Abstract
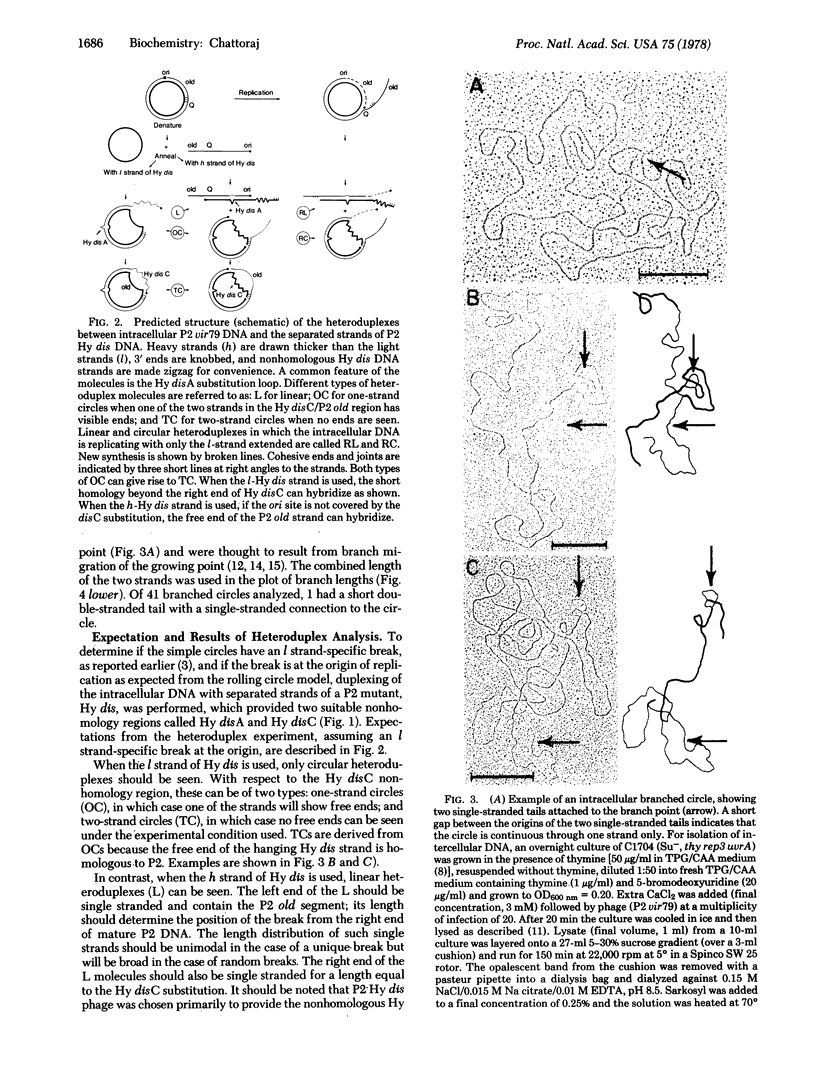
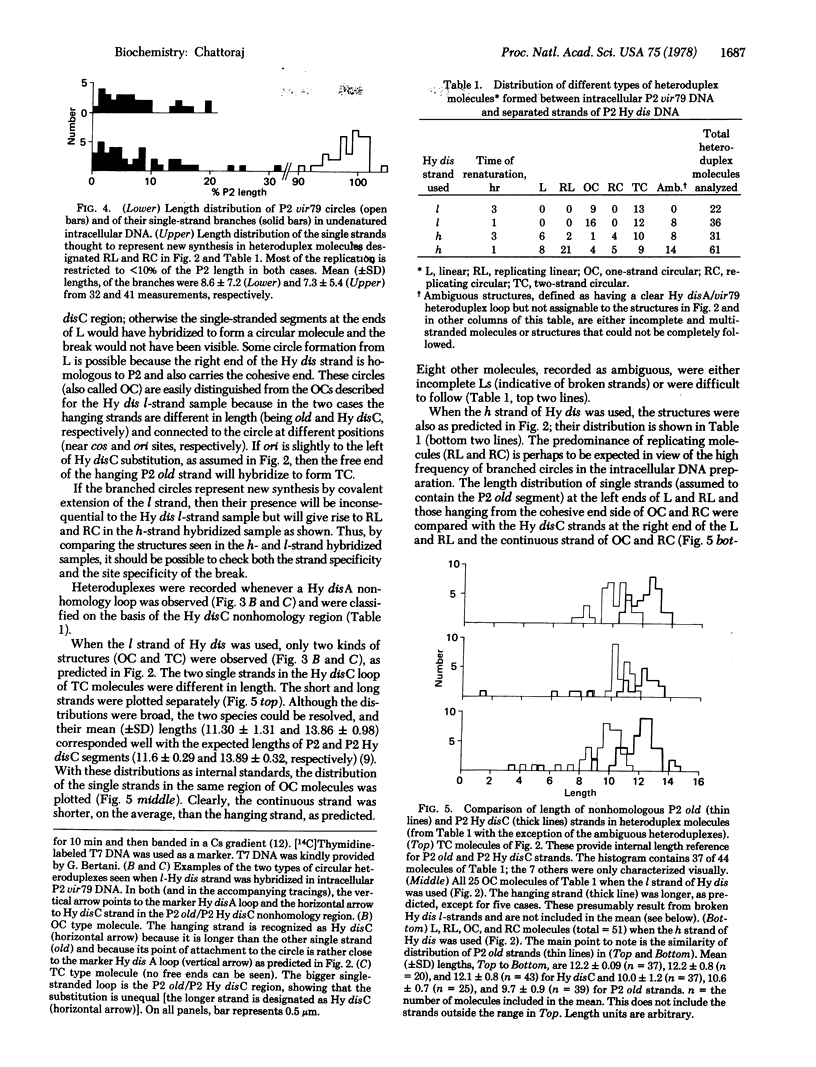
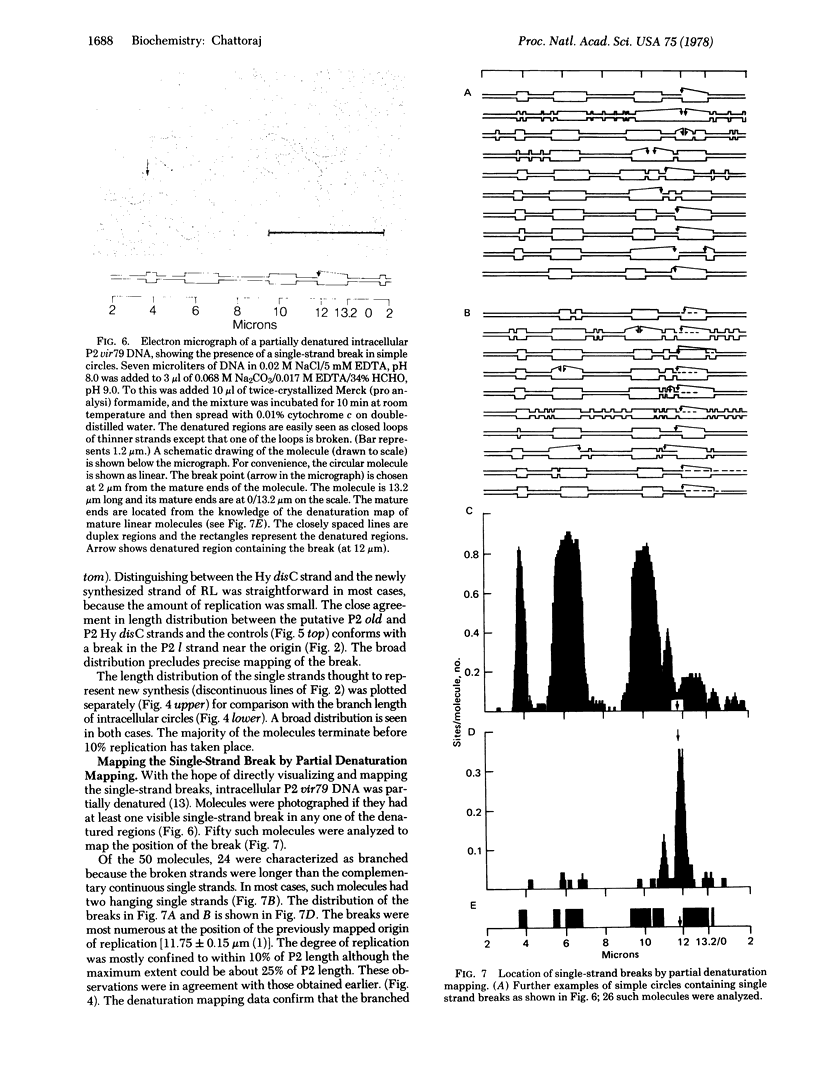
Membrane-associated P2 DNA isolated early after infection under conditions that block replication (amB in phage and rep in Escherichia coli C) was analyzed by electron microscopy. Most DNA was in the form of relaxed circles (40%) and circles with short single-stranded tails (60%). When this DNA was hybridized with separate strands of linear P2 Hy dis DNA (which provides suitable reference points along the heteroduplex molecules), an interruption was located near the previously mapped origin of P2 DNA replication in one specific strand. The same strand was sometimes extended in the direction consistent with the unidirectional mode of P2 DNA replication. Similar conclusions were reached when the intracellular DNA was analyzed after partial denaturation. These results are consistent with the rolling circle mode of DNA replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P. D., Jansz H. S. Bacteriophage phiX174 DNA synthesis in a replication-deficient host: determination of the origin of phiX DNA replication. J Mol Biol. 1976 Apr 15;102(3):633–656. doi: 10.1016/0022-2836(76)90339-9. [DOI] [PubMed] [Google Scholar]
