Abstract
Significance: Arterial blood vessels functionally and structurally adapt to altering hemodynamic forces in order to accommodate changing needs and to provide stress homeostasis. This ability is achieved at the cellular level by converting mechanical stimulation into biochemical signals (i.e., mechanotransduction). Physiological mechanical stress helps maintain vascular structure and function, whereas pathologic or aberrant stress may impair cellular mechano-signaling, and initiate or augment cellular processes that drive disease. Recent Advances: Reactive oxygen species (ROS) may represent an intriguing class of mechanically regulated second messengers. Chronically enhanced ROS generation may be induced by adverse mechanical stresses, and is associated with a multitude of vascular diseases. Although a causal relationship has clearly been demonstrated in large numbers of animal studies, an effective ROS-modulating therapy still remains to be established by clinical studies. Critical Issues and Future Directions: This review article focuses on the role of various mechanical forces (in the form of laminar shear stress, oscillatory shear stress, or cyclic stretch) as modulators of ROS-driven signaling, and their subsequent effects on vascular biology and homeostasis, as well as on specific diseases such as arteriosclerosis, hypertension, and abdominal aortic aneurysms. Specifically, it highlights the significance of the various NADPH oxidase (NOX) isoforms as critical ROS generators in the vasculature. Directed targeting of defined components in the complex network of ROS (mechano-)signaling may represent a key for successful translation of experimental findings into clinical practice. Antioxid. Redox Signal. 20, 914–928.
Introduction
The major function of large arteries is to maintain an adequate blood supply directed from the heart to peripheral organs and tissue. As such, they are subjected to various mechanical stresses that are mainly generated by a pulsatile blood flow. However, instead of being a merely passive conductive system, the vasculature is able—and required—to adapt actively to changing mechanical loads that may arise from both physiologic and pathologic hemodynamic conditions (66, 71). Consequently, arterial structure is significantly influenced by the mechanical forces that are imposed on the blood vessel wall. At a cellular level, this implies that various types of vascular cells have the ability to differentially sense mechanical stimuli (i.e., mechanosensation), and to integrate them into the cellular biochemical signaling network (i.e., mechanotransduction).
This review highlights reactive oxygen species (ROS) as intriguing messengers that may link biomechanical forces to subsequent functional and structural vascular alterations. To this end, we first provide a brief overview of ROS biochemistry, including ROS-generation and elimination. We then discuss the complex mechanical forces leading to altered ROS generation and elimination in vascular endothelial cells (ECs) and vascular smooth muscle cells (VSMCs), focusing on different types of mechanical stress, such as those hemodynamically induced by various shear profiles (laminar vs. oscillatory) or cyclic stretch. We elucidate the role of ROS in driving general processes of vascular function/dysfunction (such as endothelial dysfunction, extra-cellular matrix (ECM) remodeling, and inflammation). Finally, we discuss the contribution of mechanically modulated ROS signaling to specific vascular diseases such as arteriosclerosis, hypertension and abdominal aortic aneurysm. The roles of various NADPH oxidases (NOXs) isoforms are particularly emphasized.
ROS—Substrates of Oxidative Stress and Mediators of Redox Signaling
After the discovery of free radicals in biologic materials in 1954, ROS have long been recognized as a cause of hazardous cellular effects (28). Via oxidative damage of cellular molecules, lipids, proteins, or DNA, they are involved in gross cellular damage, promotion of mutagenesis, biologic degeneration, and aging (37). In physiologic states, ROS generation is counterbalanced by various cellular anti-oxidant enzymes to promote “redox homeostasis.” However, excessive/toxic ROS generation may induce an imbalance favoring oxidants compared with antioxidant capacity—a condition summarized by the term “oxidative stress.” However, in contrast to their nonspecific toxic effects, ROS have been recently recognized as specific mediators of cellular signaling. Here, acting as second messengers, they give rise to the term “redox signaling.”
ROS as Second Messengers
Second messengers are defined as intracellular molecules that serve as transmitters and amplifiers of chemically or mechanically generated information—typically at the plasma membrane—to cytoplasmic or nuclear targets. Their regulated production or release occurs on stimulation through an enzymatic or ion channel process (102, 121). The transience of the increase in second-messenger concentration is due to signal termination at several levels, including enzymatic removal of the second messenger (e.g., hydrolysis of the second messenger cAMP) or release from its downstream receptor. Furthermore, there is specificity of second messengers for their targets, which are most often protein kinases and protein phosphatases.
Free radicals were recognized as messenger molecules decades ago, when it was shown that the free radical nitric oxide (•NO) could activate soluble guanylate cyclase to produce cGMP from GTP (4). However, it took many more years until ROS were first identified in mechanisms of cellular signaling. As summarized next, ROS provide all aforementioned characteristics required to serve as second messengers (e.g., regulated generation, elimination, and target specificity). Moreover, due to the high diversity of ROS, ROS generators, and ROS metabolizing enzymes in specific cells and cellular compartments (e.g., cytosol, mitochondria, endoplasmatic reticulum), ROS-signaling features high spatial complexity. As such, there may be regions within the cell in which ROS accumulation is high, while other regions display diminished ROS signaling. Thus, the steady state of ROS in a given cellular compartment may be important for further downstream signaling.
At this point, it should be noted that the term “ROS-mediated signaling” in this review is used for both signaling through enhanced ROS generation and via mechanisms that decrease ROS levels.
Chemistry of ROS
The ROS family comprises various molecules that are derived from the sequential reduction of molecular oxygen and which are capable of oxidizing molecular targets (Fig. 1). Although sharing the same molecular origin, ROS exhibit a relatively wide spectrum of chemical properties (e.g., radicals vs. nonradicals), resulting in significant heterogeneity of biologic capabilities.
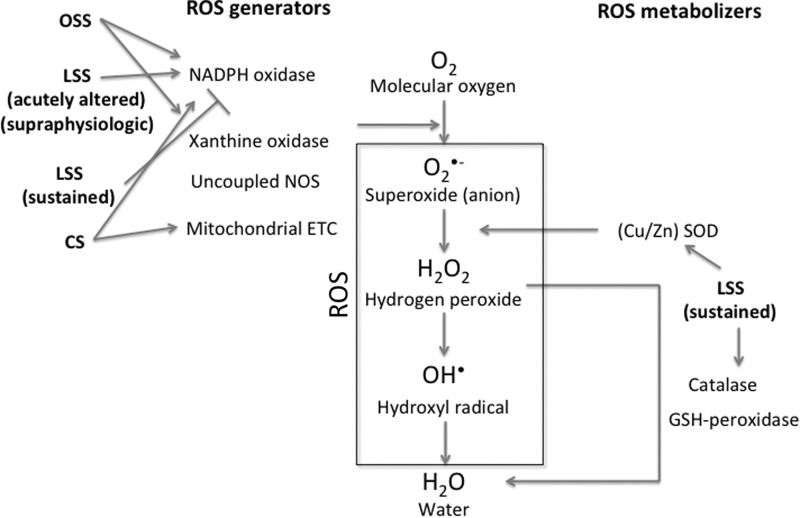
FIG. 1.
ROS metabolism. Sequential reduction of oxygen resulting in ROS generation is depicted, and sites of interference of specific ROS generators and ROS metabolizer are shown. In addition, significant interactions of different mechanical stimuli with ROS metabolism are depicted. ROS, reactive oxygen species; NOS, nitric oxide synthase; ETC, electron transport chain; SOD, superoxide dismutase; LSS, laminar shear stress; OSS, oscillatory shear stress; CS, cyclic stretch.
Unstable free radicals such as superoxide anion (O2•−) and hydroxyl radical (OH•) have short biological half lives. Consequently, these molecules may only act in close proximity to the locus of their generation. The superoxide anion, in particular, is extremely reactive and will generally irreversibly oxidize the first biomolecule it comes into contact with. Therefore, this ROS is unlikely to act in a highly targeted process as cellular signaling.
In contrast, hydrogen peroxide (H2O2), a nonradical, is uncharged, comparatively stable, and unreactive, resulting in a longer biological half life and an ability to diffuse across lipid bilayers. The limited reactivity of H2O2 enables this molecule to act on specific molecular targets. Thus, hydrogen peroxide may represent the most ideal second messenger among the group of ROS (39). In addition, it should be noted that ROS which are produced locally may elicit long-distance signaling via activation of other molecules, propagating throughout cells and tissues. Thus, in the context of mechano-sensing, the transduction of local mechanical forces by molecular entities can translate into responses far away from the initiating force.
