Abstract
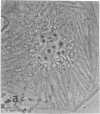
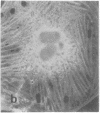
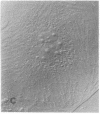
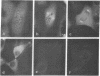
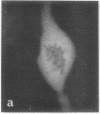
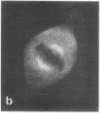
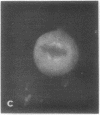
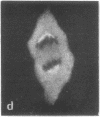
Calcium-dependent regulator protein is a low molecular weight (17,000), thermostable, calcium binding protein which is structurally homologous to skeletal muscle troponin C. This protein is present in all nonmuscle cells and has been shown to decorate stress fibers in interphase cells by indirect immunofluorescence. Using this procedure we have investigated the distribution of the protein during mitosis of eukaryotic cells. As the cells enter prophase, the distinct cytoplasmic localization disappears commensurate with the dissolution of the cytoskeleton. The regulator protein seems to be randomly distributed throughout the prophase cell, including the region around the condensed chromosomes. However, at prometaphase, it is localized in association with the half-spindles of the mitotic apparatus. Through metaphase and most of anaphase, the protein remains localized between the chromosomes and the poles of the spindle. During late anaphase the protein is also found in the interzone region but rapidly condenses into two small regions, one on each side of the midbody that separates the daughter cells. The regulator protein is not localized in the cleavage furrow during telophase, whereas actin is demonstrable in this region. Indeed, placement of the protein during mitosis is distinct from both that of actin and that of tubulin. The localization of calcium-dependent regulator protein during mitosis suggests that it may mediate the calcium effects on the mitotic apparatus and thus play a role in chromosome movement.
Keywords: calcium binding protein, mitosis, microfilaments, microtubules
Full text
PDF
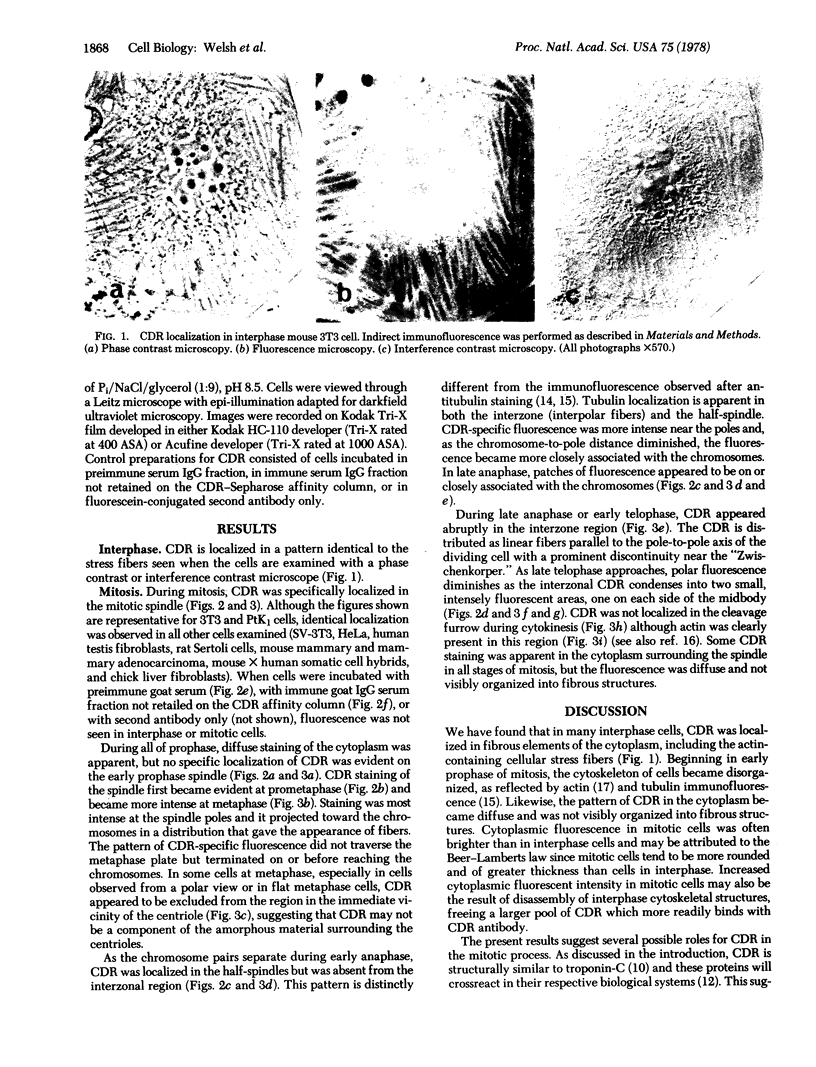
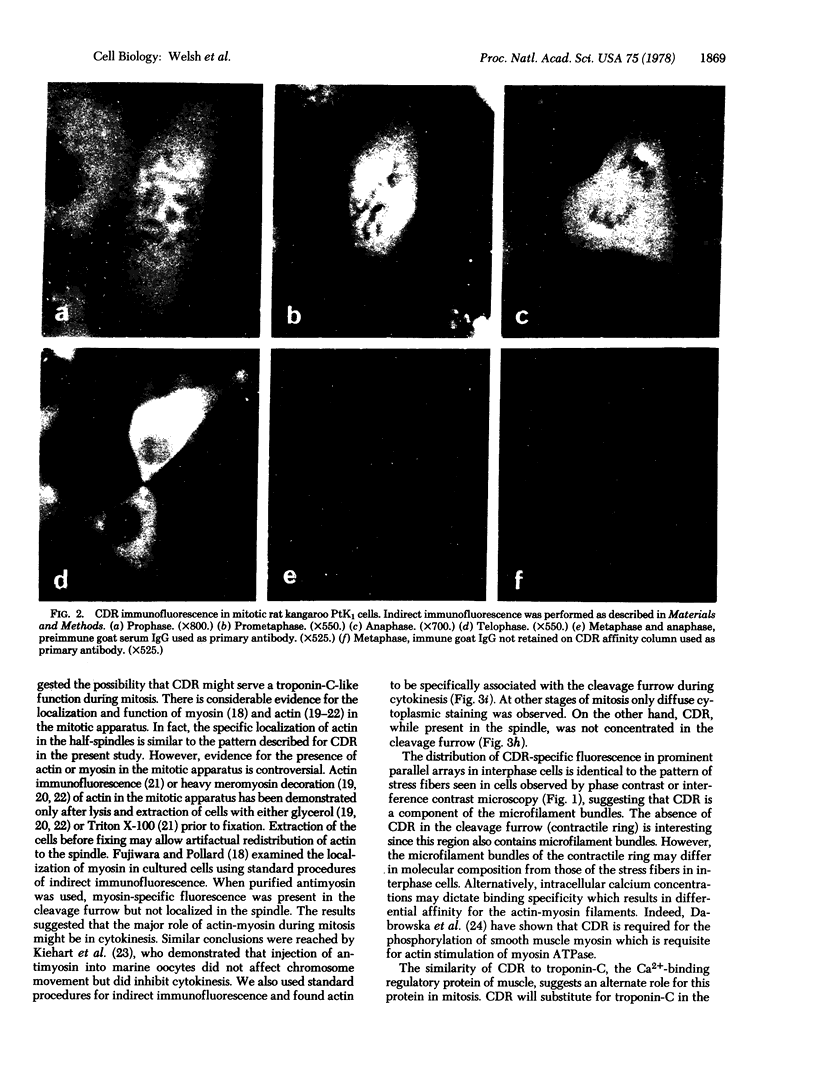
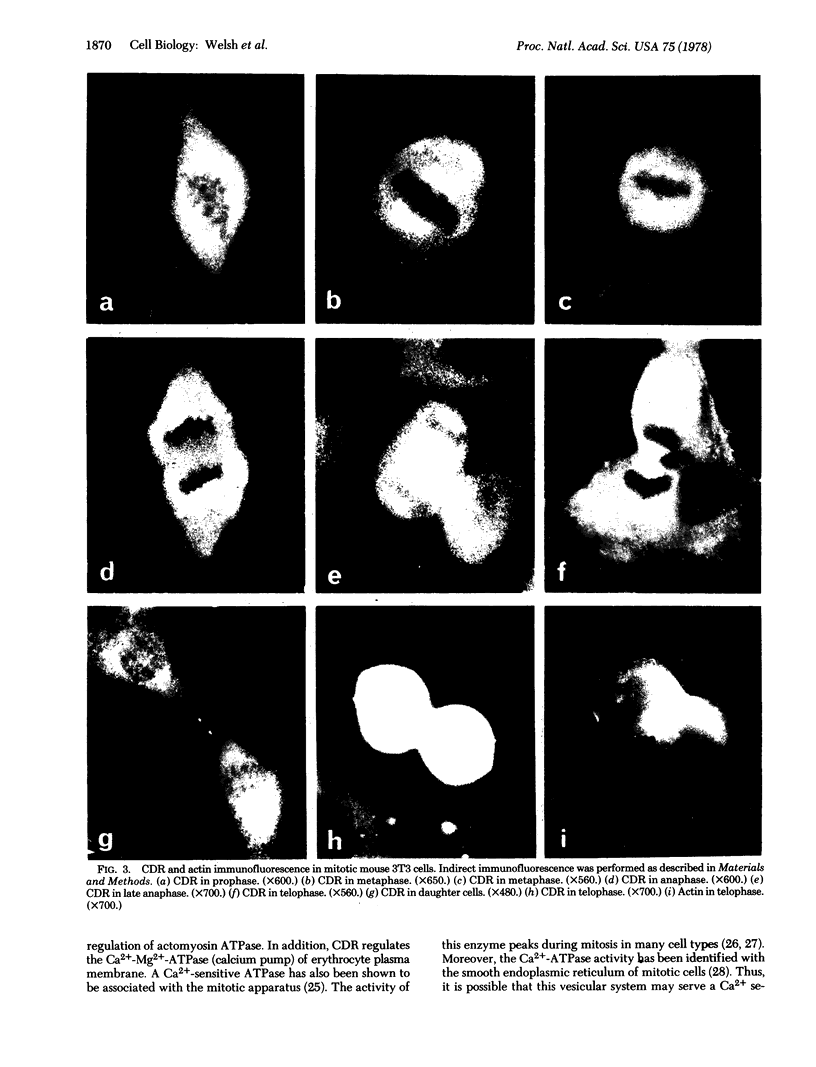

