Abstract
Acute intestinal ischemia is a medical emergency with a high mortality rate, attesting to the need for a better understanding of its pathogenesis and the development of effective therapies. The goal of this study was to delineate the relationships among intracellular and extracellular events in intestinal ischemia/reperfusion (I/R) injury, particularly the formation of reactive oxygen species (ROS), cell membrane instability associated with lipid peroxidation and the innate autoimmune response mediated by natural IgM and complement. A murine model of natural IgM-mediated intestinal I/R was used. Mice overexpressing anti-oxidant enzyme SOD1 were found to have significantly reduced intestinal tissue damage and complete blockage of IgM-mediated complement activation compared with WT controls. To determine if cell membrane instability was an event intermediate between ROS formation and natural IgM-mediated innate autoimmune response, the cell membrane stabilizer (trehalose) was administered to WT mice prior to the induction of intestinal ischemia. Treatment with trehalose significantly protected animals from I/R injury and inhibited IgM-mediated complement activation although it did not prevent membrane lipid peroxidation. These data indicate that in normal mice subjected to I/R injury, intracellular ROS formation is an event upstream of the lipid peroxidation which results in cell membrane instability. The membrane instability leads to an innate autoimmune response by natural IgM and complement. Trehalose, a nontoxic disaccharide tolerated well by animals and humans, has promise as a protective agent for patients with medical conditions related to acute intestinal ischemia.
Keywords: reactive oxygen species, cell membrane instability, natural IgM, complement, reperfusion injury, trehalose
1. Introduction
Splanchnic ischemia can occur in a wide spectrum of clinical conditions including gastrointestinal and aortic surgery, critical illness, as a consequence of hemodialysis, atherosclerosis, and the use of causative medication (e.g. alpha-adrenergic agents) (Kolkman and Geelkerken, 2005). The deadliest form is acute mesenteric ischemia, a medical emergency with a mortality rate of 70–90%, representing 30% of intestinal ischemia cases (Brandt, 2003). Immediate reperfusion is required in these cases, but may result in acute tissue injury locally and systemically (Kolkman and Geelkerken, 2005). Currently there is no clinically approved treatment. A better understanding of the pathogenesis is required if effective therapies are to be developed.
Pathogenic events occur during intestinal I/R include the production of reactive oxygen species (ROS) (Grisham et al., 1986; Li and Jackson, 2002; Watts and Kline, 2003) and damage to cell membranes (Moore et al., 1995). Ischemia also triggers an acute inflammatory response that involves natural IgM and the complement system and mimics an autoimmune response (Zhang and Carroll, 2007). In particular, a specific self-reactive IgM has been isolated which can induce intestinal I/R injury (Zhang et al., 2004). Two ischemic self-antigens (non-muscle myosin heavy chain-II, NMHC-II, isoforms A & C) were identified as targets for this pathogenic IgM (Zhang et al., 2006). In addition, autoantibodies against negatively charged phospholipids were found to induce intestinal I/R injury (Fleming et al., 2004). Thus the innate autoimmune response makes an important contribution to acute intestinal I/R injury (Zhang and Carroll, 2007). However, the relationships between the newly discovered innate autoimmunity and other known pathogenic events, i.e. ROS formation and cell membrane damage, remain unclear.
ROS are generated by multiple intracellular sources during I/R and are removed by intracellular antioxidant enzymes (Eberhardt, 2001). Specifically, superoxide dismutase-1 (SOD1) converts intracellular •O2− to H2O2 which is subsequently removed by glutathione peroxidase and catalase. Administering SOD1 or genetically over-expressing this enzyme can prevent intestinal I/Rinduced inflammatory cell recruitment (Kurose et al., 1998; Russell et al., 2000). An important consequence of excessive ROS production is cell membrane damage via lipid peroxidation (Toledo-Pereyra et al., 2004). Our hypothesis is that during intestinal I/R, ROS cause cellular changes, particularly cell membrane damage, which results in exposure of self-antigens to natural IgM. If ROS are effectively scavenged by overexpression of antioxidant enzymes, the exposure of ischemic antigens on the cell surface will be minimized or prevented and IgM-mediated complement activation will be lowered or abolished. Further, if cell membrane damage occurs between ROS formation and the innate autoimmune response involving natural IgM, effective preservation of cell membrane integrity should block IgM-mediated complement activation but not ROS formation. We believe that determining the relationships between these intracellular and extracellular pathogenic events will allow identification of specific target(s) for therapeutic intervention in intestinal I/R injury.
2. Materials and Methods
2.1. Animals and intestinal model of I/R injury
Wild type C57BL/6J and SOD1 transgenic (tg) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All experiments were performed in compliance with the requirements of the National Institute of Health and the Institutional Animal Care and Use committee of SUNY-Downstate Medical Center.
Intestinal I/R was induced surgically as previously described (Zhang et al., 2004). Briefly, male mice, 8–12 weeks old, were anesthetized (pentobarbital, 60 mg/kg, i.p.). A laparotomy was performed and a microclip (125 g of pressure; Roboz, MD) was applied to the superior mesenteric artery. After 40 minutes of ischemia, the microclip was removed and animals were kept warm for 3 hours of reperfusion. At the end of reperfusion, the ischemic segment of the jejunum was harvested and the central 4-cm section was excised for pathological analysis and immunohistochemistry. Sham control animals underwent laparotomy without the application of the microclip to the superior mesentery artery.
Trehalose (Sigma, MO) was dissolved in normal saline at a concentration of 100 mg/ml and administered i.p. at 1g/kg to WT mice (n=11) 30 minutes prior to intestinal ischemia surgery. Normal saline was used i.p. in the control animals (n=11).
For histopathology analyses, a semi-quantitative pathology score was assessed on sections stained with Hematoxylin and Eosin (H&E). The pathology score is based on a previously described procedure that directly inspects the injured villi over 4-cm of the jejunum (Zhang et al., 2004).
Pathology score = S/(V + [P × 25]) × 50% + D/(V + [P × 25]) × 100% + (P × 25)/(V + [P × 25]) × 100%
where V = total number of villi; S = number of villi which have a subepithelial space; D = number of villi which have epithelial disruption; P = the number of 10× objective power fields with complete loss of villi (each power field in unaffected intestine will have 25 villi).
2.2. Immunohistochemical analyses
Cryosections of intestinal tissues were fixed with 4% paraformaldehyde, washed with phosphate buffered saline (PBS) and blocked with 2% bovine serum albumin (BSA) buffer. To detect IgM and complement C3 deposition, the cryosections were incubated with biotin-labeled goat antimouse IgM (2.5µg/ml; SouthernBiotech, AL), followed by staining with streptavidin-Alexa-568 (4µg/ml; Molecular Probes, CA). The deposition of C3 was detected using FITC-labeled anti-C3c (Dako, CA).