Generation and Elimination of ROS
Virtually all types of vascular cells, including ECs, smooth muscle cells, and adventitial cells, are capable of producing ROS, generated from both enzymatic and nonenzymatic sources. It is important to emphasize that redox signaling is dependent on enzymatic—and, therefore, inducible/controllable—generators. With regard to vascular ROS signaling, the most relevant sources are NAPDH oxidase, xanthine oxidase (XO),and (uncoupled) NO synthase (NOS) (Fig. 1).
NADPH oxidases
The first description of the production of ROS by intact mammalian cells through an enzymatic reaction was in phagocytes (110). In those same cells—albeit years later—the first NOX was also identified, being responsible for the transfer of one electron from NADPH to oxygen forming O2•. To date, seven members (NOX1–5; DUOX1–2) have been characterized in the NOX family with the aforementioned phagocytic NOX2 being the prototype. NOX is the only family of enzymes known to generate ROS as its primary function.
All NOX enzymes utilize NADPH as an electron donor and catalyze a transfer of electrons to molecular oxygen to generate superoxide, which may be subsequently dismutated to hydrogen peroxide by superoxide dismutase (SOD). However, there are marked differences among the NOX homologues (Table 1). NOX 1, 2, 4, and 5 are variably expressed in different vascular cell types (93). NOX1 is predominantly expressed in VSMCs, and, to a lesser extent, in ECs. NOX2 (gp91phox-containing oxidase) is found in ECs as well as in vascular fibroblasts and macrophages. NOX4 is expressed in all cardiovascular cell types, and it seems to be the major isoform expressed in ECs and VSMC. NOX5 expression has been reported in ECs and VSMC. However, since it is only found in humans and not in rodents, there is little evidence regarding its functional relevance to date.
Table 1.
Characteristics of NOX Isoforms in the Vascular System
| Characteristic | ||||
|---|---|---|---|---|
| NOX isoform | NOX1 | NOX2 | NOX4 | NOX5 |
| Core protein | Nox1 | Nox2 (gp91phox) | Nox4 | Nox5 |
| p22phox-associated | + | + | + | − |
| Regulatory subunits | NOXO1 | p47phox | − | − |
| NOXA1 | p67phox | |||
| Rac | p40phox (optional) | |||
| (p47phox) | ||||
| (p67phox) | Rac | |||
| Activation/regulation | Regulatory subunits | Regulatory subunits | Transcription | Calcium binding |
| Poldip2 | ||||
| Primary ROS product | Superoxide | Superoxide | Hydrogen peroxide | Superoxide |
| Vascular cell type | VSMC | EC | Ubiquitous | EC |
| (EC) | Fibroblasts | VSMC | ||
| VSMC |
EC, endothelial cell; NOX, NADPH oxidase; ROS, reactive oxygen species; VSMC, vascular smooth muscle cell.
Apart from these cell specificities, different NOXs also have variable requirements for additional subunits in order to form a functional complex. All NOXs contain a name-defining membrane-bound core catalytic unit (Nox1–5, Duox1, and Duox2). Nox1, Nox2, and Nox4 require binding to the smaller, membrane-bound p22phox subunit. Activation of NOX1 and NOX2 by soluble elements (such as angiotensin II [AngII]) and mechanical factors (further discussed in the present article) induces the recruitment of additional cytosolic subunits such as p47phox, p67phox, Rac1, and optionally p40phox (in the case of NOX2), or the respective homologues of p47phox and p67phox, NOXO1, and NOXA1 (in the case of NOX1). NOX5 is activated by calcium binding. In contrast, NOX4 is constitutively active and may, therefore, be primarily regulated at the transcriptional level—although Poldip2 was recently discovered as a regulator of NOX4 in VSMC (113).
Finally, NOXs also differ in specific ROS production. In contrast to NOX1, NOX2, and NOX5, which mainly generate superoxide, NOX4 seems to primarily generate hydrogen peroxide (30, 83).
Xanthine oxidase
As a part of purine metabolism, XO catalyzes the oxidation of hypoxanthine and xanthine, thereby generating O2•− and H2O2 as byproducts. XO has been identified in vascular endothelium and seems to be of particular importance as an ROS-generator in ischemia/reperfusion (53).
Uncoupled NOS
NOS is the enzyme that is best known for generation of NO by catalyzing the reaction from L-arginine to L-citrulline. However, all three known isoforms, endothelial NOS (eNOS), neuronal NOS, and immunological/inducible NOS, may potentially generate ROS. Here, in a process of “NOS uncoupling,” the electron flow is diverted to molecular oxygen rather than to L-arginine—thereby generating superoxide rather than NO (2). In ECs, uncoupling can be triggered in vitro by deficiency of the co-factors tetrahydrobiopterin and L-arginine or in a low-pH medium (126). Other enzymatic sources of ROS include cyclooxygenase/lipoxygenase and various amine oxidases.
Nonenzymatic ROS generators
As opposed to controllable enzymatic ROS generation, “unintentional” nonenzymatic ROS production mainly contributes to an elevation of cellular oxidative stress. Nonenzymatic ROS sources include the mitochondrial electron chain, the cytochrome-P450 family, or free iron (Fe2+).
Elimination of ROS
Analogous to the dual role of ROS in mediating both oxidative stress and cellular signaling, ROS-reducing enzymes may be considered cellular “detoxifying” agents, altering ROS signal transduction as well as providing redox-homeostasis. Furthermore, antioxidants may also be categorized into an enzymatic as well as a nonenzymatic group.
Enzymatic antioxidants include superoxide dismutases (SOD1–3; which catalyze the dismutation of superoxide (O2•−) into H2O2 and O2), and two major types of enzymes metabolizing H2O2: catalase (which dismutates H2O2 into water and molecular oxygen) and peroxidases such as glutathione (GSH) peroxidases, thiol-dissulfide oxidoreductases, and peroxiredoxins (all of which eliminate H2O2 by using it as an oxidant for another substrate, generating water) (Fig. 2). Many of these enzymatic antioxidants are present in vascular cells—albeit in different cellular compartments (40).
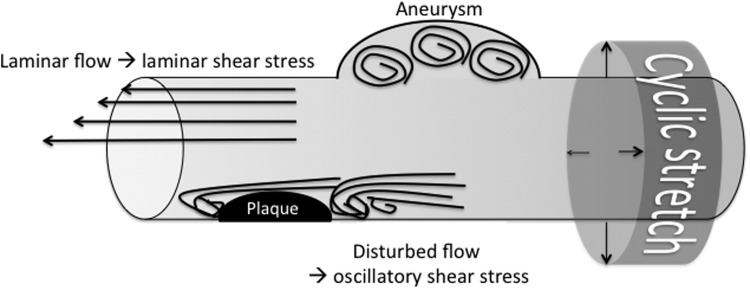
FIG. 2.
Hemodynamic impact on vascular mechanical stimulation. Regular laminar blood flow induces tangential (endothelial) shear stress; disturbed flow patterns (e.g., occurring at atherosclerotic lesions, vascular bifurcations, or aneurysmal dilatations) cause oscillatory shear stress. Pulse pressure drives cyclic circumferential stretch of the vessel wall.
In addition to enzymatic antioxidants, there are several low-molecular-weight compounds that contribute to the antioxidant defense systems. These include endogenously synthesized (e.g., uric acid, bilirubin, and coenzyme Q10) as well as dietary substances (vitamin C, vitamin E) (118).
With regard to overall ROS homeostasis, low amounts of ROS can contribute to physiological cellular functions via activation of signaling cascades or engagement of receptors that drive various cellular pathways (leading to growth proliferation, dilatation etc.), or can (when generated at high levels, or under sustained production) damage biological molecules. The damage can be contained when pathways are activated to suppress ROS production (antioxidants, etc.), repair ROS-mediated damage (nucleotide and base excision repair), or degrade the oxidative products formed (elimination by autophagosomy). Damage occurs when the oxidative load exceeds the capacity of the cell to enact these responses.
ROS in the Transmission of Hemodynamic Signals
Blood vessels are continuously exposed to mechanical stress induced by various types of hemodynamic forces (Fig. 2). The viscous drag of blood flow results in a tangential frictional force acting on the vascular luminal surface, thereby generating shear stress. Shear stress is proportional to flow rate and inversely proportional to the third power of the vessel's radius. This implies that an increase in vascular diameter effectively reduces shear stress. As discussed in subsequent sections, shear stress may appear in two distinct forms: laminar and nonlaminar (oscillatory, turbulent). In addition, the pulsatile nature of blood flow generates cyclic changes of hydrostatic intravascular pressure, inducing a (mainly circumferential) cyclic stretch/strain—where strain is generally defined as the ratio of total deformation related to the initial dimension of the vessel. In contrast to shear stress, which mainly acts on the luminal surface of the endothelium, cyclic stretch may affect all vascular layers, including VSMC.
