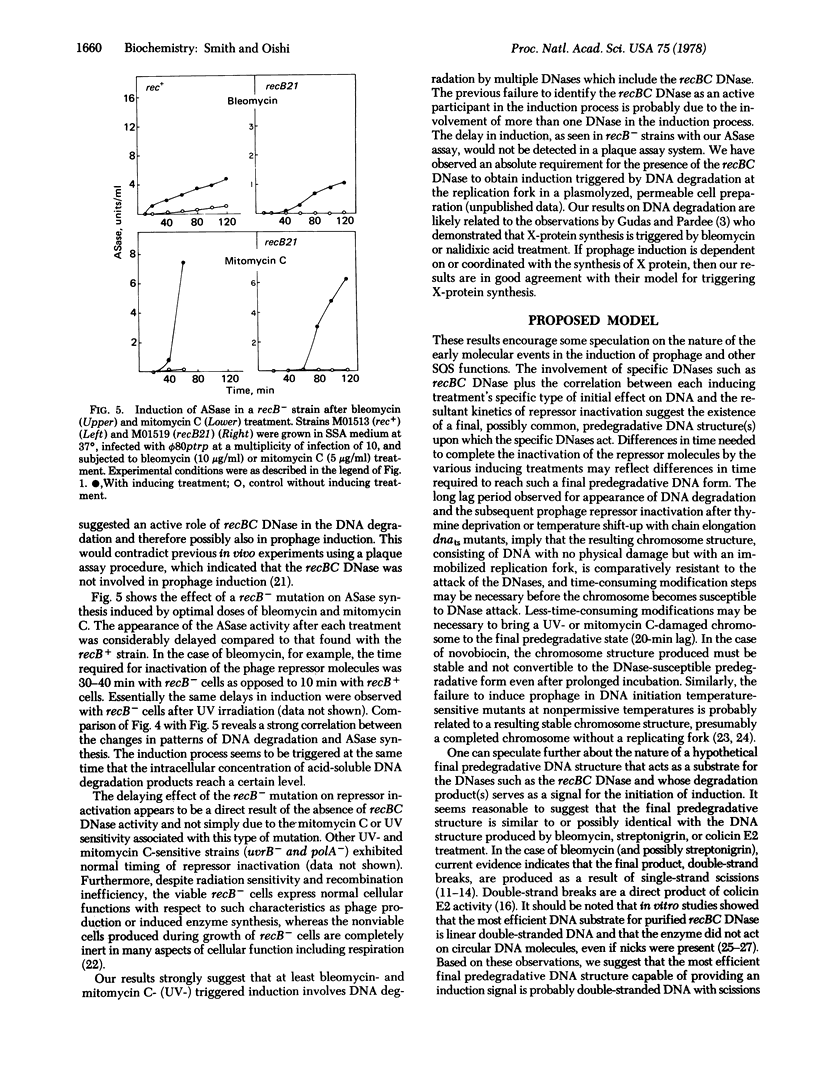
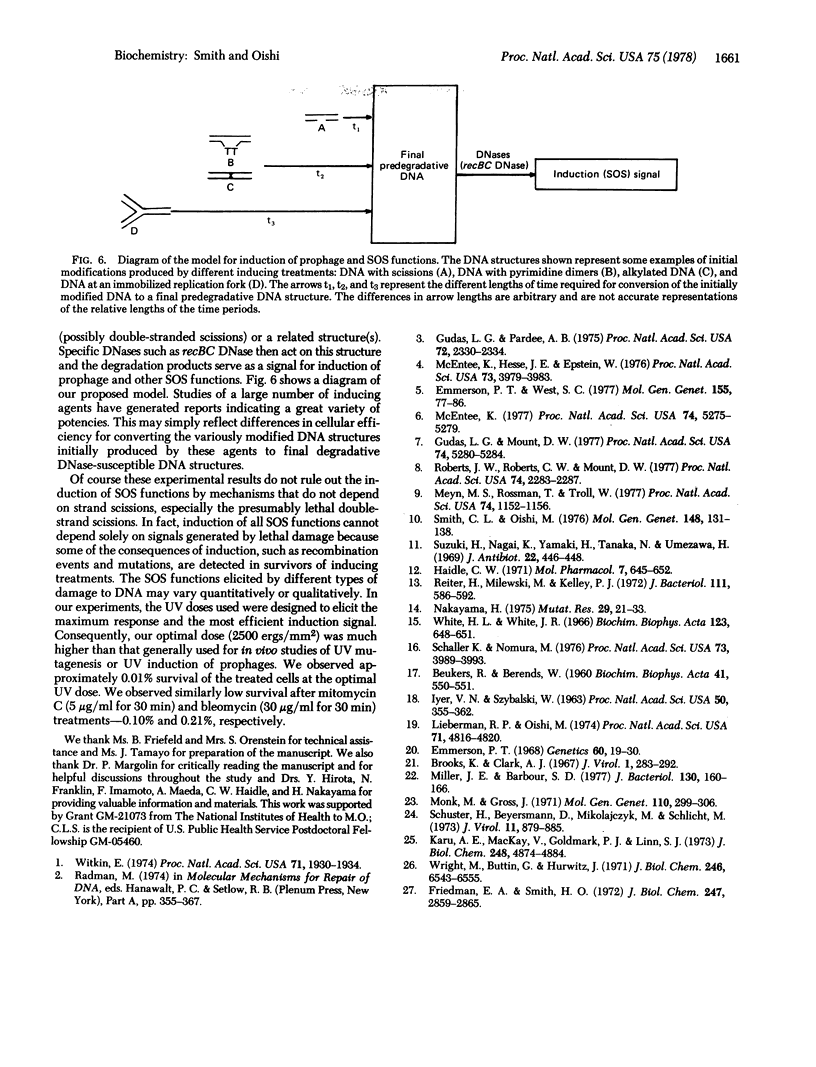
Different inducing agents and treatments produced distinctly different kinetic patterns of inactivation of prophage repressor molecules. The different patterns were related to differences in the initial altered states of DNA that were produced. The timing of appearance of DNA degradation was correlated with the time needed for repressor inactivation. These characteristics suggest that all the inducing treatments lead to the formation of a final predegradative DNA structure(s) (probably involving scissions) that is acted on by specific DNases, including the recBC DNase, to produce the signals for the induction of prophage.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUKERS R., BERENDS W. Isolation and identification of the irradiation product of thymine. Biochim Biophys Acta. 1960 Jul 15;41:550–551. doi: 10.1016/0006-3002(60)90063-9. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T. Recombination deficient mutants of Escherichia coli K12 that map between thy A and argA. Genetics. 1968 Sep;60(1):19–30. doi: 10.1093/genetics/60.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- Friedman E. A., Smith H. O. An adenosine triphosphate-dependent deoxyribonuclease from Hemophilus influenzae Rd. 3. Substrate specificity. J Biol Chem. 1972 May 10;247(9):2859–2865. [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidle C. W. Fragmentation of deoxyribonucleic acid by bleomycin. Mol Pharmacol. 1971 Nov;7(6):645–652. [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu A. E., MacKay V., Goldmark P. J., Linn S. The recBC deoxyribonuclease of Escherichia coli K-12. Substrate specificity and reaction intermediates. J Biol Chem. 1973 Jul 25;248(14):4874–4884. [PubMed] [Google Scholar]
- Lieberman R. P., Oishi M. The recBC deoxyribonuclease of Escherichia coli: isolation and characterization of the subunit proteins and reconstitution of the enzyme. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4816–4820. doi: 10.1073/pnas.71.12.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K., Hesse J. E., Epstein W. Identification and radiochemical purification of the recA protein of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3979–3983. doi: 10.1073/pnas.73.