Abstract
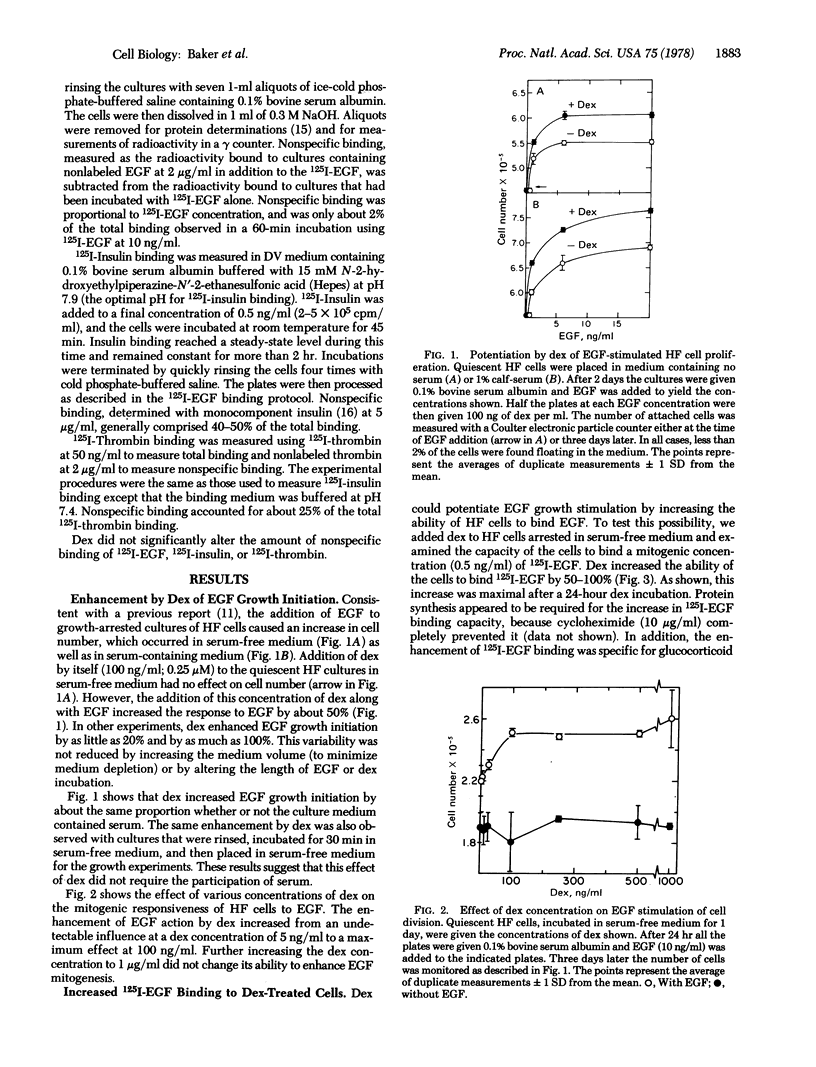
Experiments probing the mechanism by which glucocorticoids modulate cell proliferation were carried out on serum-free cell cultures of quiescent human diploid foreskin (HF) cells. Added alone, the synthetic glucocorticoid dexamethasone had no effect on cell number. However, dexamethasone enhanced the mitogenic response of HF cells to epidermal growth factor (EGF) by 50% at all EGF concentrations. The mitogenic action of EGF was maximally promoted by a dexamethasone concentration of 100 ng/ml (0.25 μM).