At the cellular level, these mechanical forces initially activate numerous sensors (e.g., molecular moieties on the plasma membrane or other cellular components). As will become apparent in the following sections, ROS subsequently play a significant role in the downstream transmission of these mechanical signals, resulting in both physiological and pathophysiological responses.
Shear stress
Shear stress arising from blood flow in the vasculature is determined by the local vascular topology. Depending on the geometric characteristics of the vessel, lumen flow may appear laminar or nonlaminar (oscillatory). Laminar shear stress typically occurs in straight vessels, whereas oscillatory shear stress is found at vascular branch points, or in other regions of complex vascular geometry such as atherosclerotic plaques or aneurysmal dilatations (Fig. 2).
Overall, shear is sensed by the endothelium and mechanotransduced into various biochemical and signaling pathways via a multistep process. The initial step is flow-induced deformation of the cell surface, followed by transmission of stress via biochemical changes, leading to changes in gene expression and remodeling of the vessels.
Unidirectional laminar shear stress
Flow-induced vasodilation represents a classical auto-regulatory mechanism that regionally adapts tissue perfusion to changes in (systemic) cardiac output (e.g., during physical exercise). At the endothelial level, this effect is promoted by laminar shear stress triggering the release of the “endothelium-derived relaxing factor”—since 1987 better known as NO (99). In addition to NO, ROS signaling is also involved in mediating the effects of laminar flow. Application of elevated intraluminal flow to intact vessel segments stimulates O2•− production both in vivo and ex vivo (68). Subsequent in vitro studies confirmed enhanced—but transient—ROS generation after the abrupt onset of laminar shear flow via the NOX system (58). In this acute setting, enhanced ROS generation mediates flow-induced vasodilation in coronary and cerebral arteries—with hydrogen peroxide being specifically responsible (88, 100). Interestingly, the acute cessation of flow/shear stress has also been demonstrated to trigger prolonged (NOX2-mediated) ROS generation in the pulmonary endothelium and circulation (20, 129). Taken together, ROS seem to be signaling messengers that are particular sensitive to acute changes in flow conditions. Their specific relevance in the context of chronically alternating flow conditions (i.e., oscillatory flow) is discussed in the next section.
In addition to transiently inducing ROS generation, laminar shear potently stimulates the expression of the cytosolic copper/zinc-containing superoxide dismutase (Cu/Zn SOD), a major cytoplasmic O2•−scavenger (61). Laminar flow also increases expression and intracellular levels of GSH peroxidase as well as cellular catalase activity, which are responsible for H2O2 scavenging in mammalian cells (58, 91). From a signaling perspective, the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) seems to be critically involved in mediating the anti-oxidant effects of pulsatile laminar shear stress [reviewed in Takabe et al. (124)]. Nrf2 is activated by pulsatile laminar shear stress in ECs, and, subsequently, binds to the antioxidant response element in the promoter region of a variety of genes, thereby enhancing the expression of antioxidant enzymes, such as heme oxygenase 1, NADPH quinone oxidoreductase 1, as well as glutamate-cysteine ligase catalysis and glutamate-cysteine ligase modifier, which regulate GSH synthesis. In addition, Nrf2 is involved in the down-regulation of endothelial NOX4 in response to long-term laminar shear (47). Anti-oxidant Nrf2 activation by pulsatile laminar shear stress seems to be dependent on NOX-derived ROS-generation, which implies (negative) feedback regulation. Underscoring the critical protective effect of Nrf2 function, Nrf2-loss-of-function studies have revealed detrimental ROS accumulation even in the presence of (normally protective) laminar shear stress (124).
Further, endothelial NO release directly contributes to anti-oxidant effects, as it causes an increase in expression of extracellular SOD in the adjacent VSMCs (42). Taken together, increased laminar shear stress may initially enhance ROS production, but reduces ROS levels in the long term due to a sustained up-regulation of anti-oxidant enzymes. In contrast, supra-physiologically elevated laminar flow (as experimentally induced by arterio-venous fistulas) was shown to enhance ROS synthesis in a p47phox-dependent, but gp91phox-independent fashion—thereby indicating a potential role for NOX1 in this specific context of mechanotransduction (22).
Oscillatory shear stress
In contrast to physiological unidirectional laminar flow, various vascular pathologies are associated with focally disturbed flow patterns. Most evidence is derived from studies investigating the pathophysiology of atherosclerosis, which typically occurs at (and also generates) locations of disturbed, nonuniform, oscillating flow (67). In this scenario of disturbed flow, ROS levels are markedly elevated. Exposure of ECs to oscillatory shear causes a sustained increase in the production of ROS which is greater than that caused by laminar shear (29). This finding is consistent with studies demonstrating that oscillatory shear stress results in an up-regulation of subunits of the NOX complex, such as p47phox, gp91phox, and Nox4 in bovine aortic ECs (59). In contrast, physiologic pulsatile flow down-regulated gp91phox, as well as Nox4 expression, accompanied by reduced superoxide production (59). Additional studies found that NOX (p47phox)-derived ROS production in oscillatory shear stress may trigger further ROS generation by XO, thus implying a mechanism for signal amplification (85).
Increasing the relevance of the in vivo results, others demonstrated ex vivo that pathologic flow reversal (equivalent to oscillatory flow) in porcine common femoral arteries induced superoxide production, and reduced nitrite production and endothelium-dependent vasodilation—all of which could be reversed by NOX/gp91phox inhibition (45).
In summary, oscillatory shear promotes a pro-oxidant milieu (enhanced ROS-generation); whereas chronic laminar flow creates an anti-oxidant environment, reducing ROS levels.
Cyclic stretch
Cyclic stretch represents another significant vascular mechanical stimulus that results from pulsatile hemodynamics. In contrast to shear stress, which typically affects the endothelial layer of the vasculature, cyclic stretch is relevant to the entire vessel wall. Therefore, cellular studies investigating the effects of cyclic stretch also include VSMC. Similar to the aforementioned opposing effects of shear stress on vascular health, cyclic stretch also has a role in both physiological and pathological vascular processes.
Physiologic levels of cyclic stretch may contribute to vascular homeostasis by inhibiting VSMC proliferation and endothelial apoptosis (24, 55). On the other hand, cyclic stretch is chronically elevated in pathological conditions such as hypertension. In this regard, cyclic stretch has been shown to activate vascular cell proliferation or ECM synthesis—both of which are features of hypertensive vascular remodeling (131).
In terms of vascular redox signaling, increased production of ROS in response to cyclic mechanical stretch was reported in ECs as well as in VSMCs (56, 84). This finding was also reproduced in a model of arterial stretch (97). Various potential sources for increased ROS production in response to mechanical stretch have been identified in ECs and VSMC, including the NOX system (Fig. 1). Mechanical stretch in VSMC induced Nox1 expression, and subsequent ROS generation was completely abrogated in p47phox knockout conditions (49). In ECs, cyclic stretch enhanced p22phox expression, and ROS production was sensitive to NOX inhibition with diphenyl iodonium (84). In contrast, physiological levels of cyclic strain down-regulated Nox4 expression and superoxide anion formation, accompanied by an increase in NO release and eNOS expression (46).
The mitochondrial system has also been identified as a stretch-responsive ROS generator (1). At an ex vivo level, high intravascular pressure (180 mmHg) was shown to induce increased NOX activity, superoxide production, and, eventually, impaired endothelium-dependent vasorelaxation (127). Although not specifically studied, one could speculate that, in fact, vascular stretch secondary to elevated intraluminal pressure induced subsequent mechanotransduction (involving the integrin-linked kinase ILK-1, and Rac-1).
In addition to hemodynamic forces, the pulmonary microvasculature is also subjected to cyclic stretch during breathing or mechanical ventilation. Here, cellular over-distension may result in endothelial damage, contributing to distinct pathologies such as ventilator-induced lung injury (104). Specific aspects of ROS-mediated mechanotransduction in the pulmonary vasculature are beyond the scope of this article and have been extensively reviewed recently (15).
ROS-Sensitive Processes of Vascular Biology
The next section highlights ROS as universal regulators of vascular biology, including endothelial and VSMC homeostasis, ECM remodeling, and inflammatory control (Fig. 3). Pathological alterations of those specific features are implied in virtually all vascular diseases. Therefore, ROS a priori qualify as significant signaling molecules for vascular pathologies.