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn M. S., Rossman T., Troll W. A protease inhibitor blocks SOS functions in Escherichia coli: antipain prevents lambda repressor inactivation, ultraviolet mutagenesis, and filamentous growth. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1152–1156. doi: 10.1073/pnas.74.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. E., Barbour S. D. Metabolic characterization of the viable, residually dividing and nondividing cell classes of recombination-deficient strains of Escherichia coli. J Bacteriol. 1977 Apr;130(1):160–166. doi: 10.1128/jb.130.1.160-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk M., Gross J. Induction of prophage lambda in a mutant of E. coli K12 defective in initiation of DNA replication at high temperature. Mol Gen Genet. 1971;110(4):299–306. doi: 10.1007/BF00438272. [DOI] [PubMed] [Google Scholar]
- Nakayama H. Phileomycin-induced lethality and DNA degradation in Escherichia coli K12. Mutat Res. 1975 Jul;29(1):21–31. doi: 10.1016/0027-5107(75)90018-4. [DOI] [PubMed] [Google Scholar]
- Reiter H., Milewskiy M., Kelley P. Mode of action of phleomycin on Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):586–592. doi: 10.1128/jb.111.2.586-592.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller K., Nomura M. Colicin E2 is DNA endonuclease. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3989–3993. doi: 10.1073/pnas.73.11.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. The molecular mechanism of virus induction. I. A procedure for the biochemical assay of prophage induction. Mol Gen Genet. 1976 Oct 18;148(2):131–138. doi: 10.1007/BF00268376. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nagai K., Yamaki H., Tanaka N., Umezawa H. On the mechanism of action of bleomycin: scission of DNA strands in vitro and in vivo. J Antibiot (Tokyo) 1969 Sep;22(9):446–448. doi: 10.7164/antibiotics.22.446. [DOI] [PubMed] [Google Scholar]
- White H. L., White J. R. Interaction of streptonigrin with DNA in vitro. Biochim Biophys Acta. 1966 Sep;123(3):648–651. doi: 10.1016/0005-2787(66)90241-3. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M., Buttin G., Hurwitz J. The isolation and characterization from Escherichia coli of an adenosine triphosphate-dependent deoxyribonuclease directed by rec B, C genes. J Biol Chem. 1971 Nov;246(21):6543–6555. [PubMed] [Google Scholar]


