Abstract
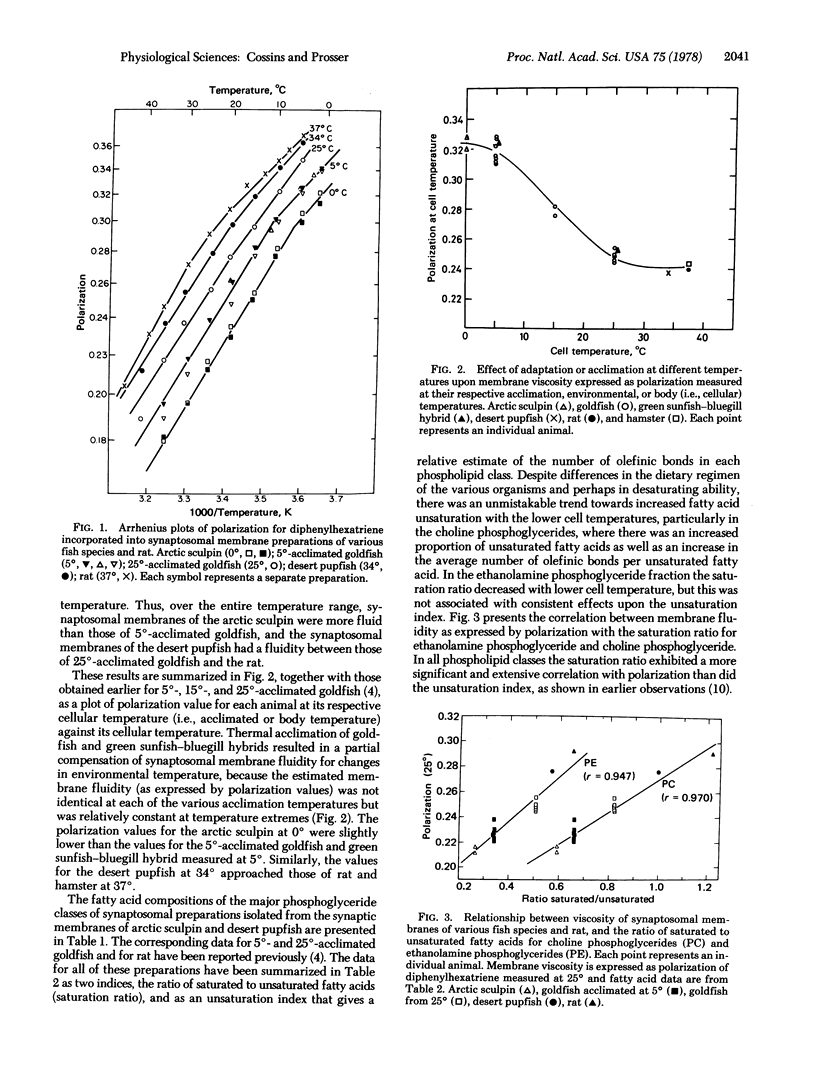
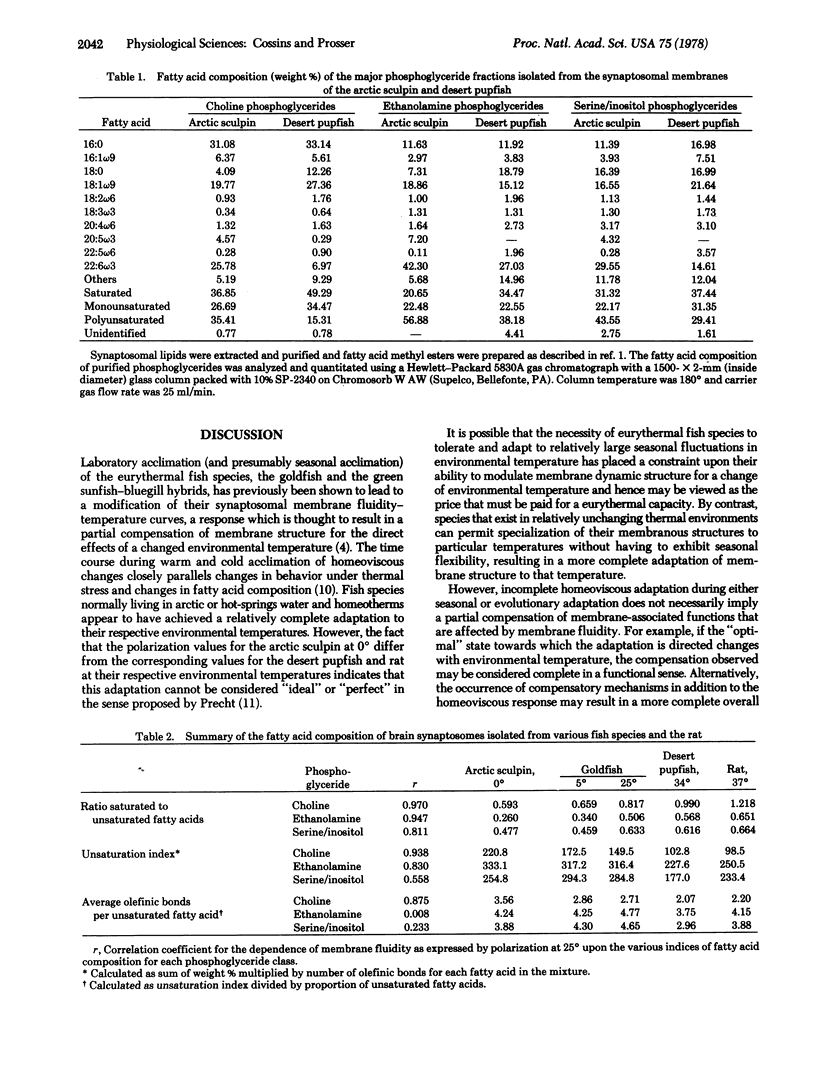
The "fluidity" of brain synaptosomal membrane preparations of arctic and hot-springs fish species, two temperature water fish species acclimated to different seasonal temperatures, and two mammals was estimated using the fluorescence polarization technique. At all measurement temperatures, the fluidity decreased in the order: arctic sculpin, 5 degrees-acclimated goldfish, 25 degrees-acclimated goldfish, desert pupfish, and rat. This correlated with increasing adaptation or body (i.e., cellular) temperatures of 0 degrees, 5 degrees, 25 degrees, 34 degrees, and 37 degrees and suggested a partial compensation of membrane fluidity for environmental temperature that occurs over the evolutionary time period as well as during laboratory (seasonal) acclimation. Evolutionary adaptation of relatively stenothermal species to constant thermal environments resulted in a more complete compensation than laboratory (seasonal) acclimation. Each compensation is accompanied by differences in the saturation of membrane phosphoglycerides. At increased cellular temperatures the proportion of saturated fatty acids increased and the unsaturation index decreased; the correlation between these indices and the measured expression of membrane dynamic structure was highly significant. It is concluded that the homeoviscous compensation of synaptic membrane function is an important component of temperature adaptation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrich M. P., Vanderkooi J. M. Temperature dependence of 1,6-diphenyl-1,3,5-hexatriene fluorescence in phophoslipid artificial membranes. Biochemistry. 1976 Mar 23;15(6):1257–1261. doi: 10.1021/bi00651a013. [DOI] [PubMed] [Google Scholar]