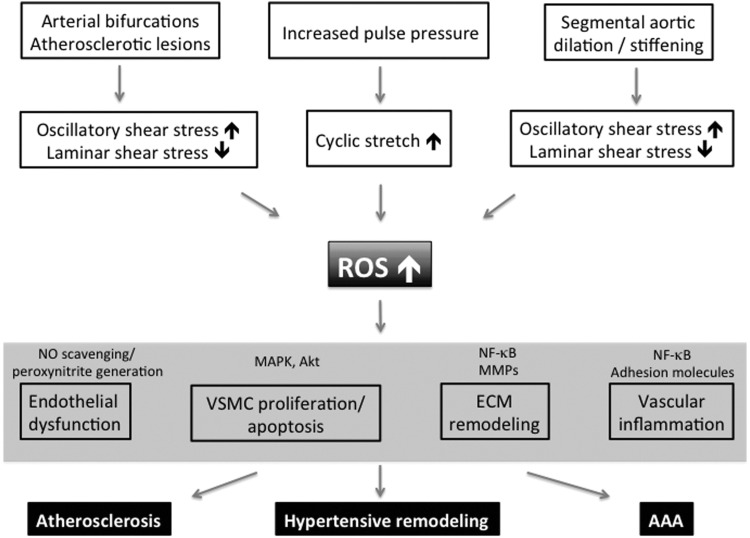
FIG. 3.
Model of mechanical ROS regulation contributing to general vascular pathology and specific diseases. Different hemodynamic vessel characteristics induce adverse mechanical stimulation of the vessel wall, thereby increasing vascular ROS levels. Elevated ROS levels target various redox-sensitive elements (shown only exemplarily) and thereby lead to abundant pathologic alterations within the vessel wall, contributing to specific diseases. MAPK, mitogen-activated protein kinases; MMPs, matrix metalloproteinases.
In order to exert their pleiotropic actions, ROS interfere with various signaling molecules. The heterogeneous group of redox-sensitive targets includes protein kinases, such as mitogen-activated protein (MAP) kinases (associated with signaling cascades controlling cell proliferation, differentiation, and death), protein phosphatases (which are critical regulators in a multitude of signaling pathways and are particularly susceptible to oxidative inactivation), small G proteins (Rac, Rho), ion channels, or transcription factors (such as NF-κB, activator protein-1 [AP-1], and Nrf2). Although it is beyond the scope of the present review to comprehensively dissect ROS downstream signaling at a molecular level, the large variety of available targets clearly indicates the great potential of ROS to interfere with nearly any cellular function.
Endothelial homeostasis
As discussed earlier, the vascular endothelium is a crucial sensor of mechanical stress (especially shear stress), subsequently initiating ROS-mediated mechanotransduction. However, ECs also represent targets of ROS signaling.
Besides modulating vascular smooth muscle tone and growth, the messenger NO is most strongly related to the maintenance of endothelial homeostasis; for example, by inhibiting platelet aggregation, and leukocyte binding to endothelium—thereby preventing crucial features of “endothelial dysfunction” (99). In a reduced definition, the term “endothelial dysfunction” is used in reference to impaired endothelium-dependent vasorelaxation, caused by a loss of NO bioactivity. Thus, on the one hand, endothelial dysfunction may obviously be a sequel of reduced NO production. On the other hand, NO levels may be low due to accelerated NO degradation. In this regard, there is evidence that ROS (superoxide) seem to be of critical significance. Even before it was identified as NO, the endothelium-derived vascular relaxing factor (EDRF) was shown to be inactivated by superoxide anion and to be stabilized by SOD (50). In line with those early findings were animal studies which suggested that impaired endothelium-dependent vascular relaxation in atherosclerotic arteries was not due to an impaired release of NO/EDRF, but rather a consequence of an accelerated inactivation by ROS—which, therefore, was partially restorable by SOD treatment (87, 92). Those studies helped define the role of ROS as agents promoting endothelial dysfunction. Subsequent animal and also human studies further promoted the significance of ROS-enhanced NO inactivation as an underlying mechanism of endothelial dysfunction in various diseases (e.g., hypertension, hypercholesterolemia, and diabetes) (21).
Apart from merely decreasing the (protective) bioavailability of NO, the rapid reaction of superoxide with NO also forms a highly reactive intermediate, peroxynitrite (ONOO−). Peroxynitrite in turn—being a strong cytotoxic oxidant—causes oxidative damage, nitration, and S-nitrosylation of biomolecules, such as proteins, lipids, and DNA, and thereby may be involved in the pathogenesis of virtually all major cardiovascular diseases (e.g., stroke, myocardial infarction, and heart failure) (10, 98). Moreover, peroxynitrite may uncouple eNOS (via oxidation of tetrahydrobiopterin), thus leading to further superoxide generation, resulting in a vicious cycle that contributes to enhanced NO depletion (41). Only recently, oxidative stress-induced S-glutathionylation of eNOS was identified as another molecular mechanism leading to eNOS uncoupling and subsequent impaired endothelium-dependent vasodilation, which can be restored by thiol-specific reducing agents (25).
The current understanding of ROS-eNOS interaction has recently been complicated by findings demonstrating paradoxical activation of eNOS due to endothelial Nox5 overexpression and subsequent superoxide generation (134). However, since this putatively protective feedback regulation is not effective in increasing the total amount of biologically active NO, it may instead contribute to deleterious, enhanced, ROS-triggered peroxynitrite generation.
Given the importance of NOXs as ROS generators in the vasculature, it seems obvious that these proteins may be crucial in inducing/mediating endothelial dysfunction. Indeed, various studies assign such a role to Nox1 as well as Nox2 (11, 32, 63). The immediate clinical relevance of impaired Nox2 signaling has been shown in patients suffering from a hereditary disorder called chronic granulomatous disease (X-CGD)—a disease affecting innate immunity and predisposing to life-threatening infections due to a functional deficiency of gp91phox. These patients, however, exhibit enhanced endothelium-dependent flow-mediated vasorelaxation, along with increased NO levels, compared with healthy subjects. In addition, CGD patients are protected from experimental ischemia/reperfusion-induced endothelial dysfunction (76).
The rather uniform picture of NOXs as mediators of vascular dysfunction has recently been challenged by experimental data gathered in transgenic mice with endothelium-targeted Nox4 overexpression. Those animals not only failed to exhibit endothelial dysfunction but—on the contrary—also demonstrated enhanced endothelium-dependent vasorelaxation primarily via increased hydrogen peroxide generation and subsequent H2O2-induced (K+-dependent) hyperpolarization (108). These findings have refined the impact of superoxide as a crucial ROS-promoting vascular dysfunction (via NO depletion and simultaneous peroxynitrite generation), and highlighted the unique role of Nox4 as an NOX that primarily produces H2O2, bypassing detrimental superoxide generation (17).
Direct evidence for the significance of mechanically induced ROS generation in the context of endothelial function and dysfunction is provided by studies demonstrating that not only NO, but also hydrogen peroxide may serve as a mechano transducer promoting flow-induced vasodilatation by means of cellular hyperpolarization (88, 100). With chronic flow overload and chronically enhanced shear or pathological flow reversal, however, increased ROS production (via up-regulation of NOX subunits p22phox, p47phox, Nox2, and Nox4 or enhanced activation of the NOX system) contributes to impaired endothelium-dependent vasorelaxation (77).
Finally, in addition to their impact on endothelial function, ROS have also been identified as inducers and mediators of endothelial apoptosis. From a biophysical perspective, it should be noted that physiologic laminar shear stress exhibits anti-apoptotic properties via anti-oxidant mechanisms (33).
VSMC hypertrophy, proliferation and apoptosis
VSMC biology represents another significant target for ROS-mediated signaling. ROS have been identified as critical transducers of AngII-induced VSMC hypertrophy, susceptible to various anti-oxidant interventions (48, 133). Further, ROS have been implicated in pro-proliferative and anti-apoptotic cellular responses. For example, PDGF-induced or phenylephrine-induced proliferation and migration were shown to be ROS (H2O2) dependent (96, 120). Overexpression of catalase was effective in inhibiting proliferation and promoting apoptosis (19). The anti-apoptotic effect of H2O2 may be mediated by canonical anti-apoptotic pathways involving MAP kinases and Akt (120).
However, different studies found ROS inducing or enhancing VSMC apoptosis (38, 73). These contrary cellular responses can be explained by the extent and location of ROS generation, as discussed in earlier sections; low levels of ROS promote growth and survival, while high levels drive oxidative stress and apoptosis.
With regard to the specific role of NOXs in governing (mal-)adaptive responses in VSMC, NOX1 (and to some extent NOX4) has been shown to modulate VSMC proliferation (119). From a mechanistic point of view, it should be noted that NOX1 seems to exert its effects via oxidative inactivation of the phosphatase SHP-2, with subsequent augmented signaling through Akt, thereby involving a central regulator of cell proliferation and cell survival (123).