2.3. Measurement of repertoire of pre-existing anti-NMHC-II IgM
The quantitative immunoassay used was a modification of a previously described method (Lee et al., 2010) to detect serum anti-NMHC II-IgM levels. A 96-well flat-bottom immulon 2HB plate (Thermo, MA) was coated with rabbit anti-NMHC-II A (1µg/ml; Covance, NJ) and incubated overnight at 4°C. The plate was washed and blocked with 1% BSA/0.05% Tween-20/PBS for 2 hours at room temperature. NMHC-II antigens in intestinal lysate from sham-treated mice diluted to a total protein concentration of 12 mg/ml were added to the coated wells and the plates incubated for 2 hours at room temperature. The wells were washed; 1:50 diluted serum from experimental mice was added and the plates were incubated for 2 hours at room temperature. To detect NMHC II-specific IgM bound to the captured Ag, alkaline phosphatase (AP)-labeled rabbit anti-mouse IgM (0.33µg/ml; Rockland, PA) was added and the plates were incubated for 1 hour at room temperature. The unbound antibodies were removed by washing and phosphatase substrate (Sigma, MO) was added to develop color. The optical density at 405nm was determined in a Multiscan microplate reader (Thermo, MA). The absorbance of each individual sample was converted to an amount (Unit) of the IgM of interest using a standard described previously (Lee et al., 2010).
2.4. ELISA method to measure serum C3 levels in mice with or without trehalose treatment
To determine serum C3 levels in animals with or without trehalose treatment, a 96-well flat-bottom MaxiSorp plate (Nunc, NY) was coated with a monoclonal anti-C3 Ab (Cedarlane, NC) and incubated overnight at 4°C. The plate was washed and blocked with blocking buffer (1% BSA/0.05% Tween-20 in PBS) for 2 hours at room temperature. The plate was washed again. Serum (1:1,000 dilution in blocking buffer) from mice with or without trehalose treatment (from Methods, Section 2.1) was added to the plate which was incubated for 1 hour at room temperature. The plate was then washed 3 times. To detect the captured C3, a goat polyclonal anti-human C3 Ab (Comptech, TX) was added and the plate was incubated for 1 hour at room temperature. After washing, a secondary antibody, rabbit anti-goat IgG-AP (Abcam, MA) was added to the plate which was incubated for 1 hour at room temperature. The unbound antibodies were washed out and phosphatase substrate was added to develop color. The optical density at 405 nm was determined in a Multiscan microplate reader.
2.5. An ELISA method to evaluate binding between natural IgM and trehalose
To determine if there was any specific interaction between IgM and trehalose, a 96-well flat-bottom MaxiSorp plate was coated with 1mg/ml trehalose and incubated overnight at 4°C. BSA (1mg/ml) was used as a negative control to coat additional wells in the same plate. Because natural IgM is known for its polyreactivity towards self-antigens (Zhang and Carroll, 2007), the binding of IgM to intracellular proteins can serve as a positive control in an ELISA assay to test IgM binding to other factors. To provide self-antigens, a soluble cytosolic protein fraction was purified from naive WT mouse heart lysate (the purification followed a published protocol (Zhang et al., 2010)) and used at a concentration of 1 mg/ml to coat additional wells. The plate was washed once and blocked with 1% BSA/0.05% Tween-20 in PBS for 1 hour at room temperature. The plate was washed once. Purified IgM from naïve WT mice at a concentration of 100 µg/ml (Millipore, CA) was added to the plate which was incubated for 1 hour at room temperature. The plate was then washed 3 times. To detect any bound IgM, AP-labeled rabbit anti-mouse IgM was added to the plate and incubated for 1 hour at room temperature. The unbound antibodies were removed by washing with 0.05% Tween-20/PBS and phosphatase substrate was added to develop color reaction. The optical density at 405 nm was determined in a Multiscan microplate reader. Experiments were performed in triplicate.
2.6. Detection of lipid peroxidation by the malondialdehyde (MDA) assay in intestinal tissue lysates
Lipid peroxidation was assessed using the LPO-586 assay kit (OxisResearch, OR), which detects MDA. MDA is produced as a byproduct of polyunsaturated fatty acid peroxidation and arachidonic acid metabolism and considered an indicator of lipid peroxidation (Halliwell and Chirico, 1993). Lipid standards used in the assays and tissue homogenates were incubated for 1 hour in a 45°C water bath with N-methyl-2-phenylindole (NM2P, dissolved in acetonitrile). The ratio of cell lysate to the volume of NM2P solution was 1:3.25.
2.7. Detection of lipid peroxidation by assaying for 4-HNE in intestinal cryosections
4-HNE is an α,β-unsaturated hydroxyalkenal that is produced by lipid peroxidation in cell membranes (Esterbauer et al., 1991). Intestinal sections were stained with an anti-4-HNE Ab (LifeSpan Biosciences, WA) followed by a FITC-labeled secondary Ab. The numbers of 4-HNE positive villi and total villi in the section were counted under a fluorescent microscope. The percentage of 4-HNE positive villi/total villi were calculated for each mouse.
2.8. Statistical analysis
Data were entered into a Microsoft Excel database and SPSS software was used for quantitative data and statistical analyses. Data were presented as means ± standard error of the mean (SEM). Comparisons between two groups for significance were performed using the independent t-test (two-tailed, unequal variance). For multi-group analyses, Levene’s test was used first to determine the homogeneity of variances. Statistical significance was then assessed by a one-way ANOVA test followed by a post hoc Bonferroni's test (when the equality of variances assumption held) or Dunnett T3 test (when the equality of variances was not met). P<0.05 was considered to be statistically significant.
