Abstract
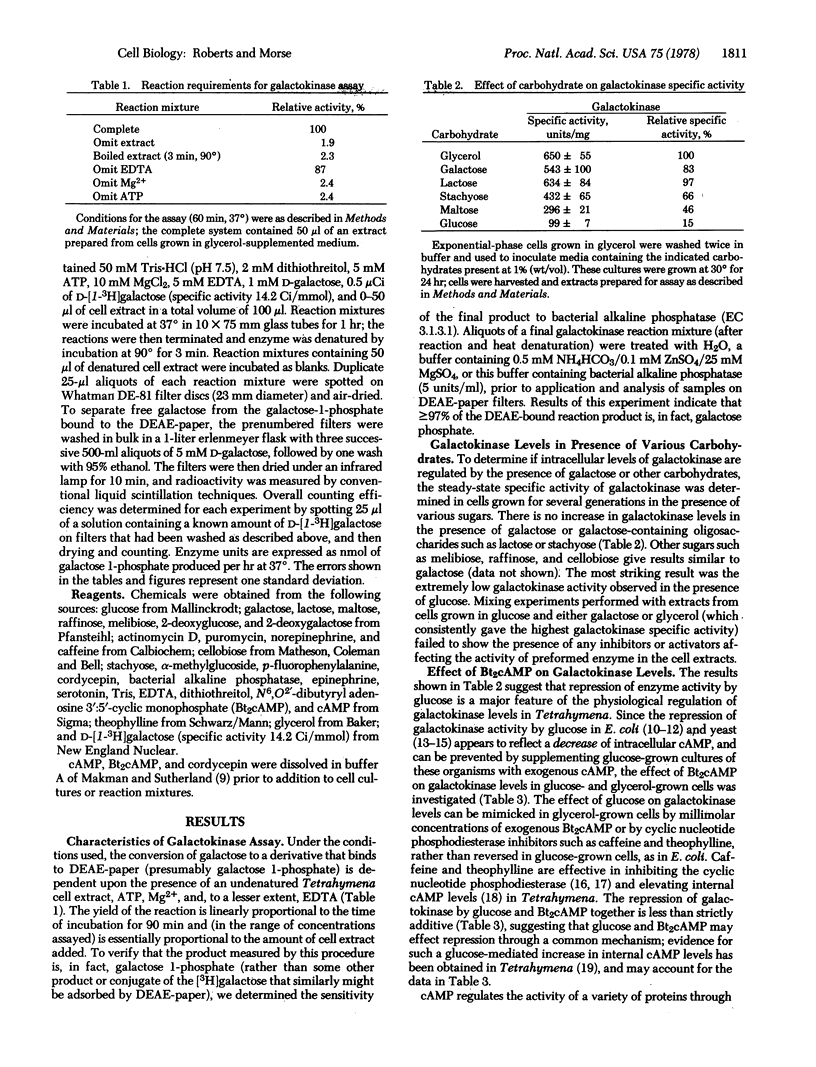
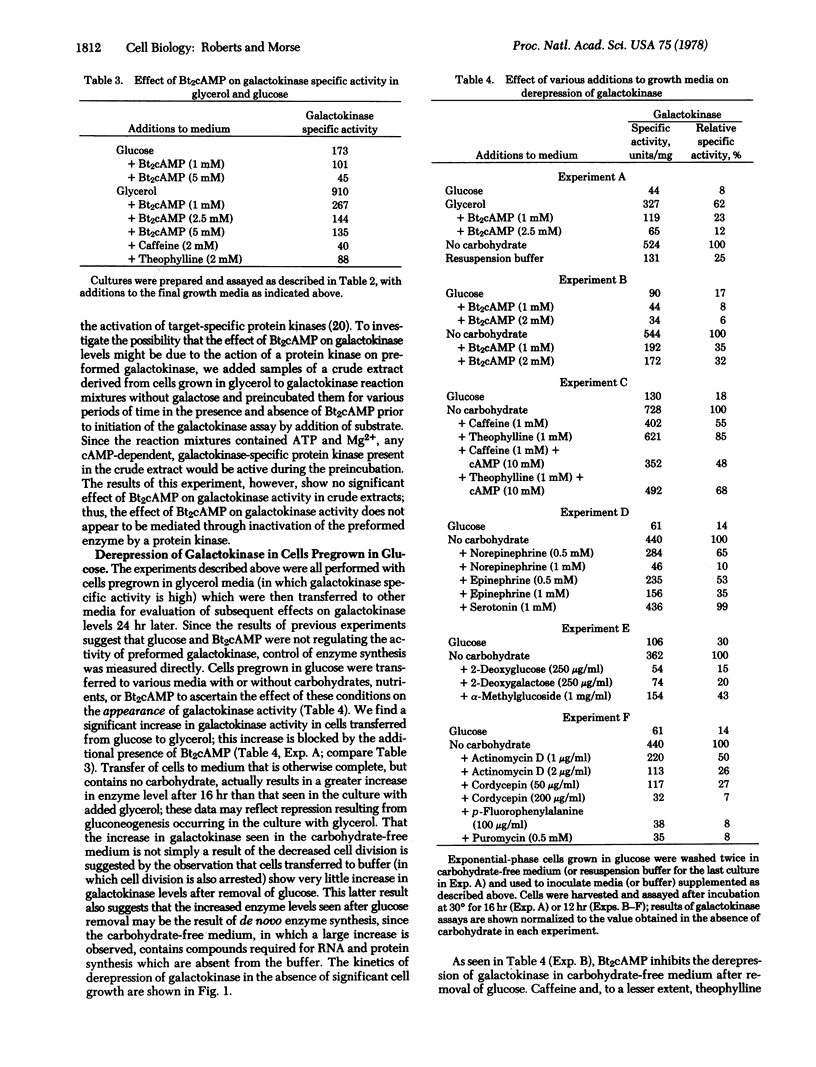
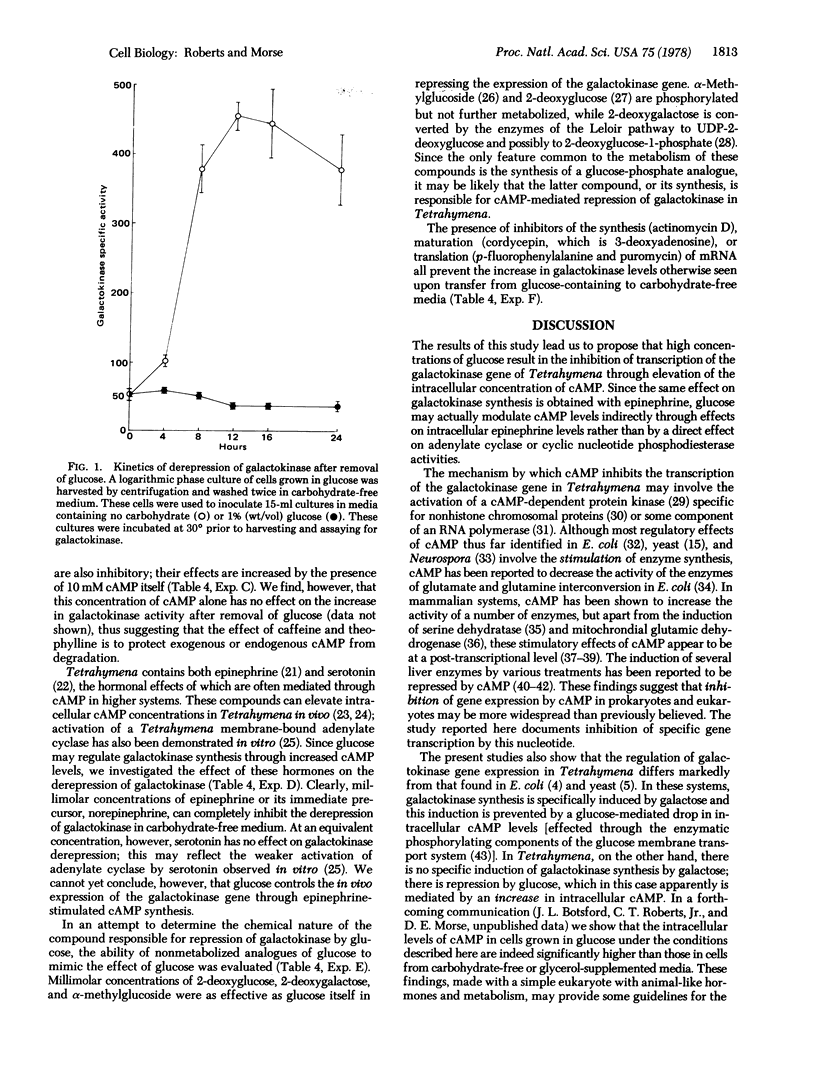
We have found evidence that transcription of the galactokinase (ATP:D-galactose 1-phosphotransferase; EC 2.7.1.6) gene is inhibited, in the animal-like protozoan Tetrahymena, by dibutyryl adenosine 3′:5′-cyclic monophosphate, glucose, and epinephrine. The specific activities of galactokinase in Tetrahymena cells grown in defined media with galactose or glycerol as the principal carbon source are equivalent; the specific activity in glucose minimal medium is [unk] the value. Thus, while there seems to be no specific induction of the enzyme by the substrate, galactose, there is a strong “repression” by glucose. This repression by glucose is mimicked, in glycerol-grown cells, by the addition of millimolar amounts of dibutyryl adenosine 3′:5′-cyclic monophosphate or phosphodiesterase inhibitors such as caffeine and theophylline. When glucose-grown cells are washed and resuspended in carbohydrate-free medium, the galactokinase specific activity increases by as much as 10-fold within 12 hr. This increase is blocked by dibutyryl adenosine 3′:5′-cyclic monophosphate and by epinephrine (synthesized by Tetrahymena, and previously shown to activate a membrane-bound adenylate cyclase in extracts of this organism), as well as by inhibitors of mRNA synthesis, maturation, and translation. Our results suggest that glucose and epinephrine can regulate transcription of the galactokinase gene by modulation of cyclic nucleotide levels. The observation that the nonmetabolized sugars 2-deoxyglucose, 2-deoxygalactose, and α-methylglucoside are as effective as glucose suggests that the sugar itself, or an immediate metabolite such as the 1-phosphate derivative, may be the effector.
Keywords: transcription, dibutyryl adenosine 3′:5′-cyclic monophosphate