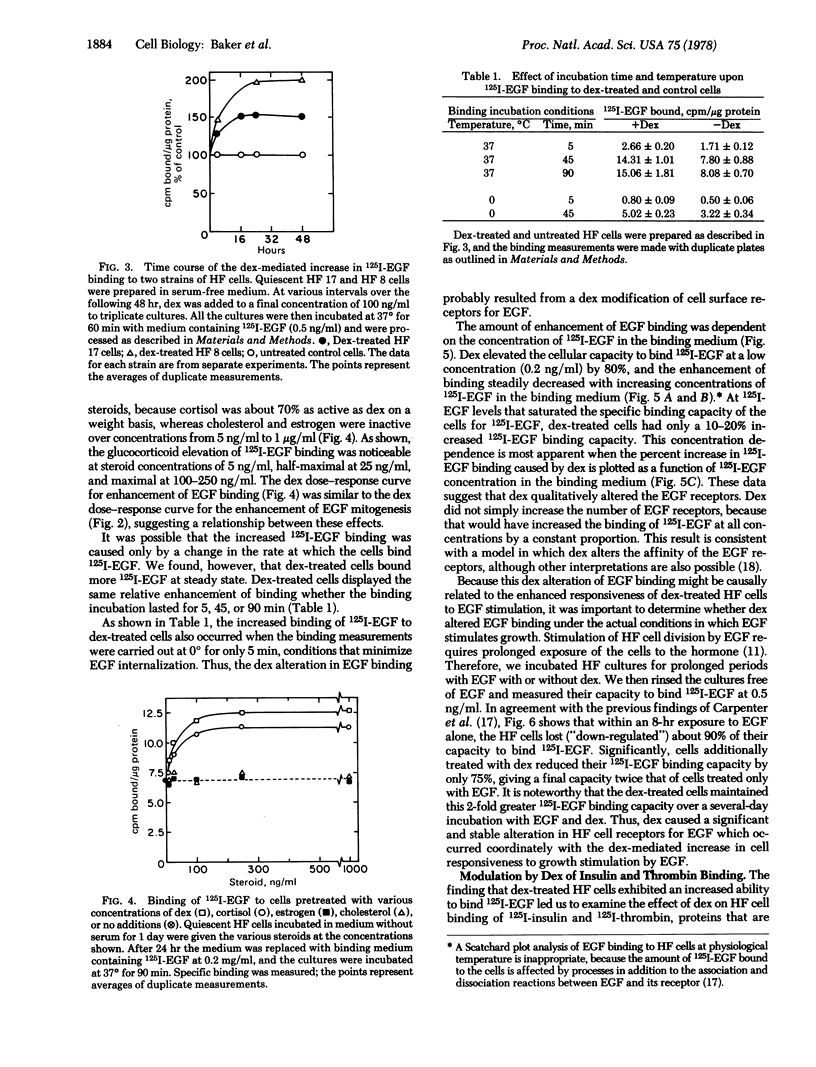
Binding studies with 125I-labeled EGF (125I-EGF) suggested that dexamethasone caused this “permissive” effect by modulating cell surface receptors for EGF. Paralleling their increased responsiveness to EGF growth stimulation, dexamethasone-treated cells exhibited a 50-100% increased ability to bind physiological concentrations of 125I-EGF. A binding increase was apparent after a 4-hr dexamethasone treatment. The dexamethasone-treated cells maintained an increased ability to bind 125I-EGF during the prolonged exposure to EGF that was required to stimulate cell division. Moreover, the increase in 125I-EGF binding exhibited a dexamethasone dose-dependence similar to that for the enhancement of EGF mitogenesis, suggesting a relationship between the dexamethasone effects on binding and growth.
An investigation of the binding increase showed that it was specific for glucocorticoids, and required protein synthesis. The enhancement of 125I-EGF binding diminished with increasing concentrations of 125I-EGF, indicating that dexamethasone caused a qualitative change in the EGF receptors (possibly a change in receptor affinity or cooperativity). The alteration in 125I-EGF binding may occur as part of a far-reaching dexamethasone-mediated change in the cell surface, because dexamethasone treatment slightly increased the ability of HF cells to bind 125I-insulin, and decreased by half their ability to bind 125I-thrombin.