3. Results
3.1. ROS formation is a pathogenic event upstream of the natural IgM-mediated complement activation that occurs in intestinal I/R injury
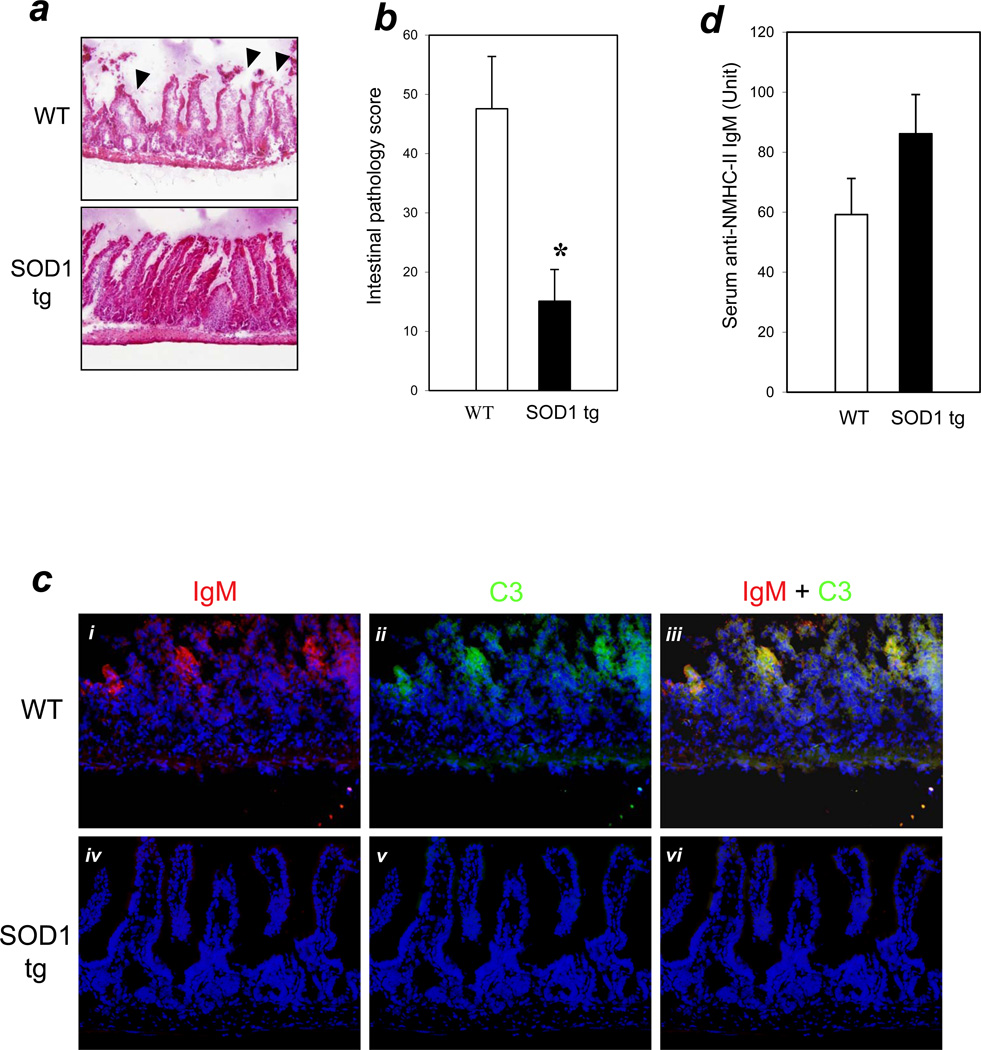
To investigate the relationship between ROS formation and the natural IgM-mediated inflammatory response, we used tg mice that overexpressed the anti-oxidant enzyme SOD1 in an intestinal I/R injury model (40 minutes ischemia / 3 hours reperfusion). Histopathological analyses of intestinal tissues showed that SOD1 tg mice had significantly reduced mucosal damage compared with WT control mice (pathology scores: SOD1 tg =15±5, n=11; WT=48±9, n=12; P < 0.01) (Figures 1a & 1b).
Figure 1. SOD1 overexpression decreased tissue injury and blocked IgM-mediated complement activation in the intestinal I/R model.
(a) Intestinal I/R (40 min./3 hrs.) was carried out and H&E staining performed as described in Methods. Arrows indicate typical pathology features of villi injury (200× magnification). (b) Bar graph: bars indicate mean pathology scores based on the degree of villi injury as described in Methods. Error bars indicate standard error of the mean (SEM). Asterisks indicate statistical significance (P<0.01; n=12 for WT, n=11 for SOD1 tg). (c) SOD1 overexpression results in the blocking of IgM and C3 binding in vivo. Panels i–iii: WT mice; panels iv-vi: SOD1 tg mice. Representative cryosections were stained with anti-IgM-biotin/streptavidin-Alexa-568 (red fluorescence in panels i and iv) and anti-C3-FITC (green fluorescence in panels ii and v). Sections were counterstained with DAPI (Violet/Blue). Panels iii and vi are merged images of (i + ii) and iv + v) respectively. Magnification=400×. (d) Serum levels of anti-NMHC-II IgM were similar in SOD1 tg and WT mice (n=5 for each group; P>0.05 between these groups).
To investigate whether SOD1 overexpression blocked natural IgM-mediated complement activation in the intestinal villi, intestinal sections of SOD1 tg and WT mice were immunostained for IgM and complement C3. WT mice were found to have co-localized depositions of IgM and C3 in the villi (Figure 1c, panels i, ii, and iii). In contrast, SOD1 tg mice had neither IgM nor C3 deposition in the villi (Figure 1c, panels iv, v, and vi). These results demonstrated that SOD1 overexpression blocked natural IgM-dependent complement activation in intestinal I/R.
To rule out the possibility that SOD1 tg mice were protected from the I/R injury due to a potential deficiency of the pathogenic natural IgM, we developed an immunoassay to quantify serum levels of anti-NMHC-II IgM. SOD1 tg mice had levels of anti-NMHC-II IgM similar to those of WT mice (86.1 ± 13.1 Unit and 59.2 ± 12 Unit, respectively, n = 5 per group, P > 0.05) (Figure 1d).
3.2. Cell membrane instability is a pathogenic event upstream of the innate autoimmune response in intestinal I/R injury
Ischemic antigens NMHC II are cytoskeletal proteins that are normally shielded by the cell membrane from extracellular immune factors. We hypothesized that cell membrane disruption by intracellular ROS formation exposed these self-antigens to pathogenic natural IgM. Thus, we speculated that cell membrane instability was an event occurring between ROS formation and the innate autoimmune response of natural IgM and complement to I/R injury.
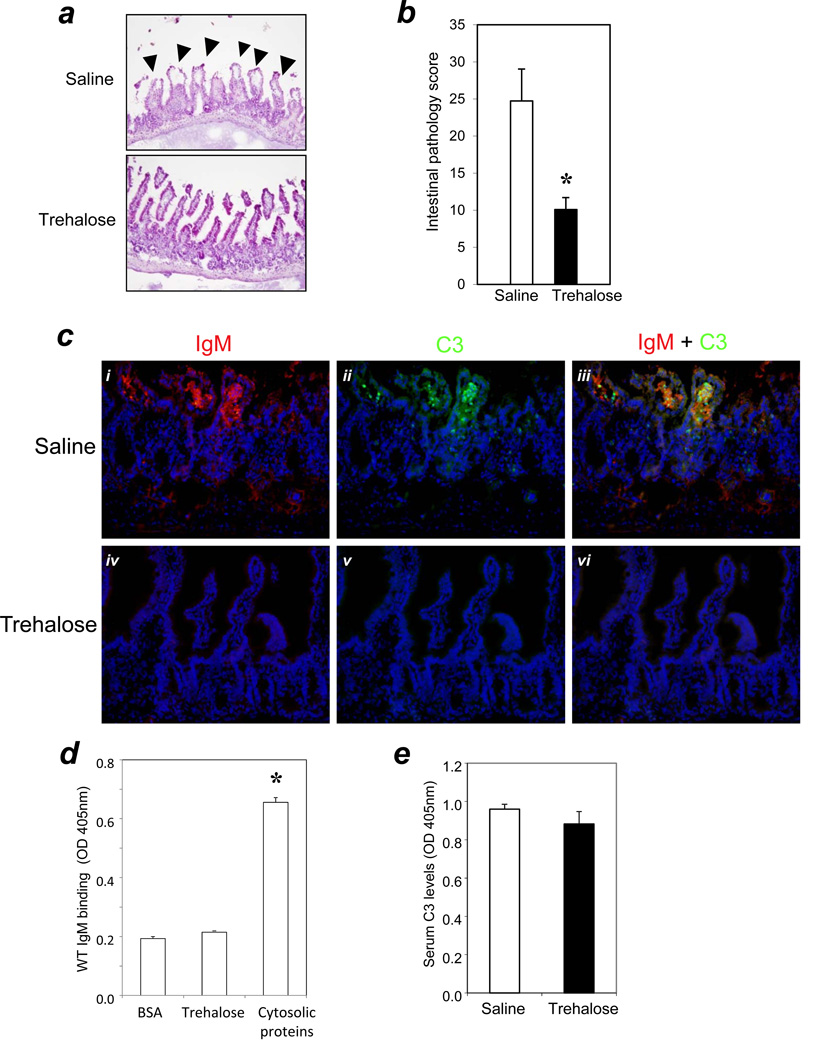
To test this hypothesis, WT mice were treated with trehalose, a cell membrane stabilizer (Beattie et al., 1997; Crowe et al., 1984; Higashiyama, 2002), prior to induction of intestinal ischemia. Saline-injected WT mice were used as controls. Histopathological analysis revealed a significant reduction in mucosal damage to the intestine in the trehalose-treated group compared with the saline control group (pathology scores: saline group = 25 ± 4, n=11; trehalose group = 10 ± 2, n=11; P < 0.01) (Figure 2a and 2b). The reduction of tissue damage was accompanied by blockage of the innate autoimmune response involving natural IgM (Figure 2c).