ECM remodeling
Vascular remodeling crucially involves qualitative and quantitative alterations of the ECM (18). The vascular ECM is synthesized by all cell types of the vascular wall and may be regarded as a composite bioactive polymer consisting of collagen and elastic fibers embedded in a viscoelastic gel of proteoglycans, various glycoproteins, hyaluronan, and water. The stability, resilience, and compliance of the vascular wall are mainly dependent on collagen and elastin. Collagen and elastin homeostasis is potently regulated by catabolic matrix metalloproteinases (MMPs), a family of enzymes that is produced by vascular cells as well as inflammatory cells. Given the regulatory power of MMPs, their own activity is controlled at multiple levels, such as transcription, translation, or zymogen activation. ROS signaling is able to interfere with these stages of MMP generation.
Cyclic stretch of VSMCs increases the transcription and release of MMP2, an effect that is dependent on p47phox (49). Similarly, induction of MMP2 by AngII requires a p47phox-containing oxidase (78). Finally, ROS are also capable of activating the proenzymes pro–MMP-9 and pro–MMP2 secreted from cultured human VSMCs (105). Mechanical force, specifically chronic supra-physiologically flow (induced by experimental creation of arterio-venous fistulas in mice), has been shown to activate MMP2 and MMP9 in a p47phox-dependent manner, ultimately resulting in adaptive vascular remodeling (22). Interestingly, gp91phox was not necessary for flow-induced remodeling. Therefore, the NOX1 complex—which may also utilize p47phox as an organizing subunit—may be the critical NOX isoform in that context (22). Later studies also identified NFκB as a shear stress-responsive transcription factor (see below) regulating MMP expression and activity in flow-induced arterial remodeling (23).
Vascular inflammation
Inflammation plays a significant role in virtually all cardiovascular diseases. One of the major pathomechanisms underlying vascular inflammation is the up-regulation in the expression of pro-inflammatory genes, such as those encoding for chemotactic cytokines or adhesion molecules. There is abundant evidence integrating ROS as signaling molecules in this process.
Much evidence in this field is derived from studies investigating the inflammatory effects of AngII stimulation. AngII has early been known to induce ROS signaling via activation of the NOX (48). Data from subsequent studies then provided a link between AngII stimulation and increased synthesis of inflammatory cytokines such as IL-6 and MCP-1, or adhesion molecules such as VCAM-1 via ROS serving as second messengers (26, 103, 112). Interestingly, the AngII type 1 receptor (AT1R) has recently been reported to be activated by shear stress. This regulation can occur independently of AngII, although subsequent signaling is ROS mediated (8, 107).
The specific importance of ROS as second messengers of mechanotransduction in the field of vascular inflammation is exemplified by studies involving central redox-sensitive “inflammatory” transcription factors, such as NFκB and AP-1. NFκB is transiently activated in ECs acutely exposed to physiological or high shear stress (14, 54, 90). In contrast pathologically low shear stress—such as the disturbed flow pattern occurring at atherosclerosis prone arterial segments—induces sustained activation of NFκB (90). Mechanistically, this phenomenon is due to a persistent activation of IKK (IKKβ in particular) that could be prevented by (upstream) NOX inhibition (89). Activation of IKK leads to consecutive phosphorylation of the inhibitor proteins IκBα and IκBβ, allowing the translocation of active NFκB into the nucleus to activate transcription of downstream genes. In addition, in accordance with the pathophysiologic concept of enhanced ROS generation due to acute changes in flow profile, NFκB and AP-1 were found to be up-regulated with acute flow cessation in a ROS-dependent fashion (129).
Moreover, cyclic stretch as well as oscillatory shear stress induced the expression of pro-inflammatory adhesion molecules as ICAM-1, VCAM-1, or E-selectin in an ROS-dependent manner, resulting in increased monocyte adhesion (27). In this regard, p47phox- and Nox1-containing NOXs have been identified as critical signaling components, leading from endothelial oscillatory shear stress to subsequent monocyte adhesion (60). However, recent in vivo studies also suggest ROS-independent mechanisms of VCAM-1 up-regulation after the onset of oscillatory shear stress (130).
The effect of ROS on vascular inflammation resulting from interference with low-density lipoproteins (LDL) is discussed next in the specific paragraph about atherosclerosis.
ROS in Specific Vascular Diseases
Atherosclerosis
The pathophysiologic impact of ROS mechanotransduction may be best exemplified in atherosclerosis. Here, locally disturbed, oscillatory shear flow (e.g., occurring at vascular bifurcations) provides a potent stimulus for regionally elevated ROS generation (as discussed in earlier sections). Locally enhanced ROS synthesis may not only trigger subsequent signaling events driving atherosclerosis but also help explain the nonuniform appearance of atherosclerotic lesions (preferentially occurring at vascular branch points) (95).
From a mechanistic point of view, in vitro studies with unidirectional, laminar shear stress showed an induction of gene transcript profiles that are considered athero-protective. Those findings matched the expression of similar genes in vivo. In contrast, oscillatory flow—as seen in atherosclerosis-prone regions (e.g., vascular branch points)—induces a pro-atherogenic expression profile (95).
With regard to the particular role of ROS, apart from interfering with the aforementioned redox-sensitive vascular pathomechanisms (endothelial dysfunction, inflammation, and remodeling—all of which contribute to atherosclerosis), superoxide promotes atherogenesis by oxidation of LDL. Oxidized LDL (oxLDL) enhances vascular inflammation by augmenting intimal macrophage infiltration (e.g., through up-regulation of chemotactic factors such as MCP-1, or enhanced expression of adhesion molecules such as VCAM-1 and ICAM-1), leading to subsequent foam-cell formation (12).
However, ROS not only oxidize LDL, but also mediate subsequent downstream cellular signaling induced by oxLDL. Macrophage activation by minimally oxidized LDL was found to be dependent on Nox2-derived ROS generation through expression regulation of pro-inflammatory cytokines interleukin-1β, interleukin-6, and CCL5/RANTES (7). Moreover, Nox4 has been identified as a mediator of oxLDL-induced macrophage cell death, which itself is known to contribute to the formation of necrotic cores in advanced atherosclerotic plaques (69). Finally, ROS signaling also mediates oxLDL-induced macrophage spreading (via oxidative inactivation of Src homology 2-containing phosphotyrosine phosphatase [SHP-2]), a mechanism that may contribute to macrophage trapping in arteriosclerotic lesions (101). The complex interplay of ROS and oxLDL is further expanded by the positive feedback regulation of oxLDL-induced NOX expression (111). With regard to aspects of mechanotransduction, it is important to emphasize that LDL oxidation has also been shown to arise through pathologic (oscillatory) shear stress, resulting in enhanced gp91phox expression and superoxide production (59).
In line with the extensive in vitro data on the significance of ROS in virtually all stages of atherogenesis, it has been demonstrated that ROS (superoxide) production is elevated in coronary artery disease and that the plaque shoulder in human coronary arteries exhibits increased levels of NOX subunits (including gp91phox, p22phox, Nox2, and Nox4) (52). Interestingly, from a cellular perspective, gp91phox expression levels correlated with the plaque macrophage content, and Nox4 correlated with the content of alpha-actin–positive cells. In addition, expression levels of gp91phox and p22phox were positively correlated with the severity of atherosclerosis (117). Nox5 was also identified as a generator of ROS in human coronary atherosclerosis (51).
In contrast to the predominant and consistent observations of increased NOX expression in atherosclerotic lesions, its functional relevance remains controversial. Atherosclerosis-prone ApoE−/− mice with additional knockout of NOX subunits p47phox or Nox2 exhibited reduced lesion formation, indicating a role for NOX2 in promoting atherosclerosis (9, 62). In contrast, other reports have described no beneficial effect of Nox2 or p47phox deficiency on plaque formation in either ApoE−/− mice or wildtype mice on a high-fat diet (57, 65). However, with regard to the role of NOX1, Nox1 knockdown or p22phox overexpression resulted in decreased and increased lesion size, respectively (64, 114). The reason for the questionable functional impact of specific Nox systems on the overall atherogenesis may be found in the complex and redundant intertwining of redox signaling, where inhibition of one redox pathway may be compensated for by another.
Restenosis after balloon angioplasty represents another specific aspect of atherosclerosis that is characterized by VSMC and myofibroblast proliferation, resulting in formation of a lumen-obstructing neo-intima. In this context, altered flow arising from the obstruction could presumably drive ROS production. Indeed, increased ROS formation as well as increased NADPH-oxidase activity has been detected in various animal models after balloon injury (6, 115). More specifically, in the setting of VSMC-dominated pathology, the (VSMC) NOX1 seems to be of particular significance. Nox1 expression increases early after balloon injury in the rat carotid artery (122). In a mouse model of arterial wire injury, Nox1 knockout resulted in reduced VSMC migration, proliferation, and apoptosis as well as in eventual attenuated neointima formation (70). Finally, Nox4 (and its recently discovered regulator Poldip2) have also been shown to modulate VSCM migration and may, therefore, qualify as regulators of neo-intima formation/restenosis (79).