- Cogan U., Shinitzky M., Weber G., Nishida T. Microviscosity and order in the hydrocarbon region of phospholipid and phospholipid-cholesterol dispersions determined with fluorescent probes. Biochemistry. 1973 Jan 30;12(3):521–528. doi: 10.1021/bi00727a026. [DOI] [PubMed] [Google Scholar]
- Cossins A. R. Adaptation of biological membranes to temperature. The effect of temperature acclimation of goldfish upon the viscosity of synaptosomal membranes. Biochim Biophys Acta. 1977 Nov 1;470(3):395–411. doi: 10.1016/0005-2736(77)90131-6. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Souza K. A. Correlation between thermal death and membrane fluidity in Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4111–4115. doi: 10.1073/pnas.71.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa Y., Iida H., Fukushima H., Oki K., Onishi S. Studies on Tetrahymena membranes: temperature-induced alterations in fatty acid composition of various membrane fractions in Tetrahymena pyriformis and its effect on membrane fluidity as inferred by spin-label study. Biochim Biophys Acta. 1974 Oct 29;367(2):134–147. doi: 10.1016/0005-2736(74)90038-8. [DOI] [PubMed] [Google Scholar]
- Sidell B. D. Turnover of cytochrome C in skeletal muscle of green sunfish (Lepomis cyanellus, R.) during thermal acclimation. J Exp Zool. 1977 Feb;199(2):233–250. doi: 10.1002/jez.1401990208. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Somero G. N., DeVries A. L. Temperature tolerance of some Antarctic fishes. Science. 1967 Apr 14;156(3772):257–258. doi: 10.1126/science.156.3772.257. [DOI] [PubMed] [Google Scholar]