Figure 2. Cell membrane stabilization reduced tissue damage and blocked IgM-mediated complement response in the intestinal I/R.
(a) Trehalose or saline were administered i.p. at a dose of 1g/kg to WT mice 30 minutes prior to I/R surgery (n=11/group). H&E staining was performed as described in Methods. Arrows indicate typical pathology features of villi injury (200× magnification). (b) Bar graph: bars indicate pathology scores. Asterisks indicate statistical significance (P<0.01). (c) Trehalose treatment blocks the deposition of IgM/C3 in WT mice. Cryosections were prepared following I/R and analyzed for IgM (panels i & iv) and C3 deposition (panels ii & v). Panels iii and vi are merged images of (i + ii) and iv + v), respectively. Panels i-iii: WT mice received saline; panels iv-vi: WT mice received trehalose. Sections were stained as in Figure 1 (200 × magnification). (d) IgM does not bind specifically to trehalose. ELISAs were performed in triplicate as described in Methods. The binding of IgM to BSA served as a negative control; the binding of IgM to the cytosolic protein fraction of naive WT mouse served as a positive control. * indicated that IgM binding to the cytosolic proteins was significantly higher (P < 0.05) than IgM binding to either BSA or trehalose. (e) Trehalose treatment did not change the systemic C3 levels in mice. Serum complement C3 levels in mice with or without trehalose treatment (n=11/group) were measured by ELISA as described in Methods.
Immunohistochemistry analyses showed that neither IgM nor C3 were deposited in the intestinal tissues of trehalose treated mice (Figure 2c: iv, v, and vi). In contrast, the saline group had IgM and C3 deposition in the villi of the intestines (Figure 2c: i, ii, and iii). Thus, these results indicated that cell membrane instability was an event upstream of the IgM-mediated innate autoimmune response.
To rule out the possibility that trehalose might directly bind IgM and thus interfere with IgM-mediated complement activation, we developed an in vitro immunoassay to evaluate any interaction between trehalose and natural IgM. Our results showed no interaction between trehalose and IgM that was more specific than that between BSA and IgM (IgM binding to trehalose, 0.215 ± 0.001 absorbance units at 405 nm; IgM binding to BSA, 0.193 ± 0.004 absorbance units at 405 nm, P > 0.05) (Figure 2d). The positive control, based on the known reactivity of natural IgM for self-antigens (Zhang and Carrol, 2007) (soluble self-proteins from heart muscle cytosol were used) bound significantly to IgM (0.655 ± 0.01 absorbance units at 405 nm; P < 0.05 compared with the binding of IgM to trehalose or BSA) (Figure 2d).
To rule out the possibility that trehalose may have consumed the circulating C3 to make it less available for activation, we used an ELISA assay to test the serum C3 levels in mice with or without trehalose treatment (serum were obtained from mice in the experiment of Figure 2a). Trehalose treatment did not change the circulating C3 levels (trehalose treatment group: 0.965 ± 0.026 absorbance units at 405 nm; saline control group: 0.906 ± 0.043 absorbance units at 405 nm, P > 0.05) (Figure 2e).
3.3. Stabilizing the cell membrane by trehalose does not prevent lipid peroxidation in intestinal I/R
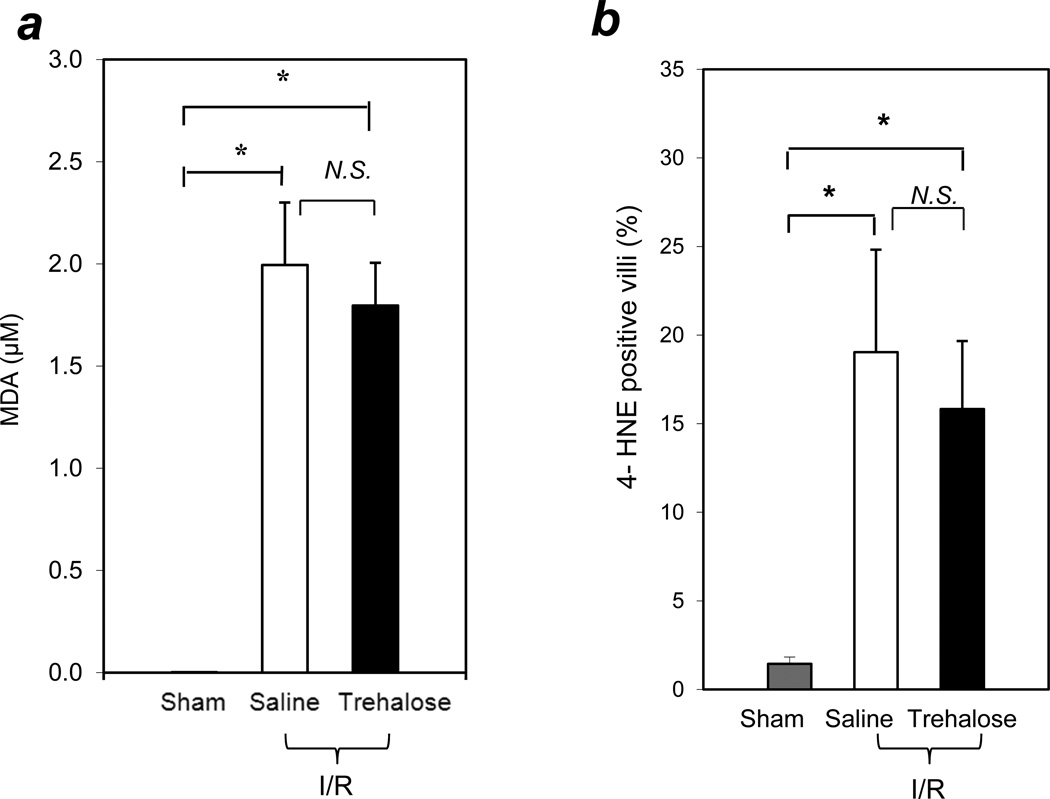
To investigate a relationship between cell membrane stabilization by trehalose and lipid peroxidation in intestinal I/R, we analyzed markers of lipid peroxidation after I/R in trehalose pretreated and saline pretreated control mice. Since a one-way ANOVA test of MDA levels with all groups showed statistical significance (P<0.01) and Levene’s test of the homogeneity of variances indicated that P<0.05, the Dunnett T3 test was used for post hoc analyses. Our results showed that after I/R, MDA levels in the intestinal tissues increased significantly in both the trehalose and saline treated groups compared with the sham operated group (Figure 3a; MDA concentrations in intestinal lysates: sham group = 0.002 ± 0.0002 µM; saline group =1.99 ± 0.31µM; trehalose-treated group = 1.8 ± 0.21µM; n=5 per group; P <0.05). There was no significant difference in MDA levels between the saline and trehalose groups.