Thus, from the perspective of mechanotransduction, it is attractive to speculate on arterial over-distension (due to balloon inflation) as an initial stimulus of NOX activation, with subsequent changes in the mechanical properties of the manipulated artery resulting in fundamentally altered ROS homeostasis.
Hypertensive arterial remodeling
Apart from hormonal stimulation with AngII, cyclic stretch represents a different mechanically triggered mechanism that is used to induce ROS generation (see above). Both AngII levels as well as cyclic stretch are elevated in various types of hypertension, thereby potentially inducing subsequent ROS signaling. In fact, ROS byproducts were increased in hypertensive patients, which was associated with reduced activity of anti-oxidant mechanisms (109). In addition, increased vascular ROS production was found to be consistently elevated in various animal models of hypertension (13, 72, 106). The functional relevance of increased ROS generation becomes evident in hypertensive arterial remodeling. Structural arterial remodeling initially represents an adaptive mechanism, increasing wall thickness and reducing wall stress in chronic hypertension, but, ultimately, may become maladaptive, contributing to a further elevation of blood pressure. In accordance with the critical role of ROS signaling in modulating nearly all components of the vascular architecture (e.g., VSMC, ECM; see above), anti-oxidant interventions (including the nonselective NOX inhibitor apocynin, and the SOD mimetic tempol) were able to attenuate hypertensive vascular remodeling in various experimental models (128).
With regard to the functional role of specific NOX homologues in hypertensive remodeling, there exists some uncertainty. Although Nox1 knockout in mice reduced the blood pressure response secondary to AngII stimulation, Nox1 deficiency was inconsistently reported to be either effective or ineffective in preventing AngII-induced vascular hypertrophy (43). Still others have found Nox1 overexpression to be a potent amplifier of AngII-induced hypertension as well as vascular hypertrophy (31).
In the arena of pulmonary hypertension (PH), gp91phox knockout has been shown to completely abolish structural remodeling in small pulmonary arteries induced by chronic hypoxia—implying a critical role for NOX/ROS signaling in PH pathogenesis (75).
Abdominal aortic aneurysms
The pathogenesis of abdominal aortic aneurysms (AAA) may provide an exquisite role for ROS-based mechanotransduction, as it exhibits significant changes in both triggers and targets of ROS signaling: a profoundly altered hemodynamic/biomechanical milieu as well as extensive inflammatory vascular remodeling. Segmental aortic dilation alters the hemodynamic flow pattern, increasing oscillatory and reducing (protective) laminar shear stress. Further, aneurysm formation is associated with markedly increased segmental stiffness (reduced circumferential strain) of the corresponding vessel wall (80). Interestingly, experimentally increased flow in the aorta during AAA formation increased laminar shear stress as well as cyclic strain and reduced AAA growth in a rodent model (94).
There are multiple lines of direct and indirect evidence indicating that ROS levels are elevated in human AAA. Early studies found reduced amounts of anti-oxidants (such as ascorbic acid, SOD, or GSH peroxidase) in AAA tissue samples compared with nonaneurysmal aorta (34, 35). Later, increased ROS levels as well as increased expression of the NOX1/NOX2 subunits p47phox and p22phox were demonstrated as local features of AAA as opposed to nondiseased aortic segments (86). Pointing toward a more universal relevance, p22phox expression was also found to be up-regulated in human thoracic aortic aneurysm (compared with healthy controls) (36).
Evidence for a functional/causative role for ROS in the pathobiology of AAA formation is derived from various animal studies. Administration of the ROS-scavenger vitamin E reduced parameters of oxidative stress, aortic macrophage infiltration, and, ultimately, ameliorated aneurysm growth or decreased the incidence of aneurysm rupture in different models of murine AAA (44, 94).
Focusing on the role of NOXs in AAA pathogenesis, NOX inhibition (by apocynin) was shown to block experimentally induced AAA formation, accompanied by decreased levels of MMP-2 and MMP-9 (132). Other experiments have further delineated the particular significance of specific NOXs in this process. Knockout of p47phox attenuated NADPH-oxidase activity, oxidative stress, aortic macrophage infiltration, and MMP-2 activity and, ultimately, reduced the incidence and severity of AngII-induced AAA in ApoE−/− mice independently from blood pressure effects (125). In addition, p47phox deletion was also protective in murine cerebral aneurysm growth (3). Although these data primarily suggest a major role for NOX2 in aneurysm pathology, others have found NOX1 deficiency to be also protective in that regard, and have offered NOX1-induced suppression of tissue inhibitors of metalloproteinase 1 as a possible mechanism (43). Therefore, both NOX1 and NOX2 may promote AAA development, while p47phox may also act as an important subunit of NOX1 in AAA disease.
Newly discovered small noncoding microRNAs have emerged as powerful cellular regulators involved in tissue remodeling (116). We recently reported that AAA formation is associated with increased expression of miR-21, a key modulator of proliferation and apoptosis, as well as a reduced expression of miR-29b, governing ECM remodeling (81, 82). Interestingly, Lin et al. found that VSMC stimulation with hydrogen peroxide also induced miR-21 upregulation and miR-29b downregulation (74). Thus, it is tempting to speculate that the increased ROS generation observed in AAA may also serve to regulate miR-based tissue remodeling.
Conclusions and Future Directions
ROS can be considered critical mediators involved in the pathomechanism of numerous vascular diseases. This results from findings demonstrating that (a) ROS levels are elevated in response to pathologic hemodynamic stresses which accompany multiple vascular diseases, (b) ROS govern significant processes of structural vascular remodeling (VSMC proliferation/apoptosis, matrix remodeling, and inflammation) that characterize most vascular pathologies, and (c) ROS levels are elevated in most vascular diseases. Therefore, despite distinct differences between specific vascular pathologies, ROS-based signaling represents an intriguing universal mechanism that promotes disease (Fig. 3). Consequently various anti-oxidative interventions “silencing” ROS-derived signals (e.g., inhibition of ROS-generating enzymes such as NOX, application of antioxidants as vitamin C and E) have been found to be effective in attenuating atherosclerosis, hypertensive arterial remodeling, or AAA formation in animal models.
In striking contrast, randomized clinical trials have substantially failed to provide convincing evidence of a protective effect of antioxidant treatment in human cardiovascular diseases (5, 16). However, this may not necessarily be regarded as a general failure of the ROS hypothesis but may simply reflect the numerous open questions regarding antioxidant treatment. These include concerns of dosage (e.g., dosage per bodyweight in animal experiment was usually much higher than in human trials), of specificity of anti-oxidant substance used (e.g., antioxidant vitamins do not scavenge H2O2; differing potency of specific vitamin E isomers; and insufficient targeting of specific cellular compartments), or of efficacy (e.g., parameters of ROS reduction were mostly not tested as endpoints).
Furthermore, in some cases, it might be beneficial to selectively inhibit specific ROS generators (e.g., NOX1 or NOX2, as opposed to NOX4, to counteract endothelial dysfunction). However, corresponding clinically applicable agents are still lacking. In other situations—due to the complex and redundant ROS signaling network—more global approaches would be desirable. Given the major importance of ROS-based signaling in animal models of vascular diseases, these issues should be incorporated into the design of future clinical trials.
Abbreviations Used
- AAA
abdominal aortic aneurysms
- AP-1
activator protein-1
- EC
endothelial cell
- ECM
extra-cellular matrix
- EDRF
endothelium-derived vascular relaxing factor
- eNOS
endothelial NOS
- ETC
electron transport chain
- GSH
glutathione
- LDL
low-density lipoproteins
- MAP
mitogen-activated protein
- MMPs
matrix metalloproteinases
- NO
nitric oxide
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- oxLDL
oxidized LDL
- PH
pulmonary hypertension
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- VSMC
vascular smooth muscle cell
- XO
xanthine oxidase
Acknowledgments
This work was supported by research grants from NIH (1R01HL105299 to P.S. Tsao) and Deutsche Forschungsgemeinschaft (RA 2179/1-1 to U. Raaz).