Keywords: permissive effects, human diploid fibroblasts, cell proliferation, insulin, thrombin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P. L., Tomkins G. M. Hormone induced modification of the cell surface. Nature. 1969 Oct 25;224(5217):344–345. doi: 10.1038/224344a0. [DOI] [PubMed] [Google Scholar]
- Boyd K. S., Melnykovych G., Fiskin A. M. A replica technique for visualizing the distribution of lanthanum-binding sites over HeLa surfaces. Cytobiologie. 1976 Dec;14(1):91–101. [PubMed] [Google Scholar]
- Braun T., Hechter O. Glucocorticoid regulation of ACTH sensitivity of adenyl cyclase in rat fat cell membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):995–1001. doi: 10.1073/pnas.66.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Friedmann N., Wong E. H., Brineaux J. P., Corbin J. D., Park C. R. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J Biol Chem. 1972 Jun 10;247(11):3579–3588. [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Gospodarowicz D., Handley H. H. Stimulation of division of Y1 adrenal cells by a growth factor isolated from bovine pituitary glands. Endocrinology. 1975 Jul;97(1):102–107. doi: 10.1210/endo-97-1-102. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Arquilla E. R. Monoiodoinsulin. Preparation, purification, and characterization of a biologically active derivative substituted predominantly on tyrosine A14. J Biol Chem. 1974 Jan 10;249(1):21–32. [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Jones K. L., Addison J. Studies of human diploid fibroblast growth. I. Responses of normal and hypopituitary cells to fibroblast growth factor, insulin, and serum. J Clin Endocrinol Metab. 1976 Oct;43(4):721–729. doi: 10.1210/jcem-43-4-721. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin B. M., Wasiewski W. W., Fenton J. W., 2nd, Detwiler T. C. Equilibrium binding of thrombin to platelets. Biochemistry. 1976 Nov 2;15(22):4886–4893. doi: 10.1021/bi00667a021. [DOI] [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Stimulation of DNA synthesis and cell division in a chemically defined medium: effect of epidermal growth factor, insulin and vitamin B12 on resting cultures of 3T6 cells. Biochem Biophys Res Commun. 1976 Nov 22;73(2):271–278. doi: 10.1016/0006-291x(76)90703-8. [DOI] [PubMed] [Google Scholar]
- Nagaiah K., MacDonnell P., Guroff G. Induction of tyrosine hydroxlase synthesis in rat superior cervical ganglia in vitro by nerve growth factor and dexamethasone. Biochem Biophys Res Commun. 1977 Apr 25;75(4):832–837. doi: 10.1016/0006-291x(77)91457-7. [DOI] [PubMed] [Google Scholar]
- RUHMANN A. G., BERLINER D. L. EFFECT OF STEROIDS ON GROWTH OF MOUSE FIBROBLASTS IN VITRO. Endocrinology. 1965 May;76:916–927. doi: 10.1210/endo-76-5-916. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schmidtke J., Wienker T., Flügel M., Engel W. In vitro inhibition of cyclic AMP phosphodiesterase by cortisol. Nature. 1976 Aug 12;262(5569):593–594. doi: 10.1038/262593a0. [DOI] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Sodoyez J. C., Sodoyez-Goffaux F., Goff M. M., Zimmerman A. E., Arquilla E. R. [127-I]- or carrier-free [125-I]monoiodoinsulin. J Biol Chem. 1975 Jun 10;250(11):4268–4277. [PubMed] [Google Scholar]
- Temin H. M. Studies on carcinogenesis by avian sarcoma viruses. VI. Differential multiplication of uninfected and of converted cells in response to insulin. J Cell Physiol. 1967 Jun;69(3):377–384. doi: 10.1002/jcp.1040690314. [DOI] [PubMed] [Google Scholar]
- Thrash C. R., Cunningham D. D. Stimulation of division of density inhibited fibroblasts by glucocorticoids. Nature. 1973 Apr 6;242(5397):399–401. doi: 10.1038/242399a0. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