Figure 3. Trehalose treatment did not reduce lipid oxidation in intestinal I/R.
(a) No reduction of MDA by trehalose treatment. Three groups of WT mice were studied: (i) sham-operated, (ii) I/R with saline treatment, and (iii) I/R with trehalose treatment; (n=5 per group). Intestinal lysates were prepared and MDA levels were measured as described in Methods. (b) Intestinal cryosections were stained with an anti-4 HNE Ab and a FITC-labeled secondary Ab (n=8 for the sham group; n=12 for the saline treated I/R group; n=11 for the trehalose treated I/R group). Bars indicate percent of 4-HNE positive villi in the intestinal sections. * indicates P<0.05; N.S. indicates not significant (P >0.05).
To confirm that lipid peroxidation was not changed by trehalose treatment, we examined the generation of 4-HNE in intestinal tissues. A one-way ANOVA test of the percent of 4-HNE positive villi with all groups showed statistical significance (P<0.05) and Levene’s test of the homogeneity of variances indicated that P<0.05, so the Dunnett T3 test was used for post hoc analyses. The percent of 4-HNE positive villi increased significantly in both the saline treated I/R group and the trehalose treated I/R group compared with the sham group (Figure 3b; % 4-HNE positive villi: sham group = 1.4 ± 0.4 %, n=8; saline group =19.0 ± 5.8 %, n=12; trehalose treated group = 15.8 ± 3.8 %, n=11; P < 0.05). There was no significant difference in the percent of 4-HNE positive villi between the saline and trehalose I/R groups. These findings indicated that stabilizing the cell membrane with trehalose did not alter the lipid peroxidation present after I/R. We hypothesize that trehalose stabilizes the membrane against the instability resulting from lipid peroxidation.
4. Discussion
This study has established that ROS formation resulting from I/R is an event upstream of the innate autoimmune response by natural IgM that occurs in intestinal I/R injury (Figure 1). Cell membrane lipid peroxidation due to the formation of ROS occurs whether or not the disaccharide trehalose is present (Figure 3). In the absence of trehalose, cytoplasmic self-antigens break through the destabilized membrane and can be bound by circulating natural IgM (Figure 1c, upper panel, Figure 2c, upper panel). In the presence of trehalose, despite the existence of membrane lipid peroxidation, trehalose stabilizes (covers) the cell membrane, preventing interaction of IgM and self-antigen and subsequent binding of C3 (Figure 2, lower panel). Taken together, our results support the hypothesis that during intestinal I/R, intracellular ROS formation leads to cell membrane damage, which in turn exposes self-antigens to circulating natural IgM followed by complement activation. An important implication is the establishment of a link in the pathogenesis of intestinal I/R between a major intracellular event and the extracellular innate autoimmune response.
The mechanism of intestinal I/R injury appears to be multi-factorial. In addition to the three pathogenic events investigated in this study, ROS, an innate immune response and lipid peroxidation, other players were recently implicated in intestinal I/R injury, e.g., IL-17A (Lee et al., 2013), free fatty acids (Qin et al., 2012), and Toll-like receptor (Watanabe et al., 2012). Questions remain, however. For instance, TLR4 deficient mice sustained less tissue damage and C3 deposition after I/R than WT mice, but additional complement inhibition by CR2-Crry did not further reduce tissue damage (Pope et al., 2010). Thus, TLR4 may function upstream of complement activation in intestinal I/R. It remains unclear whether TLR4 functions upstream of lipid peroxidation related cell membrane instability. This could be investigated by administering trehalose to TLR4 deficient mice to determine if there is further reduction of tissue damage.
An implication of our findings is that stabilization of the cell membrane may provide a new way to intervene in the innate autoimmune response in I/R. In the past, inhibitors directed against terminal complement factors, e.g. C5, have been tested as a way to prevent I/R injury. However, attempts to curtail C5 activation have not had improved outcomes in clinical trials of patients with ischemic heart disease (Testa et al., 2008). A possible explanation is that C5 operates in the common pathway of complement. Activation of upstream complement factors may affect pathways traditionally thought to be separate from the complement pathway, e.g. coagulation (Amara et al., 2008; Opal and Esmon, 2003), thus influencing clinical outcomes. Our results showed that treatment with trehalose significantly blocked IgM deposition, the initiating step in complement activation in intestinal I/R injury leading to tissue damage (Figure 2). Thus, stabilizing the cell membrane with trehalose despite ROS-induced lipid peroxidation was able to effectively prevent the IgM-mediated complement response and tissue damage, providing a method upstream of C5 for preventing intestinal I/R injury.
Targeting cell membrane instability could also be an alternative approach to targeting the effects of ROS. A number of pre-clinical studies reported that SOD1 could reduce tissue damage in heart ischemia models (Ambrosio and Flaherty, 1992; Gross et al., 1986; Hangaishi et al., 2001; Kanamasa et al., 2001; Nishikawa et al., 1991; Otani et al., 1986; Wang et al., 1998), but others found that it failed to show protection (Gallagher et al., 1986; Jones et al., 2003; Klein et al., 1988; Matsuda et al., 1991; Nejima et al., 1989; Ooiwa et al., 1991; Patel et al., 1990; Przyklenk and Kloner, 1989; Richard et al., 1988; Uraizee et al., 1987; Watanabe et al., 1993). Attempts to use ROS scavengers to treat I/R injury in clinical settings have been unsuccessful (Flaherty et al., 1994; Murohara et al., 1991). A possible explanation is that intracellular ROS are generated rapidly in I/R as demonstrated recently in our myocardial I/R model (Charlagorla et al., 2013). Thus the delivery of anti-oxidants into cells after the onset of ischemia may be either too late or provide insufficient amounts. Barriers to the delivery of anti-oxidants include timely entry into ischemia tissue, passage through the cell membrane, and achieving effective concentrations in ischemic tissue without raising systemic drug concentrations to toxic levels. Our results showed that trehalose pretreatment decreased tissue injury despite the ROS-induced membrane damage in intestinal I/R. Thus, even though tissues suffered an initial ischemic attack with ROS formation, targeting cell membrane instability using trehalose was able to offer significant protection at the local tissues level.