References
- 1.Ali MH, Pearlstein DP, Mathieu CE, and Schumacker PT. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Andrew PJ. and Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res 43: 521–531, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, and Hashimoto N. Reactive oxygen species modulate growth of cerebral aneurysms: a study using the free radical scavenger edaravone and p47phox(−/−) mice. Lab Invest 89: 730–741, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Arnold WP, Mittal CK, Katsuki S, and Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci U S A 74: 3203–3207, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asplund K. Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Intern Med 251: 372–392, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Azevedo LC, Pedro MA, Souza LC, de Souza HP, Janiszewski M, da Luz PL, and Laurindo FR. Oxidative stress as a signaling mechanism of the vascular response to injury: the redox hypothesis of restenosis. Cardiovasc Res 47: 436–445, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bae YS, Lee JH, Choi SH, Kim S, Almazan F, Witztum JL, and Miller YI. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ Res 104: 210–218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barauna VG, Magalhaes FC, Campos LC, Reis RI, Kunapuli SP, Costa-Neto CM, Miyakawa AA, and Krieger JE. Shear stress-induced Ang II AT1 receptor activation: G-protein dependent and independent mechanisms. Biochem Biophys Res Commun 434: 647–652, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Barry-Lane PA, Patterson C, van der Merwe M, Hu Z, Holland SM, Yeh ET, and Runge MS. p47phox is required for atherosclerotic lesion progression in ApoE(−/−) mice. J Clin Invest 108: 1513–1522, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman JS, Beckman TW, Chen J, Marshall PA, and Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A 87: 1620–1624, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, Volpi E, and Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA, Bamshad B, Esterson M, and Fogelman AM. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest 85: 1260–1266, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beswick RA, Zhang H, Marable D, Catravas JD, Hill WD, and Webb RC. Long-term antioxidant administration attenuates mineralocorticoid hypertension and renal inflammatory response. Hypertension 37: 781–786, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, and Chien S. Fluid shear stress activation of IkappaB kinase is integrin-dependent. J Biol Chem 273: 30544–30549, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal 11: 1651–1667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleys J, Miller ERr, Pastor-Barriuso R, Appel LJ, and Guallar E. Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr 84: 880–887; quiz 954–955, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Brandes RP, Takac I, and Schroder K. No superoxide—no stress?: Nox4, the good NADPH oxidase! Arterioscler Thromb Vasc Biol 31: 1255–1257, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Briones AM, Arribas SM, and Salaices M. Role of extracellular matrix in vascular remodeling of hypertension. Curr Opin Nephrol Hypertens 19: 187–194, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Brown MR, Miller FJJ, Li WG, Ellingson AN, Mozena JD, Chatterjee P, Engelhardt JF, Zwacka RM, Oberley LW, Fang X, Spector AA, and Weintraub NL. Overexpression of human catalase inhibits proliferation and promotes apoptosis in vascular smooth muscle cells. Circ Res 85: 524–533, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Browning EA, Chatterjee S, and Fisher AB. Stop the flow: a paradigm for cell signaling mediated by reactive oxygen species in the pulmonary endothelium. Annu Rev Physiol 74: 403–424, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H. and Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Castier Y, Brandes RP, Leseche G, Tedgui A, and Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res 97: 533–540, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Castier Y, Ramkhelawon B, Riou S, Tedgui A, and Lehoux S. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal 11: 1641–1649, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Chapman GB, Durante W, Hellums JD, and Schafer AI. Physiological cyclic stretch causes cell cycle arrest in cultured vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H748–H754, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, and Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen XL, Tummala PE, Olbrych MT, Alexander RW, and Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res 83: 952–959, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Cheng JJ, Wung BS, Chao YJ, and Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension 31: 125–130, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Commoner B, Townsend J, and Pake GE. Free radicals in biological materials. Nature 174: 689–691, 1954 [DOI] [PubMed] [Google Scholar]
- 29.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, and Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res 82: 1094–1101, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, and Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation 112: 2668–2676, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Dikalova AE, Gongora MC, Harrison DG, Lambeth JD, Dikalov S, and Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol 299: H673–H679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimmeler S, Hermann C, Galle J, and Zeiher AM. Upregulation of superoxide dismutase and nitric oxide synthase mediates the apoptosis-suppressive effects of shear stress on endothelial cells. Arterioscler Thromb Vasc Biol 19: 656–664, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Dubick MA, Hunter GC, Casey SM, and Keen CL. Aortic ascorbic acid, trace elements, and superoxide dismutase activity in human aneurysmal and occlusive disease. Proc Soc Exp Biol Med 184: 138–143, 1987 [DOI] [PubMed] [Google Scholar]
- 35.Dubick MA, Keen CL, DiSilvestro RA, Eskelson CD, Ireton J, and Hunter GC. Antioxidant enzyme activity in human abdominal aortic aneurysmal and occlusive disease. Proc Soc Exp Biol Med 220: 39–45, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Ejiri J, Inoue N, Tsukube T, Munezane T, Hino Y, Kobayashi S, Hirata K, Kawashima S, Imajoh-Ohmi S, Hayashi Y, Yokozaki H, Okita Y, and Yokoyama M. Oxidative stress in the pathogenesis of thoracic aortic aneurysm: protective role of statin and angiotensin II type 1 receptor blocker. Cardiovasc Res 59: 988–996, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Finkel T. and Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Fiorani M, Cantoni O, Tasinato A, Boscoboinik D, and Azzi A. Hydrogen peroxide-and fetal bovine serum-induced DNA synthesis in vascular smooth muscle cells: positive and negative regulation by protein kinase C isoforms. Biochim Biophys Acta 1269: 98–104, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Fisher AB. Redox signaling across cell membranes. Antioxid Redox Signal 11: 1349–1356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forman HJ, Torres M, and Fukuto J. Redox signaling. Mol Cell Biochem 234–235: 49–62, 2002 [PubMed] [Google Scholar]
- 41.Forstermann U. Janus-faced role of endothelial NO synthase in vascular disease: uncoupling of oxygen reduction from NO synthesis and its pharmacological reversal. Biol Chem 387: 1521–1533, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, and Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest 105: 1631–1639, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, and Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett 580: 497–504, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Gavrila D, Li WG, McCormick ML, Thomas M, Daugherty A, Cassis LA, Miller FJJ, Oberley LW, Dellsperger KC, and Weintraub NL. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: 1671–1677, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godbole AS, Lu X, Guo X, and Kassab GS. NADPH oxidase has a directional response to shear stress. Am J Physiol Heart Circ Physiol 296: H152–H158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goettsch C, Goettsch W, Arsov A, Hofbauer LC, Bornstein SR, and Morawietz H. Long-term cyclic strain downregulates endothelial Nox4. Antioxid Redox Signal 11: 2385–2397, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Goettsch C, Goettsch W, Brux M, Haschke C, Brunssen C, Muller G, Bornstein SR, Duerrschmidt N, Wagner AH, and Morawietz H. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res Cardiol 106: 551–561, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Griendling KK, Minieri CA, Ollerenshaw JD, and Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 49.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, and Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80–e86, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Gryglewski RJ, Palmer RM, and Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature 320: 454–456, 1986 [DOI] [PubMed] [Google Scholar]
- 51.Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, and Harrison DG. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52: 1803–1809, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, and Channon KM. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26: 333–339, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab Rev 36: 363–375, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Hay D. Activation of NF-κB nuclear transcription factor by flow in human endothelial cells. Biochim Biophys Acta Mol Cell Res 1642: 33–44, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Hipper A. and Isenberg G. Cyclic mechanical strain decreases the DNA synthesis of vascular smooth muscle cells. Pflugers Arch 440: 19–27, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Howard AB, Alexander RW, Nerem RM, Griendling KK, and Taylor WR. Cyclic strain induces an oxidative stress in endothelial cells. Am J Physiol 272: C421–C427, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, and Finkel T. Vascular effects following homozygous disruption of p47phox: an essential component of NADPH Oxidase. Circulation 101: 1234–1236, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, and Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol 175: 156–162, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, and Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, and Harrison DG, Jo H. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J Biol Chem 278: 47291–47298, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Inoue N, Ramasamy S, Fukai T, Nerem RM, and Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, and Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am J Physiol Heart Circ Physiol 298: H24–H32, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, and Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation 109: 1795–1801, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Khatri JJ, Johnson C, Magid R, Lessner SM, Laude KM, Dikalov SI, Harrison DG, Sung HJ, Rong Y, and Galis ZS. Vascular oxidant stress enhances progression and angiogenesis of experimental atheroma. Circulation 109: 520–525, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Kirk EA, Dinauer MC, Rosen H, Chait A, Heinecke JW, and LeBoeuf RC. Impaired superoxide production due to a deficiency in phagocyte NADPH oxidase fails to inhibit atherosclerosis in mice. Arterioscler Thromb Vasc Biol 20: 1529–1535, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Kojda G. and Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 67: 187–197, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Ku DN, Giddens DP, Zarins CK, and Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 68.Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, and da Luz PL. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res 74: 700–709, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Lee CF, Qiao M, Schroder K, Zhao Q, and Asmis R. Nox4 is a novel inducible source of reactive oxygen species in monocytes and macrophages and mediates oxidized low density lipoprotein-induced macrophage death. Circ Res 106: 1489–1497, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MY, San Martin A, Mehta PK, Dikalova AE, Garrido AM, Datla SR, Lyons E, Krause KH, Banfi B, Lambeth JD, Lassegue B, and Griendling KK. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler Thromb Vasc Biol 29: 480–487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lehoux S. and Tedgui A. Signal transduction of mechanical stresses in the vascular wall. Hypertension 32: 338–345, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Lerman LO, Nath KA, Rodriguez-Porcel M, Krier JD, Schwartz RS, Napoli C, and Romero JC. Increased oxidative stress in experimental renovascular hypertension. Hypertension 37: 541–546, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Li PF, Dietz R, and von Harsdorf R. Reactive oxygen species induce apoptosis of vascular smooth muscle cell. FEBS Lett 404: 249–252, 1997 [DOI] [PubMed] [Google Scholar]