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. L., Shapiro J. A. The galactose operon of E. coli K-12. I. Structural and pleiotropic mutations of the operon. Genetics. 1969 Jun;62(2):231–247. doi: 10.1093/genetics/62.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson P. F., Blunt S., Brown S. P. Amniotic-cell galactokinase activity: stimulation by galactose. Lancet. 1973 Jan 13;1(7794):106–107. doi: 10.1016/s0140-6736(73)90508-4. [DOI] [PubMed] [Google Scholar]
- Blum J. J. On the regulation of glycogen metabolism in Tetrahymena. Arch Biochem Biophys. 1970 Mar;137(1):65–74. doi: 10.1016/0003-9861(70)90411-x. [DOI] [PubMed] [Google Scholar]
- Chacko C. M., McCrone L., Nadler H. L. A study of galactokinase and glucose 4-epimerase from normal and galactosemic skin fibroblasts. Biochim Biophys Acta. 1972 Oct 12;284(2):552–555. doi: 10.1016/0005-2744(72)90153-2. [DOI] [PubMed] [Google Scholar]
- Csaba G., Nagy S. U., Lantos T. Are biogenic amines acting on tetrahymena through a cyclic amp mechanism? Acta Biol Med Ger. 1976;35(2):259–261. [PubMed] [Google Scholar]
- Csaba G., Nagy U. Effect of vertebrate hormones on the cyclic AMP level in Tetrahymena. Acta Biol Med Ger. 1976;35(10):1399–1401. [PubMed] [Google Scholar]
- Douglas H. C., Hawthorne D. C. Regulation of genes controlling synthesis of the galactose pathway enzymes in yeast. Genetics. 1966 Sep;54(3):911–916. doi: 10.1093/genetics/54.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Thayer J. P. Cyclic AMP-induced tyrosinase synthesis in Neurospora crassa. Biochem Biophys Res Commun. 1974 Dec 11;61(3):977–982. doi: 10.1016/0006-291x(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Janakidevi K., Dewey V. C., Kidder G. W. Serotonin in protozoa. Arch Biochem Biophys. 1966 Mar;113(3):758–759. doi: 10.1016/0003-9861(66)90259-1. [DOI] [PubMed] [Google Scholar]
- Janakidevi K., Dewey V. C., Kidder G. W. The biosynthesis of catecholamines in two genera of protozoa. J Biol Chem. 1966 Jun 10;241(11):2576–2578. [PubMed] [Google Scholar]