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande W. Z., Lazarides E., McIntosh J. R. A comparison of the distribution of actin and tubulin in the mammalian mitotic spindle as seen by indirect immunofluorescence. J Cell Biol. 1977 Mar;72(3):552–567. doi: 10.1083/jcb.72.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Dabrowska R., Aromatorio D., Sherry J. M., Hartshorne D. J. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1263–1272. doi: 10.1016/0006-291x(77)91429-2. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Jackson R. L., Johnson J. D., Means A. R. Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. J Biol Chem. 1977 Dec 10;252(23):8415–8422. [PubMed] [Google Scholar]
- Dedman J. R., Potter J. D., Means A. R. Biological cross-reactivity of rat testis phosphodiesterase activator protein and rabbit skeletal muscle troponin-C. J Biol Chem. 1977 Apr 10;252(7):2437–2440. [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Fuseler J. W. Temperature dependence of anaphase chromosome velocity and microtubule depolymerization. J Cell Biol. 1975 Dec;67(3):789–800. doi: 10.1083/jcb.67.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. The role of membranes in the ogranization of the mitotic apparatus. Exp Cell Res. 1975 Sep;94(2):409–425. doi: 10.1016/0014-4827(75)90507-8. [DOI] [PubMed] [Google Scholar]
- Hinkley R., Telser A. Heavy meromyosin-binding filaments in the mitotic apparatus of mammaliam cells. Exp Cell Res. 1974 May;86(1):161–164. doi: 10.1016/0014-4827(74)90662-4. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y., Uenishi K., Miyamoto E. Multiple cyclic nucleotide phosphodiesterase activities from rat tissues and occurrence of a calcium-plus-magnesium-ion-dependent phosphodiesterase and its protein activator. Biochem J. 1975 Jan;146(1):109–120. doi: 10.1042/bj1460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Petzelt C., Williams R. O., Meza I. A Ca-activated ATPase in the mitotic apparatus of the sea urchin egg (isolated by a new method). Exp Cell Res. 1972 Feb;70(2):325–332. doi: 10.1016/0014-4827(72)90143-7. [DOI] [PubMed] [Google Scholar]