- 74.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, and Zhang C. Involvement of MicroRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem 284: 7903–7913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu JQ, Zelko IN, Erbynn EM, Sham JS, and Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 290: L2–L10, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, de Groot E, MacAllister RJ, Kuijpers TW, and Deanfield JE. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation 121: 2310–2316, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Lu X, Guo X, Wassall CD, Kemple MD, Unthank JL, and Kassab GS. Reactive oxygen species cause endothelial dysfunction in chronic flow overload. J Appl Physiol 110: 520–527, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, and Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 328: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, and Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacSweeney ST, Young G, Greenhalgh RM, and Powell JT. Mechanical properties of the aneurysmal aorta. Br J Surg 79: 1281–1284, 1992 [DOI] [PubMed] [Google Scholar]
- 81.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, and Tsao PS. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med 4: 122ra22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, and Tsao PS. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 122: 497–506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Matsushita H, Lee KH, and Tsao PS. Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem Suppl Suppl 36: 99–106, 2001 [DOI] [PubMed] [Google Scholar]
- 85.McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, and Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285: H2290–H2297, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Miller FJ. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol 22: 560–565, 2002 [DOI] [PubMed] [Google Scholar]
- 87.Minor RLJ, Myers PR, Guerra RJ, Bates JN, and Harrison DG. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest 86: 2109–2116, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miura H. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: 31e–40e, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, and Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of ROS for persistent activation of NF-kappaB in endothelial cells. Am J Physiol Cell Physiol 292: C362–C371, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Mohan S, Mohan N, and Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol 273: C572–C578, 1997 [DOI] [PubMed] [Google Scholar]
- 91.Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, and Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res 97: 637–644, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Mugge A, Elwell JH, Peterson TE, Hofmeyer TG, Heistad DD, and Harrison DG. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res 69: 1293–1300, 1991 [DOI] [PubMed] [Google Scholar]
- 93.Muller G. and Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal 11: 1711–1731, 2009 [DOI] [PubMed] [Google Scholar]
- 94.Nakahashi TK, Hoshina K, Tsao PS, Sho E, Sho M, Karwowski JK, Yeh C, Yang RB, Topper JN, and Dalman RL. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol 22: 2017–2022, 2002 [DOI] [PubMed] [Google Scholar]
- 95.Nigro P, Abe J, and Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal 15: 1405–1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishio E. and Watanabe Y. The involvement of reactive oxygen species and arachidonic acid in alpha 1-adrenoceptor-induced smooth muscle cell proliferation and migration. Br J Pharmacol 121: 665–670, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oeckler RA. Stretch enhances contraction of bovine coronary arteries via an NAD(P)H oxidase-mediated activation of the extracellular signal-regulated kinase mitogen-activated protein kinase cascade. Circ Res 92: 23–31, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Pacher P, Beckman JS, and Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palmer RM, Ferrige AG, and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 100.Paravicini TM, Miller AA, Drummond GR, and Sobey CG. Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol-3 kinase, NADPH-oxidase, and nitric oxide synthase. J Cereb Blood Flow Metab 26: 836–845, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Park YM, Febbraio M, and Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 119: 136–145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petersen OH, Michalak M, and Verkhratsky A. Calcium signalling: past, present and future. Cell Calcium 38: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Pueyo ME, Gonzalez W, Nicoletti A, Savoie F, Arnal J-F, and Michel J-B. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor- b activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol 20: 645–651, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Raaz U, Kuhn H, Wirtz H, and Hammerschmidt S. Rapamycin reduces high-amplitude, mechanical stretch-induced apoptosis in pulmonary microvascular endothelial cells. Microvasc Res 77: 297–303, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, and Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, and Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97: 1916–1923, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramkhelawon B, Rivas D, and Lehoux S. Shear stress activates extracellular signal-regulated kinase 1/2 via the angiotensin II type 1 receptor. FASEB J 27: 3008–3016, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, and Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 109.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A, and Saez GT. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 41: 1096–1101, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Rosen GM, Pou S, Ramos CL, Cohen MS, and Britigan BE. Free radicals and phagocytic cells. FASEB J 9: 200–209, 1995 [DOI] [PubMed] [Google Scholar]
- 111.Rueckschloss U, Galle J, Holtz J, Zerkowski H-R, and Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme a reductase inhibitor therapy. Circulation 104: 1767–1772, 2001 [DOI] [PubMed] [Google Scholar]
- 112.Schieffer B, Luchtefeld M, Braun S, Hilfiker A, Hilfiker-Kleiner D, and Drexler H. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res 87: 1195–1201, 2000 [DOI] [PubMed] [Google Scholar]
- 113.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, and Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, and Miller FJJ. Role for Nox1 NADPH oxidase in atherosclerosis. Atherosclerosis 216: 321–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, and Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol 21: 739–745, 2001 [DOI] [PubMed] [Google Scholar]
- 116.Small EM, Frost RJ, and Olson EN. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121: 1022–1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, and Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Stocker R. and Keaney JFJ. Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, and Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature 401: 79–82, 1999 [DOI] [PubMed] [Google Scholar]
- 120.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, and Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270: 296–299, 1995 [DOI] [PubMed] [Google Scholar]
- 121.Sutherland EW. Studies on the mechanism of hormone action. Science 177: 401–408, 1972 [DOI] [PubMed] [Google Scholar]
- 122.Szocs K. Upregulation of Nox-Based NAD(P)H Oxidases in Restenosis After Carotid Injury. Arterioscler Thromb Vasc Biol 22: 21–27, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Tabet F, Schiffrin EL, Callera GE, He Y, Yao G, Ostman A, Kappert K, Tonks NK, and Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res 103: 149–158, 2008 [DOI] [PubMed] [Google Scholar]
- 124.Takabe W, Warabi E, and Noguchi N. Anti-atherogenic effect of laminar shear stress via Nrf2 activation. Antioxid Redox Signal 15: 1415–1426, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Thomas M, Gavrila D, McCormick ML, Miller FJJ, Daugherty A, Cassis LA, Dellsperger KC, and Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation 114: 404–413, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, and Pritchard KAJ. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95: 9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, Antenucci G, Mascio G, Bettarini U, Landolfi A, Iorio L, Maffei A, and Lembo G. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betaPIX/Rac-1 pathway. Hypertension 54: 1028–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 128.Virdis A, Neves MF, Amiri F, Touyz RM, and Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens 22: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 129.Wei Z, Costa K, Al-Mehdi AB, Dodia C, Muzykantov V, and Fisher AB. simulated ischemia in flow-adapted endothelial cells leads to generation of reactive oxygen species and cell signaling. Circ Res 85: 682–689, 1999 [DOI] [PubMed] [Google Scholar]
- 130.Willett NJ, Kundu K, Knight SF, Dikalov S, Murthy N, and Taylor WR. Redox signaling in an in vivo murine model of low magnitude oscillatory wall shear stress. Antioxid Redox Signal 15: 1369–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams B. Mechanical influences on vascular smooth muscle cell function. J Hypertens 16: 1921–1929, 1998 [DOI] [PubMed] [Google Scholar]
- 132.Xiong W, Mactaggart J, Knispel R, Worth J, Zhu Z, Li Y, Sun Y, Baxter BT, and Johanning J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 202: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, and Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension 32: 488–495, 1998 [DOI] [PubMed] [Google Scholar]
- 134.Zhang Q, Malik P, Pandey D, Gupta S, Jagnandan D, Belin de Chantemele E, Banfi B, Marrero MB, Rudic RD, Stepp DW, and Fulton DJ. Paradoxical activation of endothelial nitric oxide synthase by NADPH oxidase. Arterioscler Thromb Vasc Biol 28: 1627–1633, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]