- Jost J. P., Hsie A., Hughes S. D., Ryan L. Role of cyclic adenosine 3',5'-monophosphate in the induction of hepatic enzymes. I. Kinetics of the induction of rat liver serine dehydratase by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1970 Jan 25;245(2):351–357. [PubMed] [Google Scholar]
- Jungmann R. A., Kranias E. G. Cyclic AMP-mediated protein kinase activation and its regulatory effect on mammalian RNA polymerase. Adv Biochem Psychopharmacol. 1976;15:413–428. [PubMed] [Google Scholar]
- Keppler D. O., Rudigier J. F., Bischoff E., Decker K. F. The trapping of uridine phosphates by D-galactosamine. D-glucosamine, and 2-deoxy-D-galactose. A study on the mechanism of galactosamine hepatitis. Eur J Biochem. 1970 Dec;17(2):246–253. doi: 10.1111/j.1432-1033.1970.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Halvorson H. O. Studies on the positive regulatory gene, GAL4, in regulation of galactose catabolic enzymes in Saccharomyces cerevisiae. Mol Gen Genet. 1974;135(3):203–212. doi: 10.1007/BF00268616. [DOI] [PubMed] [Google Scholar]
- Krone W., Marquardt W., Seitz H. J., Tarnowski W. Effect of cordycepin and cycloheximide on the induction of phosphoenolpyruvate carboxykinase by dexamethasone or N6, O2'-dibutyrylcyclic AMP in the isolated perfused rat liver. FEBS Lett. 1975 Sep 1;57(1):64–67. doi: 10.1016/0014-5793(75)80153-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakshmanan M. R., Nepokroeff C. M., Porter J. W. Control of the synthesis of fatty-acid synthetase in rat liver by insulin, glucagon, and adenosine 3':5' cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3516–3519. doi: 10.1073/pnas.69.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- Majumder G. C., Shrago E., Elson C. E. Changes in cyclic AMP-dependent protein dinase activity in Tetrahymena pyriformis during the growth cycle. Biochim Biophys Acta. 1975 Apr 19;384(2):399–412. doi: 10.1016/0005-2744(75)90041-8. [DOI] [PubMed] [Google Scholar]
