Abstract
The effect of rat spinal cord explants and cell-free nerve extract on acetylcholine receptor site density and distribution was studied using 125I- and rhodamine-labeled α-bungarotoxin on L6, a cloned rat muscle cell line. Control L6 myotubes have a low and uniform distribution of acetylcholine receptors (20 ± 3 sites per μm2 in the present study). The addition of spinal cord explants caused an increase in average receptor site density of about 6 times on myotubes within 2 mm of the explant, while a smaller increase of 3 times was observed at distances greater than 5 mm. The formation of high-density patches of receptors was also stimulated. These observations suggested that a diffusible substance originating from the explant was responsible for these changes. Cell-free homogenates of the central nervous system were prepared and found to produce the same effects. The effect of the homogenate was not strongly dependent on the age of the fetus from which the tissue was isolated, and fetal liver had little or no effect. The active component(s) appears to be a protein(s) with a molecular weight of about 100,000. Because the nerve homogenates make the L6 cells resemble primary muscle cultures, we suggest that a common factor is responsible for regulating the acetylcholine receptor in the two types of muscle culture. The normally acetylcholine receptor-poor L6 cells may provide a more sensitive assay for these factors than do primary muscle cultures.
Keywords: neurotrophic phenomena, α-bungarotoxin binding, L6 cells
Full text
PDF

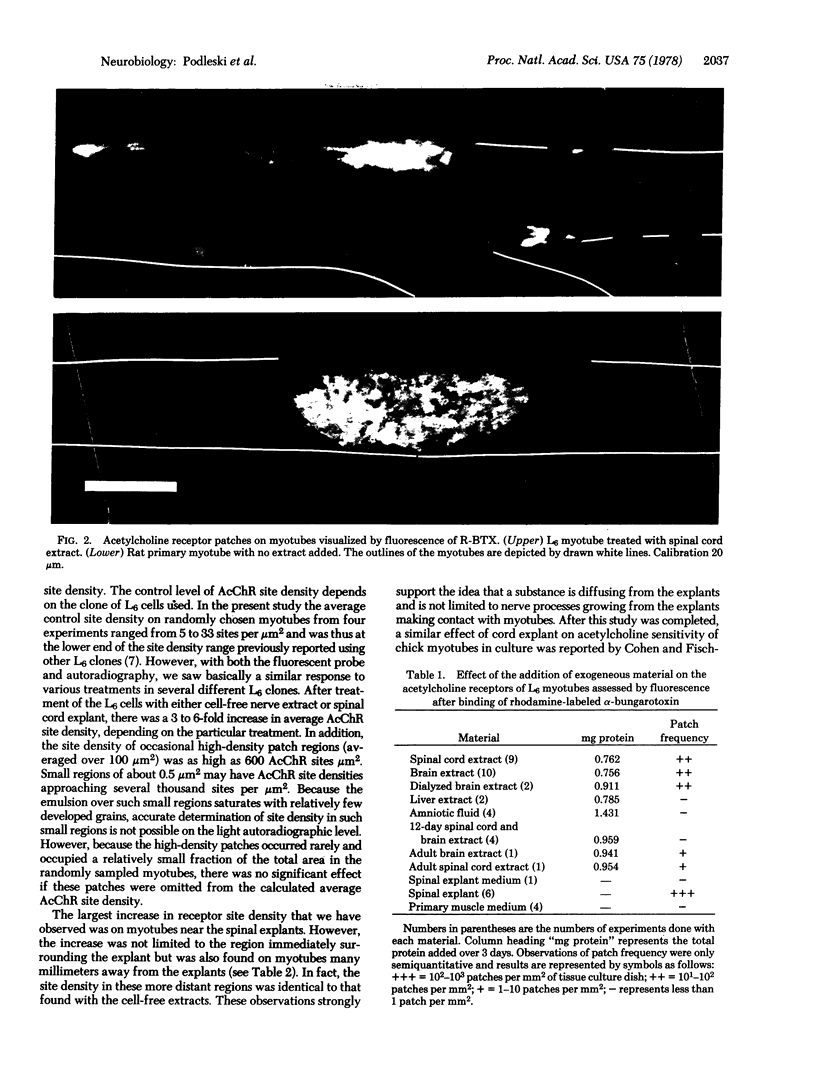


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Cohen M. W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):757–773. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Cohen M. W., Zorychta E. Effects of innervation on the distribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977 Jul;268(3):731–756. doi: 10.1113/jphysiol.1977.sp011879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen R. C. The possible role of cyclic AMP in the neurotrophic control of skeletal muscle. J Physiol. 1975 May;247(2):343–361. doi: 10.1113/jphysiol.1975.sp010935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. A., Fischbach G. D. Clusters of acetylcholine receptors located at identified nerve-muscle synapses in vitro. Dev Biol. 1977 Aug;59(1):24–38. doi: 10.1016/0012-1606(77)90237-8. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Heinemann S. Synapse formation between clonal muscle cells and rat spinal cord explants. Nature. 1974 Dec 13;252(5484):593–594. doi: 10.1038/252593a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lentz T. L. Effect of brain extracts on cholinesterase activity of cultured skeletal muscle. Exp Neurol. 1974 Dec;45(3):520–526. doi: 10.1016/0014-4886(74)90157-5. [DOI] [PubMed] [Google Scholar]
- Nelson P. G. Nerve and muscle cells in culture. Physiol Rev. 1975 Jan;55(1):1–61. doi: 10.1152/physrev.1975.55.1.1. [DOI] [PubMed] [Google Scholar]
- Oh T. H., Johnson D. D., Kim S. U. Neurotrophic effect on isolated chick embryo muscle in culture. Science. 1972 Dec 22;178(4067):1298–1300. doi: 10.1126/science.178.4067.1298. [DOI] [PubMed] [Google Scholar]
- Oh T. H. Neurotrophic effects: characterization of the nerve extract that stimulates muscle development in culture. Exp Neurol. 1975 Feb;46(2):432–438. doi: 10.1016/0014-4886(75)90147-8. [DOI] [PubMed] [Google Scholar]
- Oh T. H. Neurotropic effects of sciatic nerve extracts on muscle development in culture. Exp Neurol. 1976 Feb;50(2):376–386. doi: 10.1016/0014-4886(76)90012-1. [DOI] [PubMed] [Google Scholar]
- Patrick J., Heinemann S. F., Lindstrom J., Schubert D., Steinbach J. H. Appearance of acetylcholine receptors during differentiation of a myogenic cell line. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2762–2766. doi: 10.1073/pnas.69.10.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Singer M., Maier C. E., McNutt W. S. Neurotrophic activity of brain extracts in forelimb regeneration of the urodele, Triturus. J Exp Zool. 1976 May;196(2):131–150. doi: 10.1002/jez.1401960202. [DOI] [PubMed] [Google Scholar]
- Steinbach J. H., Harris A. J., Patrick J., Schubert D., Heinemann S. Nerve-muscle interaction in vitro. Role of acetylcholine. J Gen Physiol. 1973 Sep;62(3):255–270. doi: 10.1085/jgp.62.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]





