Abstract
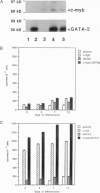
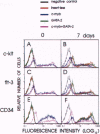
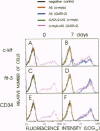
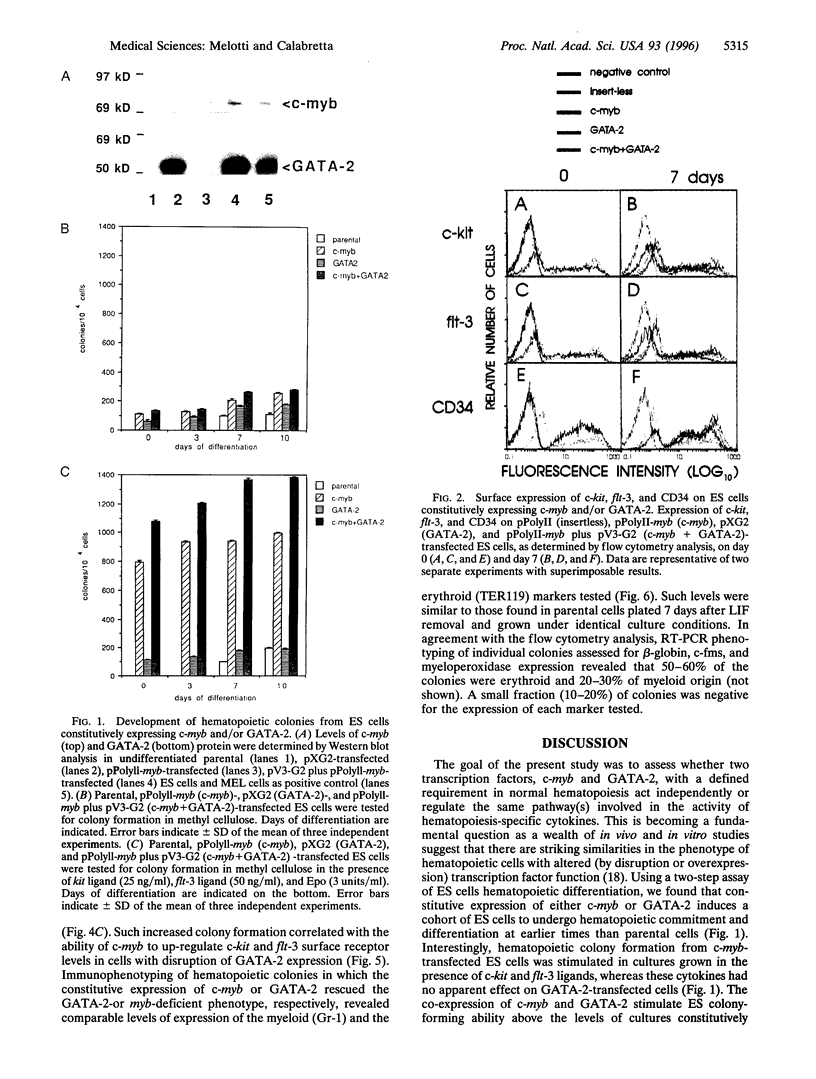
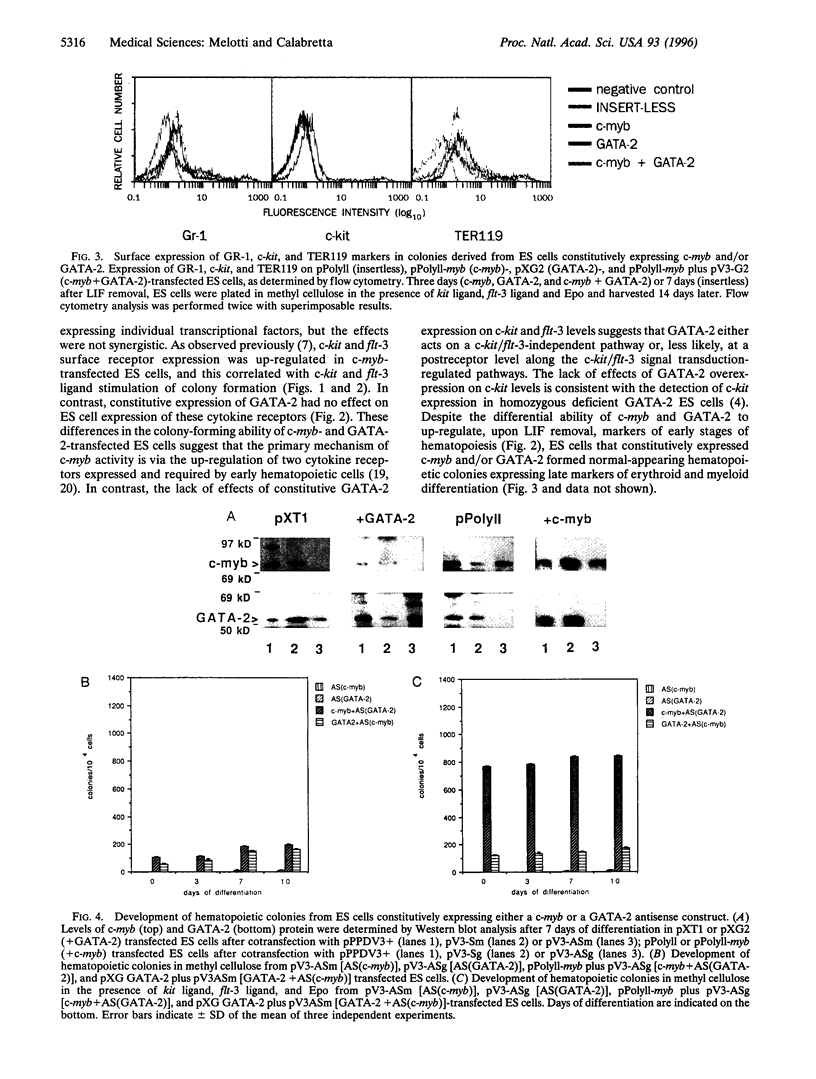
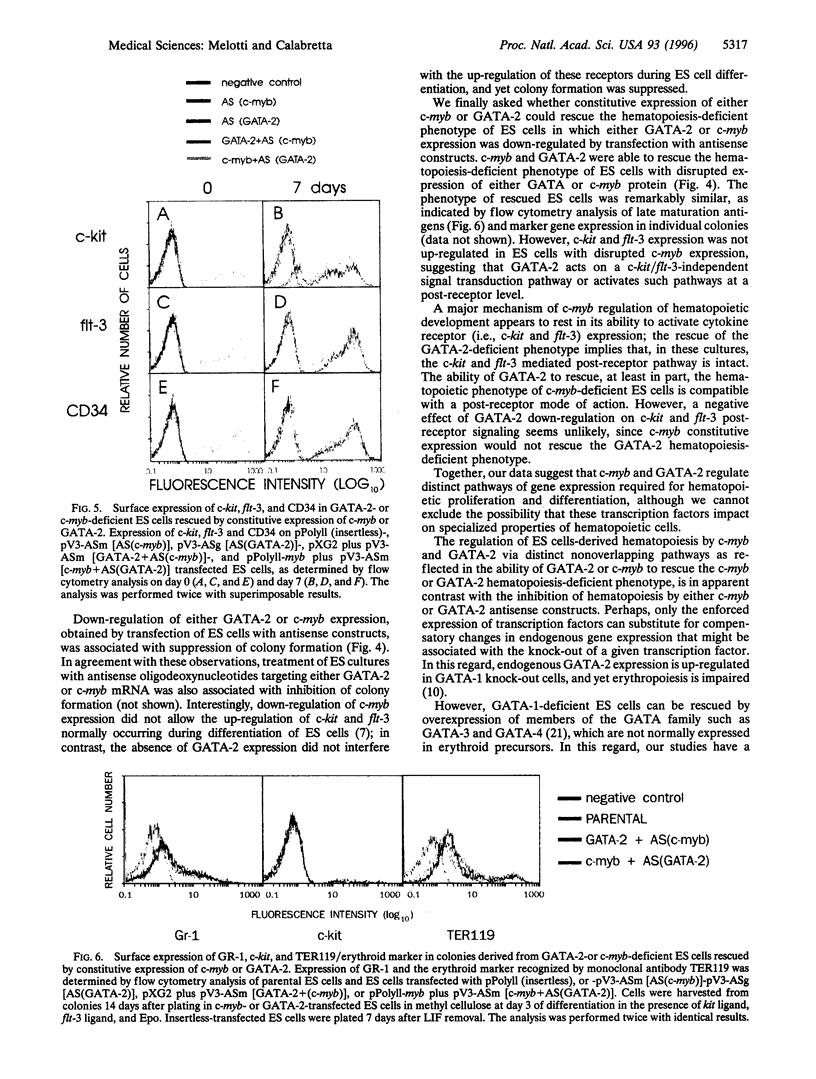
The transcription factors c-myb and GATA-2 are both required for blood cell development in vivo and in vitro. However, very little is known on their mechanism(s) of action and whether they impact on complementary or overlapping pathways of hematopoietic proliferation and differentiation. We report here that embryonic stem (ES) cells transfected with c-myb or GATA-2 cDNAs, individually or in combination, underwent hematopoietic commitment and differentiation in the absence of added hematopoietic growth factors but that stimulation with c-kit and flt-3 ligands enhanced colony formation only in the c-myb transfectants. This enhancement correlated with c-kit and flt-3 surface receptor up-regulation in c-myb-(but not GATA-2-) transfected ES cells. Transfection of ES cells with either a c-myb or a GATA-2 antisense construct abrogated erythromyeloid colony-forming ability in methyl cellulose; however, introduction of a full-length GATA-2 or c-myb cDNA, respectively, rescued the hematopoiesis-deficient phenotype, although only c-myb-rescued ES cells expressed c-kit and flt-3 surface receptors and formed increased numbers of hematopoietic colonies upon stimulation with the cognate ligands. These results are in agreement with previous studies indicating a fundamental role of c-myb and GATA-2 in hematopoiesis. Of greater importance, our studies suggest that GATA-2 and c-myb exert their roles in hematopoietic gene regulation through distinct mechanisms of action in nonoverlapping pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender T. P., Kuehl W. M. Murine myb protooncogene mRNA: cDNA sequence and evidence for 5' heterogeneity. Proc Natl Acad Sci U S A. 1986 May;83(10):3204–3208. doi: 10.1073/pnas.83.