- Miller Z., Varmus H. E., Parks J. S., Perlman R. L., Pastan I. Regulation of gal messenger ribonucleic acid synthesis in Escherichia coli by 3',5'-cyclic adenosine monophosphate. J Biol Chem. 1971 May 10;246(9):2898–2903. [PubMed] [Google Scholar]
- Nanney D. L., McCoy J. W. Characterization of the species of the Tetrahymena pyriformis complex. Trans Am Microsc Soc. 1976 Oct;95(4):664–682. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Interaction of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system with adenylate cyclase of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2920–2924. doi: 10.1073/pnas.72.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan S., Chou S. C. Cyclic 3',5'-adenosine monophosphate in Tetrahymena pyriformis. Experientia. 1973;29(7):814–814. doi: 10.1007/BF01946303. [DOI] [PubMed] [Google Scholar]
- Ramanathan S., Chou S. C. Cyclic nucleotide phosphodiesterase from Tetrahymena. Comp Biochem Physiol B. 1973 Sep 15;46(1):93–97. doi: 10.1016/0305-0491(73)90048-5. [DOI] [PubMed] [Google Scholar]
- Rozensweig Z., Kindler S. H. Epinephrine and serotonin activation of adenyl cyclase from Tetrahymena pyriformis. FEBS Lett. 1972 Sep 15;25(2):221–223. doi: 10.1016/0014-5793(72)80489-7. [DOI] [PubMed] [Google Scholar]
- Rudack D., Davie B., Holten D. Regulation of rat liver glucose 6- phosphate dehydrogenase levels by adenosine 3', 5' -monophosphate. J Biol Chem. 1971 Dec 25;246(24):7823–7824. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Schlanderer G., Dellweg H. Cyclid AMP and catabolite repression in yeasts, In Schizosaccharomyces pombe glucose lowers both intracellular adenosine 3':5'-monophosphate levels and the activity of catabolite-sensitive enzymes. Eur J Biochem. 1974 Nov 1;49(1):305–316. doi: 10.1111/j.1432-1033.1974.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Spelsberg T. C., Kleinsmith L. J. Nonhistone chromosomal proteins and gene regulation. Science. 1974 Mar 1;183(4127):817–824. doi: 10.1126/science.183.4127.817. [DOI] [PubMed] [Google Scholar]