Trehalose, a nonreducing disaccharide comprised of two glucose molecules linked in an α, α−1, 1-glucoside bond, is synthesized by fungi, plants and invertebrate animals (Crowe et al., 1984; da Costa Morato Nery et al., 2008; Eleutherio et al., 1993; Herdeiro et al., 2006; Higashiyama, 2002). It is the storage disaccharide of fungi (including yeast) and the blood sugar of insects. Humans and mice contain the enzyme trehalase bound to the microvillous membrane of intestinal epithelial cells, where it hydrolyzes trehalose present in large amounts in foods such as mushrooms into two molecules of glucose. In our animal model, we administered trehalose i.p., thus bypassing intestinal trehalase hydrolysis. Trehalose has good water solubility (50 g/l), high thermo-stability, and a wide pH-stability (no degradation at 100 °C or at pH values from 3.5~10 for 24 h) (Higashiyama, 2002). As a nonreducing sugar, in the absence of exposure to enzymes with the function of trehalase, it is considerably more inert than other common disaccharides (Newman et al., 1993). At the molecular level, trehalose can interact with dipalmitoyl phosphatidylcholine (DPPC) by hydrogen bonding between the hydroxyl groups in trehalose and the polar head groups of DPPC (Crowe et al., 1984). A number of cell biology studies have shown that trehalose can increase the survival of cells by covering the outer cell membrane (da Costa Morato Nery et al., 2008; Eleutherio et al., 1993; Herdeiro et al., 2006). For instance, yeast cells are well known for their ability to survive complete dehydration, a phenomenon that is directly linked to the presence of trehalose on both sides of the cell membranes. Mutant yeast strains which lack the ability to translocate trehalose from the cytosol to the outer cell membrane cannot survive dehydration although they contain accumulated endogenous trehalose. When these mutants were dehydrated in the presence of exogenous trehalose, they showed increased survival. Trehalose thus appears to bind extremely well to the hydrophilic surfaces of cell membranes. The ability of trehalose to stabilize cell membranes has been used for preservation of various types of mammalian cells (Barbas and Mascarenhas, 2009; Chen et al., 2004; Cui et al., 2007; He et al., 2008; Isowa et al., 1996; Satpathy et al., 2004) as well as tissues before transplantation (Beattie et al., 1997; Fukuse et al., 1996; Yokomise et al., 1996). It has been used in a rat model of septic shock (Minutoli et al., 2007), in which trehalose administration (1g/kg, i.p.) was safe and tolerated with significant reduction in mortality. The median lethal dose (LD50) for oral ingestion of trehalose in rats is 4.6g/kg according to the manufacturer (Sigma, MO). In humans, with average body weight of 51kg, ingestion of 30g trehalose was reported to be well tolerated and produced no obvious side effects (Oku and Nakamura, 2000). These properties of trehalose make it a good candidate for potential applications in acute intestinal I/R injury or intestinal surgical procedures involving intestinal ischemia. Although studies remain to be done to determine how much trehalose enters the blood when given orally, trehalose could be given by intravenous injection which would bypass hydrolysis by intestinal trehalase.
In summary, our study demonstrated that, following induction of I/R injury in intestinal tissue, intracellular ROS formation produces membrane lipid peroxidation leading to membrane instability. The disaccharide trehalose stabilizes such membranes, despite the lipid peroxidation. In the absence of trehalose, an innate autoimmune response involving natural IgM and complement occurs resulting in gross tissue damage. Stabilizing ROS peroxidized cell membranes using trehalose holds promise as an interventional approach to minimize intestinal I/R injury.
Highlights.
Intracellular ROS formation is an event upstream of the lipid peroxidation which results in cell membrane instability.
Cell membrane instability leads to an innate autoimmune response by natural IgM and complement.
Trehalose protects against I/R injury by blocking IgM-mediated complement activation.
Acknowledgments
We thank Dr. James Cottrell for continued support, Drs. Ira Kass and Julie Rushbrook for editing the manuscript. Research is partly supported by SUNY-Downstate Dean’s Award for Pilot Project (MZ) and NIH grant 1R21HL088527 (MZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
H.L. designed, performed and analyzed experiments and wrote the paper; E.H.K and Z. K. did quantification of IHC images; M.L., N.W., and J.B. assisted in experiments; M.Z. designed and analyzed experiments and wrote the paper.
Competing financial interests
The authors declare no competing financial interests.
Reference
- Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M. Interaction between the coagulation and complement system. Advances in experimental medicine and biology. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio G, Flaherty JT. Effects of the superoxide radical scavenger superoxide dismutase, and of the hydroxyl radical scavenger mannitol, on reperfusion injury in isolated rabbit hearts. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 1992;6:623–632. doi: 10.1007/BF00052564. [DOI] [PubMed] [Google Scholar]
- Barbas JP, Mascarenhas RD. Cryopreservation of domestic animal sperm cells. Cell Tissue Bank. 2009;10:49–62. doi: 10.1007/s10561-008-9081-4. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Crowe JH, Lopez AD, Cirulli V, Ricordi C, Hayek A. Trehalose: a cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. 1997;46:519–523. doi: 10.2337/diab.46.3.519. [DOI] [PubMed] [Google Scholar]
- Brandt JL. In: Mesenteric Vascular Disease. Friedman SL, editor. New York: The McGraw-Hill Companies, Inc; 2003. Section I.9. [Google Scholar]
- Charlagorla P, Liu J, Patel M, Rushbrook JI, Zhang M. Loss of plasma membrane integrity, complement response and formation of reactive oxygen species during early myocardial ischemia/reperfusion. Mol Immunol. 2013;56:507–512. doi: 10.1016/j.molimm.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Nakamura T, Wada H. Development of new organ preservation solutions in Kyoto University. Yonsei Med J. 2004;45:1107–1114. doi: 10.3349/ymj.2004.45.6.1107. [DOI] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Chapman D. Preservation of Membranes in Anhydrobiotic Organisms: The Role of Trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Cui XD, Gao DY, Fink BF, Vasconez HC, Pu LL. Cryopreservation of human adipose tissues. Cryobiology. 2007;55:269–278. doi: 10.1016/j.cryobiol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- da Costa Morato Nery D, da Silva CG, Mariani D, Fernandes PN, Pereira MD, Panek AD, Eleutherio EC. The role of trehalose and its transporter in protection against reactive oxygen species. Biochimica et biophysica acta. 2008;1780:1408–1411. doi: 10.1016/j.bbagen.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Eberhardt M. In: Pathologcial processes involving reactive oxygen metabolites. Eberhardt M, editor. Boca Raton, FL: CRC Press, LLC; 2001. pp. 365–379. [Google Scholar]