- Petzelt C., Auel D. Synthesis and activation of mitotic Ca2+-adenosinetriphosphatase during the cell cycle of mouse mastocytoma cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1610–1613. doi: 10.1073/pnas.74.4.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzelt C., von Ledebur-Villiger M. Ca2+-stimulated ATPase during the early development of parthenogenetically activated eggs of the sea urchin Paracentrotus lividus. Exp Cell Res. 1973 Sep;81(1):87–94. doi: 10.1016/0014-4827(73)90114-6. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Presence of actin during chromosomal movement. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2451–2455. doi: 10.1073/pnas.72.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Ellis G. W., Inoué S. Microtubular origin of mitotic spindle form birefringence. Demonstration of the applicability of Wiener's equation. J Cell Biol. 1975 Dec;67(3):501–517. doi: 10.1083/jcb.67.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A., Milsted A., Goldman R. D. Myosin subfragment binding for the localization of actin-like microfilaments in cultured cells. A light and electron microscope study. J Cell Biol. 1977 Sep;74(3):794–815. doi: 10.1083/jcb.74.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1688–1692. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoake J. A., Song S. Y., Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Distribution and developmental changes of the enzyme and its protein activator in mammalian tissues and cells. Biochim Biophys Acta. 1974 Apr 25;341(2):402–411. doi: 10.1016/0005-2744(74)90233-2. [DOI] [PubMed] [Google Scholar]
- Stevens F. C., Walsh M., Ho H. C., Teo T. S., Wang J. H. Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. J Biol Chem. 1976 Aug 10;251(15):4495–4500. [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Waisman D., Stevens F. C., Wang J. H. The distribution of the Ca++-dependent protein activator of cyclic nucleotide phosphodiesterase in invertebrates. Biochem Biophys Res Commun. 1975 Aug 4;65(3):975–982. doi: 10.1016/s0006-291x(75)80481-5. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Teo T. S., Ho H. C., Stevens F. C. Bovine heart protein activator of cyclic nucleotide phosphodiesterase. Adv Cyclic Nucleotide Res. 1975;5:179–194. [PubMed] [Google Scholar]