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson R. J., Bensinger W. I., Hill R. S., Andrews R. G., Garcia-Lopez J., Kalamasz D. F., Still B. J., Spitzer G., Buckner C. D., Bernstein I. D. Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma. Blood. 1991 Apr 15;77(8):1717–1722. [PubMed] [Google Scholar]
- Blobel G. A., Simon M. C., Orkin S. H. Rescue of GATA-1-deficient embryonic stem cells by heterologous GATA-binding proteins. Mol Cell Biol. 1995 Feb;15(2):626–633. doi: 10.1128/mcb.15.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J., Briddell R. A., Srour E. F., Leemhuis T. B., Hoffman R. Role of c-kit ligand in the expansion of human hematopoietic progenitor cells. Blood. 1992 Feb 1;79(3):634–641. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- DeAngelis T., Ferber A., Baserga R. Insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the platelet-derived growth factor receptor. J Cell Physiol. 1995 Jul;164(1):214–221. doi: 10.1002/jcp.1041640126. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Krause D. S., Ito T., Fackler M. J., Smith O. M., Collector M. I., Sharkis S. J., May W. S. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994 Aug 1;84(3):691–701. [PubMed] [Google Scholar]
- Lyman S. D., James L., Vanden Bos T., de Vries P., Brasel K., Gliniak B., Hollingsworth L. T., Picha K. S., McKenna H. J., Splett R. R. Molecular cloning of a ligand for the flt3/flk-2 tyrosine kinase receptor: a proliferative factor for primitive hematopoietic cells. Cell. 1993 Dec 17;75(6):1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- Melotti P., Calabretta B. Induction of hematopoietic commitment and erythromyeloid differentiation in embryonal stem cells constitutively expressing c-myb. Blood. 1996 Mar 15;87(6):2221–2234. [PubMed] [Google Scholar]
