Abstract
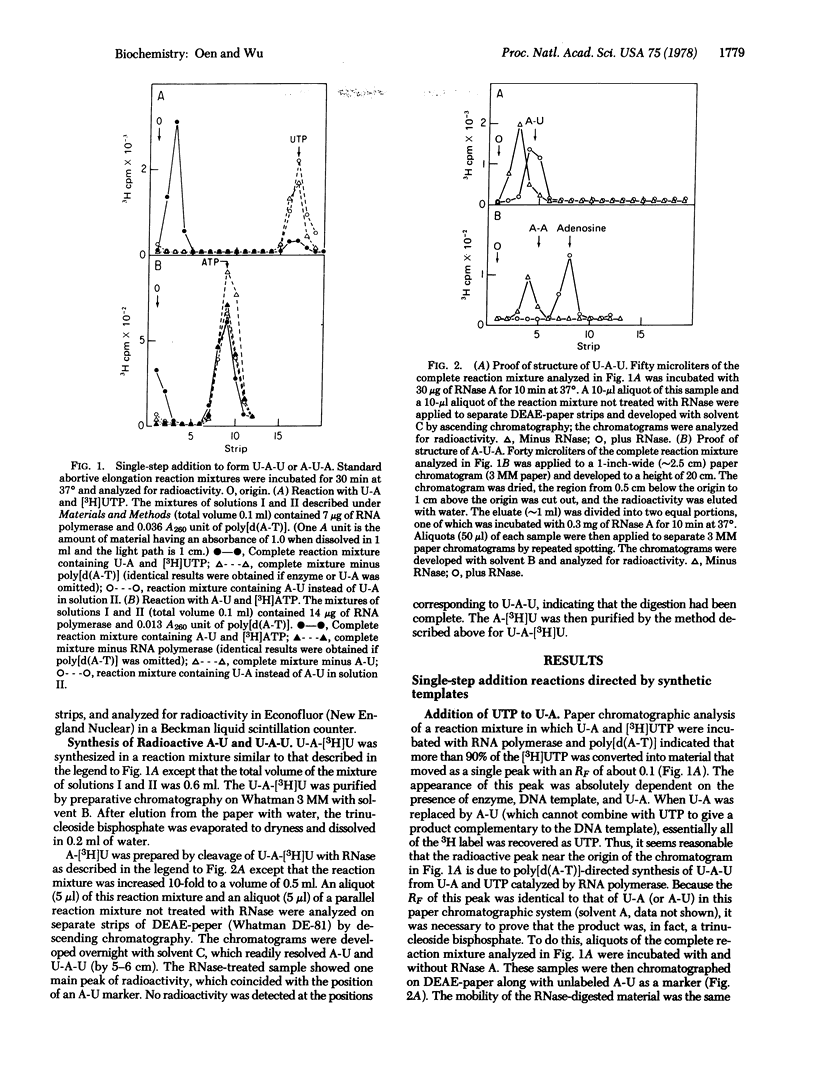
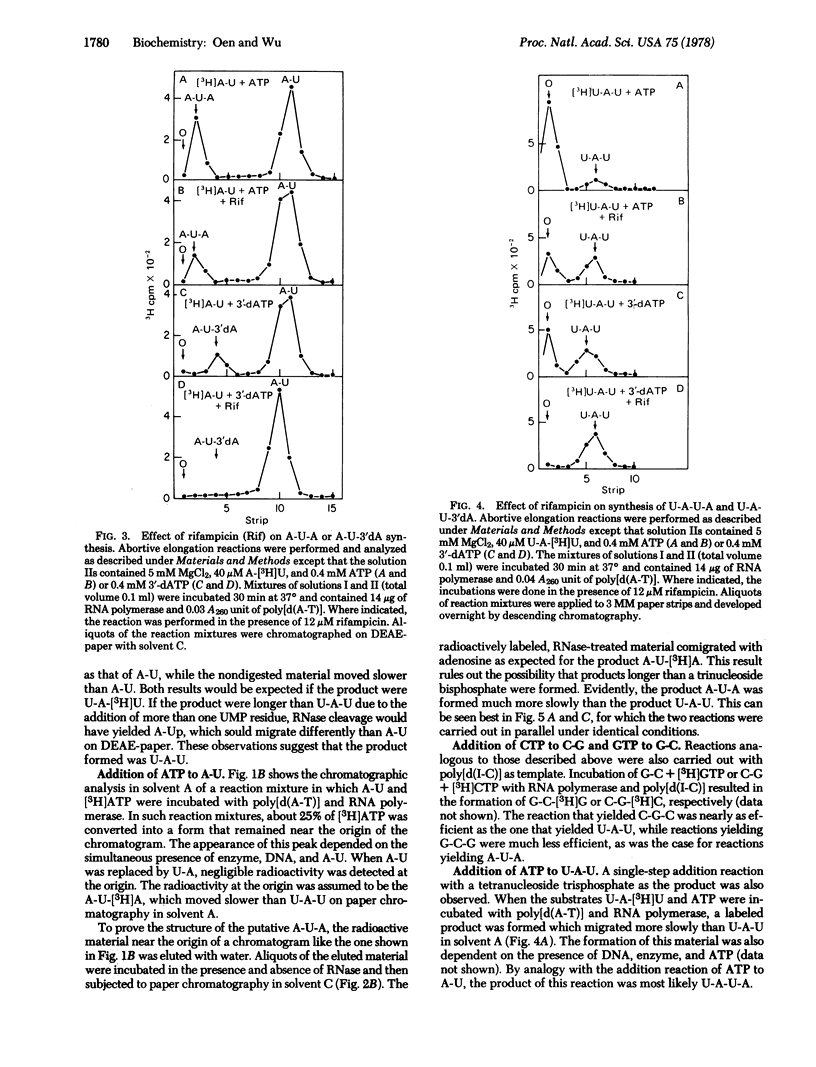
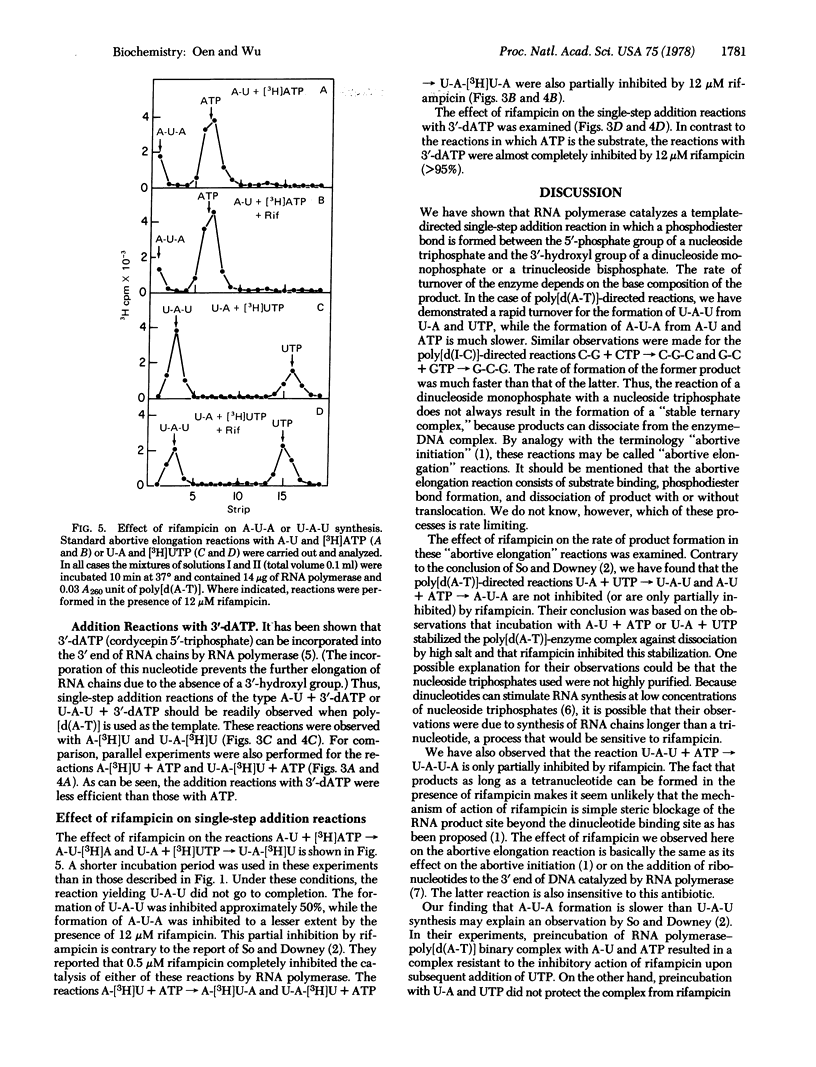
The addition of a single nucleotide to a short oligonucleotide, catalyzed by RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) in the presence of synthetic DNA templates, has been studied. The reactions A-U + ATP leads to A-U-A and U-A + UTP leads to U-A-U occur in the presence of poly[d(A-T)], while the reactions G-C + GTP leads to G-C-G and C-G + CTP leads to C-G-C take place in the presence of poly[d(I-C)]. These reactions proceed with a turnover of enzyme. The products U-A-U and C-G-C are formed rapidly, while A-U-A and G-C-G are formed much more slowly. Another poly[d(A-T)]-dependent reaction, which occurs with a turnover of enzyme, is U-A-U + ATP leads to U-A-U-A. All of these reactions are only partially inhibited by rifampicin. ATP can be replaced by 3'-deoxyadenosine 5'-triphosphate in the reactions A-U + ATP leads to A-U-A and U-A-U + ATP leads to