- Bertani G. Deletions in bacteriophage P2. Circularity of the genetic map and its orientation relative to the DNA denaturation map. Mol Gen Genet. 1975;136(2):107–137. doi: 10.1007/BF00272034. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Electron microscope heteroduplex mapping of P2 Hy dis bacteriophage DNA. Virology. 1973 Sep;55(1):174–182. doi: 10.1016/s0042-6822(73)81019-0. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Inman R. B. Origin and direction of replication of bacteriophage 186 DNA. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1768–1771. doi: 10.1073/pnas.70.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Younghusband H. B., Inman R. B. Physical mapping of bacteriophage P2 mutations and their relation to the genetic map. Mol Gen Genet. 1975;136(2):139–149. doi: 10.1007/BF00272035. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Scott J. F., Kornberg A. Enzymatic replication of viral and complementary strands of duplex DNA of phage phiX174 proceeds by seprate mechanisms. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3151–3155. doi: 10.1073/pnas.73.9.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXXIX. The structure of a DNA form with restricted binding of intercalating dyes observed during synthesis of phiX single-stranded DNA. J Mol Biol. 1976 Apr 25;102(4):723–741. doi: 10.1016/0022-2836(76)90288-6. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Cis-limited action of the gene-A product of bacteriophage phiX174 and the essential bacterial site (E. coli-electron microscopy-cis-acting protein-specifically-nicked RF). Proc Natl Acad Sci U S A. 1972 Feb;69(2):475–479. doi: 10.1073/pnas.69.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisselsoder J. Strand-specific discontinuity in republicating P2 DNA. J Mol Biol. 1976 Jan 5;100(1):13–22. doi: 10.1016/s0022-2836(76)80030-7. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Vegetative DNA of temperate coliphage P2. Mol Gen Genet. 1971;110(2):178–196. doi: 10.1007/BF00332647. [DOI] [PubMed] [Google Scholar]
- Ljungquist E. Association of nonreplicating P2 DNA to fast-sedimenting cell material following infection with satellite phage P4. Virology. 1976 Sep;73(2):402–412. doi: 10.1016/0042-6822(76)90401-3. [DOI] [PubMed] [Google Scholar]
- Ljungquist E. Interaction of phage P2 DNA with some fast-sedimenting host components during infection. Virology. 1973 Mar;52(1):120–129. doi: 10.1016/0042-6822(73)90403-0. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Dueber J. Location of the origin-terminus of the viral strand of the duplex replicative form of bacteriophage G4 DNA. J Mol Biol. 1977 Jul 15;113(4):652–661. doi: 10.1016/0022-2836(77)90228-5. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]