- Mucenski M. L., McLain K., Kier A. B., Swerdlow S. H., Schreiner C. M., Miller T. A., Pietryga D. W., Scott W. J., Jr, Potter S. S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991 May 17;65(4):677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Transcription factors and hematopoietic development. J Biol Chem. 1995 Mar 10;270(10):4955–4958. doi: 10.1074/jbc.270.10.4955. [DOI] [PubMed] [Google Scholar]
- Perrotti D., Melotti P., Skorski T., Casella I., Peschle C., Calabretta B. Overexpression of the zinc finger protein MZF1 inhibits hematopoietic development from embryonic stem cells: correlation with negative regulation of CD34 and c-myb promoter activity. Mol Cell Biol. 1995 Nov;15(11):6075–6087. doi: 10.1128/mcb.15.11.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Scott E. W., Simon M. C., Anastasi J., Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994 Sep 9;265(5178):1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Shivdasani R. A., Mayer E. L., Orkin S. H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995 Feb 2;373(6513):432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- Tsai F. Y., Keller G., Kuo F. C., Weiss M., Chen J., Rosenblatt M., Alt F. W., Orkin S. H. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994 Sep 15;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- Warren A. J., Colledge W. H., Carlton M. B., Evans M. J., Smith A. J., Rabbitts T. H. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994 Jul 15;78(1):45–57. doi: 10.1016/0092-8674(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Keller G., Orkin S. H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994 May 15;8(10):1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]