U-A-U-A, though the rate of formation of the products becomes somewhat slower. The reactions involving 3'-deoxyadenosine 5'-triphosphate are almost completely inhibited by rifampicin, indicating that the 3'-hydroxyl group is necessary for these reactions to occur in the presence of rifampicin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong V. W., Eckstein F. Interaction of substrate analogues with Escherichia coli DNA-dependent RNA polymerase. Eur J Biochem. 1976 Nov 1;70(1):33–38. doi: 10.1111/j.1432-1033.1976.tb10952.x. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Gerard G. F., Rottman F., Boezi J. A. 2'-O-methyladenosine 5'-triphosphate. A substrate for deoxyribonucleic acid dependent ribonucleic acid polymerase of Pseudomonas putida. Biochemistry. 1971 May 25;10(11):1974–1981. doi: 10.1021/bi00787a003. [DOI] [PubMed] [Google Scholar]
- Kumar S. A., Krakow J. S., Ward D. C. ATP analogues as initiation and elongation nucleotides for bacterial DNA-dependent RNA polymerase. Biochim Biophys Acta. 1977 Jul 15;477(2):112–124. doi: 10.1016/0005-2787(77)90227-1. [DOI] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Nath K., Hurwitz J. Covalent attachment of ribonucleotides at 3'-hydroxyl ends of deoxyribonucleic acid catalyzed by deoxyribonucleic acid-dependent ribonucleic acid polymerase of Escherichia coli. J Biol Chem. 1974 Apr 25;249(8):2605–2615. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- So A. G., Downey K. M. Studies on the mechanism of ribonucleic acid synthesis. II. Stabilization of the deoxyribonucleic acid-ribonucleic acid polymerase complex by the formation of a single phosphodiester bond. Biochemistry. 1970 Nov 24;9(24):4788–4793. doi: 10.1021/bi00826a024. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Jolly J. F., Boezi J. A. Purification and characterization of bacteriophage gh-I-induced deoxyribonucleic acid-dependent ribonucleic acid polymerase from Pseudomonas putida. J Biol Chem. 1975 Mar 10;250(5):1723–1733. [PubMed] [Google Scholar]