- Eleutherio EC, Araujo PS, Panek AD. Role of the trehalose carrier in dehydration resistance of Saccharomyces cerevisiae. Biochimica et biophysica acta. 1993;1156:263–266. doi: 10.1016/0304-4165(93)90040-f. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free radical biology & medicine. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N, et al. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- Fleming SD, Egan RP, Chai C, Girardi G, Holers VM, Salmon J, Monestier M, Tsokos GC. Anti-phospholipid antibodies restore mesenteric ischemia/reperfusion-induced injury in complement receptor 2/complement receptor 1-deficient mice. J Immunol. 2004;173:7055–7061. doi: 10.4049/jimmunol.173.11.7055. [DOI] [PubMed] [Google Scholar]
- Fukuse T, Hirata T, Ueda M, Hitomi S, Wada H. Effects of Euro-Collins, University of Wisconsin, and new extracellular-type trehalase-containing Kyoto solutions in an ex vivo rat lung preservation model. Transplantation. 1996;62:1212–1217. doi: 10.1097/00007890-199611150-00004. [DOI] [PubMed] [Google Scholar]
- Gallagher KP, Buda AJ, Pace D, Gerren RA, Shlafer M. Failure of superoxide dismutase and catalase to alter size of infarction in conscious dogs after 3 hours of occlusion followed by reperfusion. Circulation. 1986;73:1065–1076. doi: 10.1161/01.cir.73.5.1065. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. The American journal of physiology. 1986;251:G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Farber NE, Hardman HF, Warltier DC. Beneficial actions of superoxide dismutase and catalase in stunned myocardium of dogs. The American journal of physiology. 1986;250:H372–H377. doi: 10.1152/ajpheart.1986.250.3.H372. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. The American journal of clinical nutrition. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. discussion 724S–725S. [DOI] [PubMed] [Google Scholar]
- Hangaishi M, Nakajima H, Taguchi J, Igarashi R, Hoshino J, Kurokawa K, Kimura S, Nagai R, Ohno M. Lecithinized Cu, Zn-superoxide dismutase limits the infarct size following ischemia-reperfusion injury in rat hearts in vivo. Biochemical and biophysical research communications. 2001;285:1220–1225. doi: 10.1006/bbrc.2001.5319. [DOI] [PubMed] [Google Scholar]
- He X, Park EY, Fowler A, Yarmush ML, Toner M. Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: a study using murine embryonic stem cells. Cryobiology. 2008;56:223–232. doi: 10.1016/j.cryobiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdeiro RS, Pereira MD, Panek AD, Eleutherio EC. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochimica et biophysica acta. 2006;1760:340–346. doi: 10.1016/j.bbagen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Higashiyama T. Novel functions and applications of trehalose. Pure Appl. Chem. 2002;74:1263–1269. [Google Scholar]
- Isowa N, Hitomi S, Wada H. Trehalose-containing solutions enhance preservation of cultured endothelial cells. Ann Thorac Surg. 1996;61:542–545. doi: 10.1016/0003-4975(95)01061-0. [DOI] [PubMed] [Google Scholar]
- Jones SP, Hoffmeyer MR, Sharp BR, Ho YS, Lefer DJ. Role of intracellular antioxidant enzymes after in vivo myocardial ischemia and reperfusion. American journal of physiology. Heart and circulatory physiology. 2003;284:H277–H282. doi: 10.1152/ajpheart.00236.2002. [DOI] [PubMed] [Google Scholar]
- Kanamasa K, Ishida N, Ishikawa K. Protective effect of PEG-SOD against early coronary reperfusion injury assessed in reperfused and non-reperfused ischaemic areas of the same heart. Acta Cardiol. 2001;56:181–186. doi: 10.2143/AC.56.3.2005638. [DOI] [PubMed] [Google Scholar]
- Klein HH, Pich S, Lindert S, Buchwald A, Nebendahl K, Kreuzer H. Intracoronary superoxide dismutase for the treatment of "reperfusion injury", A blind randomized placebo-controlled trial in ischemic, reperfused porcine hearts. Basic Res Cardiol. 1988;83:141–148. doi: 10.1007/BF01907268. [DOI] [PubMed] [Google Scholar]
- Kolkman J, Geelkerken R. In: Splanchnic Ischemia. Fink MP, editor. Elsevier Sauders; 2005. pp. 2021–2029. [Google Scholar]
- Kurose I, Wolf RE, Grisham MB, Granger DN. Hypercholesterolemia enhances oxidant production in mesenteric venules exposed to Ischemia/Reperfusion. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1583–1588. doi: 10.1161/01.atv.18.10.1583. [DOI] [PubMed] [Google Scholar]
- Lee H, Green DJ, Lai L, Hou YJ, Jensenius JC, Liu D, Cheong C, Park CG, Zhang M. Early complement factors in the local tissue immunocomplex generated during intestinal ischemia/reperfusion injury. Mol Immunol. 2010;47:972–971. doi: 10.1016/j.molimm.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Kim M, Kim JY, Brown KM, Ham A, D'Agati VD, Mori-Akiyama Y. Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. American journal of physiology. Gastrointestinal and liver physiology. 2013;304:G12–G25. doi: 10.1152/ajpgi.00201.2012. [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. American journal of physiology. Cell physiology. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Fujiwara H, Kawamura A, Ishida M, Takemura G, Kida M, Uegaito T, Fujiwara Y, Fujiwara T, Kawai C. Failure to reduce infarct size by intracoronary infusion of recombinant human superoxide dismutase at reperfusion in the porcine heart: immunohistochemical and histological analysis. Journal of molecular and cellular cardiology. 1991;23:1287–1296. doi: 10.1016/0022-2828(91)90085-z. [DOI] [PubMed] [Google Scholar]
- Minutoli L, Altavilla D, Bitto A, Polito F, Bellocco E, Lagana G, Giuliani D, Fiumara T, Magazu S, Ruggeri P, Guarini S, Squadrito F. The disaccharide trehalose inhibits proinflammatory phenotype activation in macrophages and prevents mortality in experimental septic shock. Shock. 2007;27:91–96. doi: 10.1097/01.shk.0000235092.76292.bc. [DOI] [PubMed] [Google Scholar]
- Moore RM, Muir WW, Granger DN. Mechanisms of gastrointestinal ischemia-reperfusion injury and potential therapeutic interventions: a review and its implications in the horse. J Vet Intern Med. 1995;9:115–132. doi: 10.1111/j.1939-1676.1995.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Murohara Y, Yui Y, Hattori R, Kawai C. Effects of superoxide dismutase on reperfusion arrhythmias and left ventricular function in patients undergoing thrombolysis for anterior wall acute myocardial infarction. Am J Cardiol. 1991;67:765–767. doi: 10.1016/0002-9149(91)90538-v. [DOI] [PubMed] [Google Scholar]