- Stern E. S., Krooth R. S. Studies on the regulation of the three enzymes of the Leloir pathway in cultured mammalian cells. II. A search for quantitative interrelationships between the three enzyme activities. J Cell Physiol. 1975 Aug;86(1):105–110. doi: 10.1002/jcp.1040860112. [DOI] [PubMed] [Google Scholar]
- Sy J., Richter D. Content of cyclic 3',5'-adenosine monophosphate and adenylyl cyclase in yeast at various growth conditions. Biochemistry. 1972 Jul 18;11(15):2788–2791. doi: 10.1021/bi00765a009. [DOI] [PubMed] [Google Scholar]
- Tao M., Schweiger M. Stimulation of galactokinase synthesis in Escherichia coli by adenosine 3',5'-cyclic monophosphate. J Bacteriol. 1970 Apr;102(1):138–141. doi: 10.1128/jb.102.1.138-141.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureta T., Radojković J., Niemeyer H. Inhibition by catecholamines of the induction of rat liver glucokinase. J Biol Chem. 1970 Sep 25;245(18):4819–4824. [PubMed] [Google Scholar]
- Van Wijk R., Konijn T. M. Cyclic 3', 5'-amp in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971 Mar 5;13(3):184–186. doi: 10.1016/0014-5793(71)80231-4. [DOI] [PubMed] [Google Scholar]
- Weiner N. The role of cyclic AMP in the activation of tyrosine hydroxylase during nerve stimulation [proceedings]. Psychopharmacol Bull. 1977 Jan;13(1):42–44. [PubMed] [Google Scholar]
- Winkler H. H. A hexose-phosphate transport system in Escherichia coli. Biochim Biophys Acta. 1966 Mar 28;117(1):231–240. doi: 10.1016/0304-4165(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Wolfe J. Cell division, ciliary regeneration and cyclic AMP in a unicellular system. J Cell Physiol. 1973 Aug;82(1):39–48. doi: 10.1002/jcp.1040820105. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]
- Zacchello F., Benson P. F., Brown S., Croll P., Giannelli F. Induction of galactokinase in fibroblasts from heterozygous and homozygous subjects. Nat New Biol. 1972 Sep 20;239(90):95–96. doi: 10.1038/newbio239095a0. [DOI] [PubMed] [Google Scholar]