- Nejima J, Knight DR, Fallon JT, Uemura N, Manders WT, Canfield DR, Cohen MV, Vatner SF. Superoxide dismutase reduces reperfusion arrhythmias but fails to salvage regional function or myocardium at risk in conscious dogs. Circulation. 1989;79:143–153. doi: 10.1161/01.cir.79.1.143. [DOI] [PubMed] [Google Scholar]
- Newman YM, Ring SG, Colaco C. The role of trehalose and other carbohydrates in biopreservation. Biotechnol Genet Eng Rev. 1993;11:263–294. doi: 10.1080/02648725.1993.10647903. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Yamamoto S, Ataka K, Nakamura K. The effect of superoxide dismutase and catalase on myocardial reperfusion injury in the isolated rat heart. The Japanese journal of surgery. 1991;21:423–432. doi: 10.1007/BF02470970. [DOI] [PubMed] [Google Scholar]
- Oku T, Nakamura S. Estimation of intestinal trehalase activity from a laxative threshold of trehalose and lactulose on healthy female subjects. Eur J Clin Nutr. 2000;54:783–788. doi: 10.1038/sj.ejcn.1601091. [DOI] [PubMed] [Google Scholar]
- Ooiwa H, Stanley A, Felaneous-Bylund AC, Wilborn W, Downey JM. Superoxide dismutase conjugated to polyethylene glycol fails to limit myocardial infarct size after 30 min ischemia followed by 72 h of reperfusion in the rabbit. Journal of molecular and cellular cardiology. 1991;23:119–125. doi: 10.1016/0022-2828(91)90099-8. [DOI] [PubMed] [Google Scholar]
- Opal SM, Esmon CT. Bench-to-bedside review: functional relationships between coagulation and the innate immune response and their respective roles in the pathogenesis of sepsis. Crit Care. 2003;7:23–38. doi: 10.1186/cc1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani H, Umemoto M, Kagawa K, Nakamura Y, Omoto K, Tanaka K, Sato T, Nonoyama A, Kagawa T. Protection against oxygen-induced reperfusion injury of the isolated canine heart by superoxide dismutase and catalase. The Journal of surgical research. 1986;41:126–133. doi: 10.1016/0022-4804(86)90017-x. [DOI] [PubMed] [Google Scholar]
- Patel BS, Jeroudi MO, O'Neill PG, Roberts R, Bolli R. Effect of human recombinant superoxide dismutase on canine myocardial infarction. The American journal of physiology. 1990;258:H369–H380. doi: 10.1152/ajpheart.1990.258.2.H369. [DOI] [PubMed] [Google Scholar]
- Pope MR, Hoffman SM, Tomlinson S, Fleming SD. Complement regulates TLR4-mediated inflammatory responses during intestinal ischemia reperfusion. Molecular immunology. 2010;48:356–364. doi: 10.1016/j.molimm.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K, Kloner RA. "Reperfusion injury" by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circulation research. 1989;64:86–96. doi: 10.1161/01.res.64.1.86. [DOI] [PubMed] [Google Scholar]
- Qin X, Dong W, Sharpe SM, Sheth SU, Palange DC, Rider T, Jandacek R, Tso P, Deitch EA. Role of lipase-generated free fatty acids in converting mesenteric lymph from a noncytotoxic to a cytotoxic fluid. American journal of physiology. Gastrointestinal and liver physiology. 2012;303:G969–G978. doi: 10.1152/ajpgi.00290.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard VJ, Murry CE, Jennings RB, Reimer KA. Therapy to reduce free radicals during early reperfusion does not limit the size of myocardial infarcts caused by 90 minutes of ischemia in dogs. Circulation. 1988;78:473–480. doi: 10.1161/01.cir.78.2.473. [DOI] [PubMed] [Google Scholar]
- Russell J, Epstein CJ, Grisham MB, Alexander JS, Yeh KY, Granger DN. Regulation of E-selectin expression in postischemic intestinal microvasculature. American journal of physiology. Gastrointestinal and liver physiology. 2000;278:G878–G885. doi: 10.1152/ajpgi.2000.278.6.G878. [DOI] [PubMed] [Google Scholar]
- Satpathy GR, Torok Z, Bali R, Dwyre DM, Little E, Walker NJ, Tablin F, Crowe JH, Tsvetkova NM. Loading red blood cells with trehalose: a step towards biostabilization. Cryobiology. 2004;49:123–136. doi: 10.1016/j.cryobiol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Testa L, Van Gaal WJ, Bhindi R, Biondi-Zoccai GG, Abbate A, Agostoni P, Porto I, Andreotti F, Crea F, Banning AP. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. The Journal of thoracic and cardiovascular surgery. 2008;136:884–893. doi: 10.1016/j.jtcvs.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004;9:81–83. [PubMed] [Google Scholar]
- Uraizee A, Reimer KA, Murry CE, Jennings RB. Failure of superoxide dismutase to limit size of myocardial infarction after 40 minutes of ischemia and 4 days of reperfusion in dogs. Circulation. 1987;75:1237–1248. doi: 10.1161/01.cir.75.6.1237. [DOI] [PubMed] [Google Scholar]
- Wang P, Chen H, Qin H, Sankarapandi S, Becher MW, Wong PC, Zweier JL. Overexpression of human copper, zinc-superoxide dismutase (SOD1) prevents postischemic injury. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4556–4560. doi: 10.1073/pnas.95.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe BI, Premaratne S, Limm W, Mugiishi MM, McNamara JJ. High- and low-dose superoxide dismutase plus catalase does not reduce myocardial infarct size in a subhuman primate model. Am Heart J. 1993;126:840–846. doi: 10.1016/0002-8703(93)90697-8. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kobata A, Tanigawa T, Nadatani Y, Yamagami H, Watanabe K, Tominaga K, Fujiwara Y, Takeuchi K, Arakawa T. Activation of the MyD88 signaling pathway inhibits ischemia-reperfusion injury in the small intestine. American journal of physiology. Gastrointestinal and liver physiology. 2012;303:G324–G334. doi: 10.1152/ajpgi.00075.2012. [DOI] [PubMed] [Google Scholar]
- Watts JA, Kline JA. Bench to bedside: the role of mitochondrial medicine in the pathogenesis and treatment of cellular injury. Acad Emerg Med. 2003;10:985–997. doi: 10.1111/j.1553-2712.2003.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Yokomise H, Inui K, Wada H, Ueda M, Hitomi S. Long-term cryopreservation can prevent rejection of canine tracheal allografts with preservation of graft viability. The Journal of thoracic and cardiovascular surgery. 1996;111:930–934. doi: 10.1016/s0022-5223(96)70366-5. [DOI] [PubMed] [Google Scholar]
- Zhang GX, Kimura S, Murao K, Obata K, Matsuyoshi H, Takaki M. Inhibition of cytochrome c release by 10-N-nonyl acridine orange, a cardiolipin-specific dye, during myocardial ischemia-reperfusion in the rat. American journal of physiology. Heart and circulatory physiology. 2010;298:H433–H439. doi: 10.1152/ajpheart.00938.2009. [DOI] [PubMed] [Google Scholar]
- Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203:141–152. doi: 10.1084/jem.20050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD, Jr, Carroll MC. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:3886–3891. doi: 10.1073/pnas.0400347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44:103–110. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]