Abstract
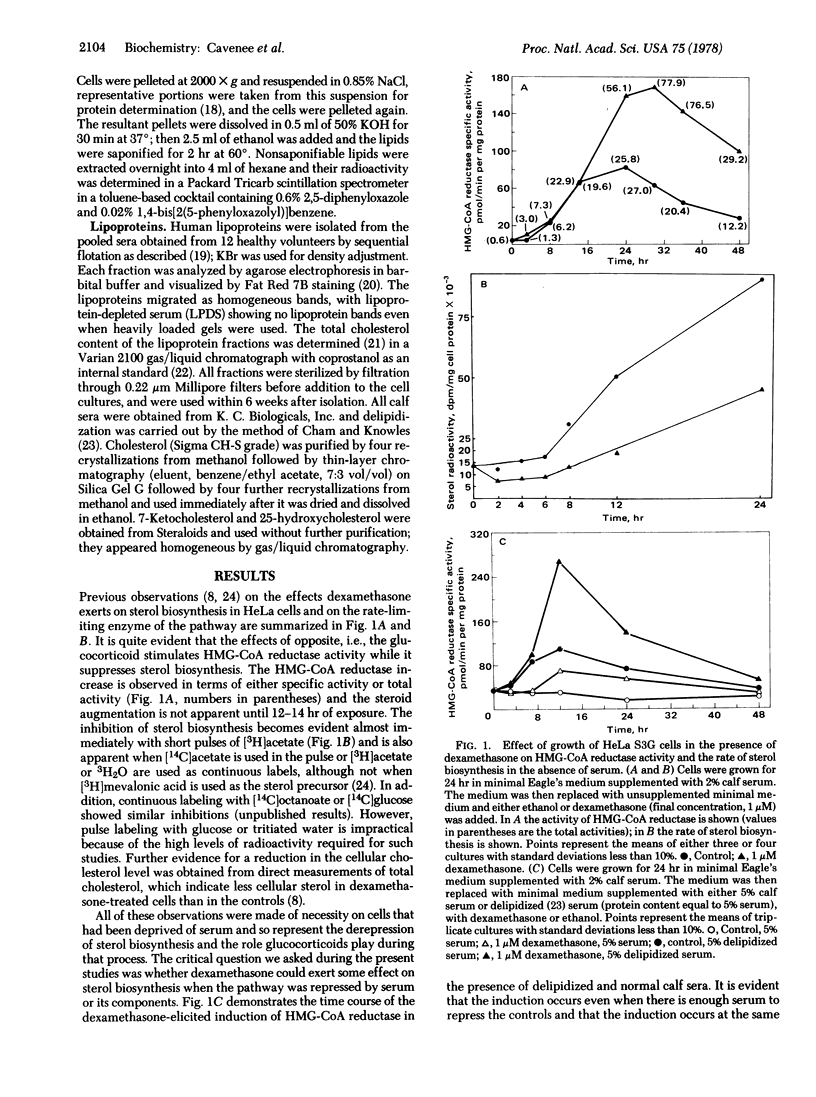
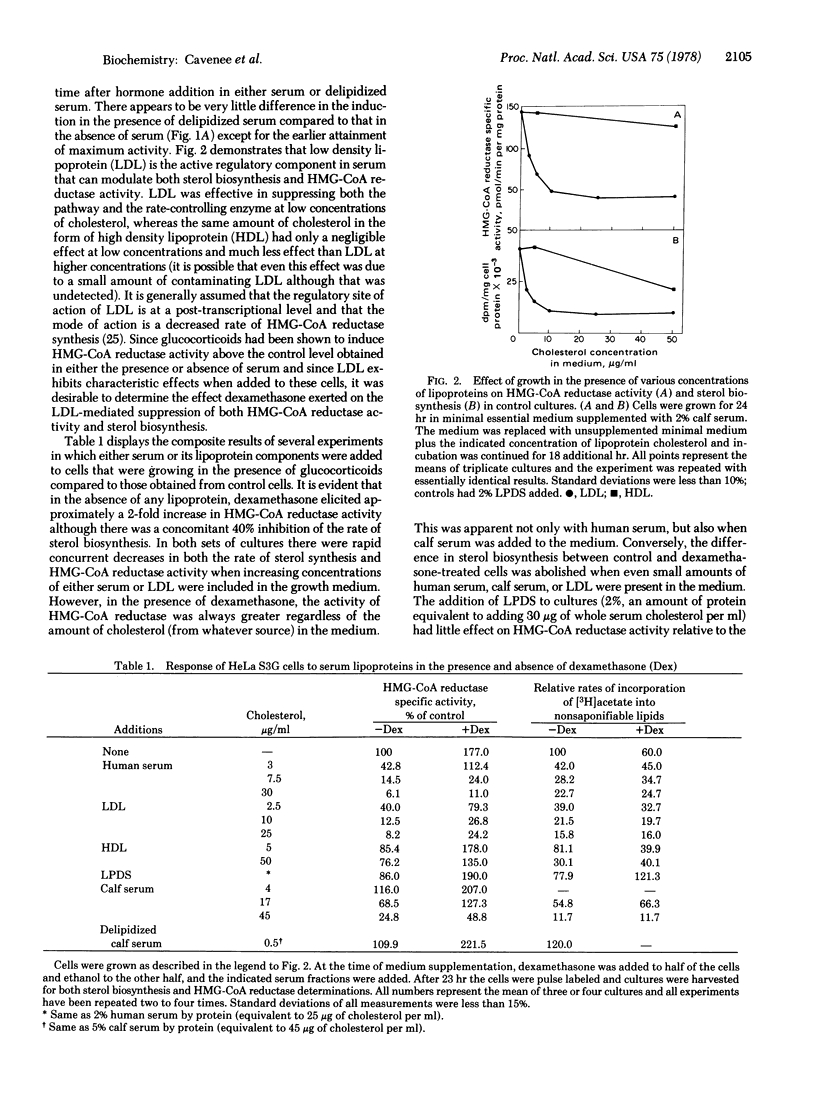
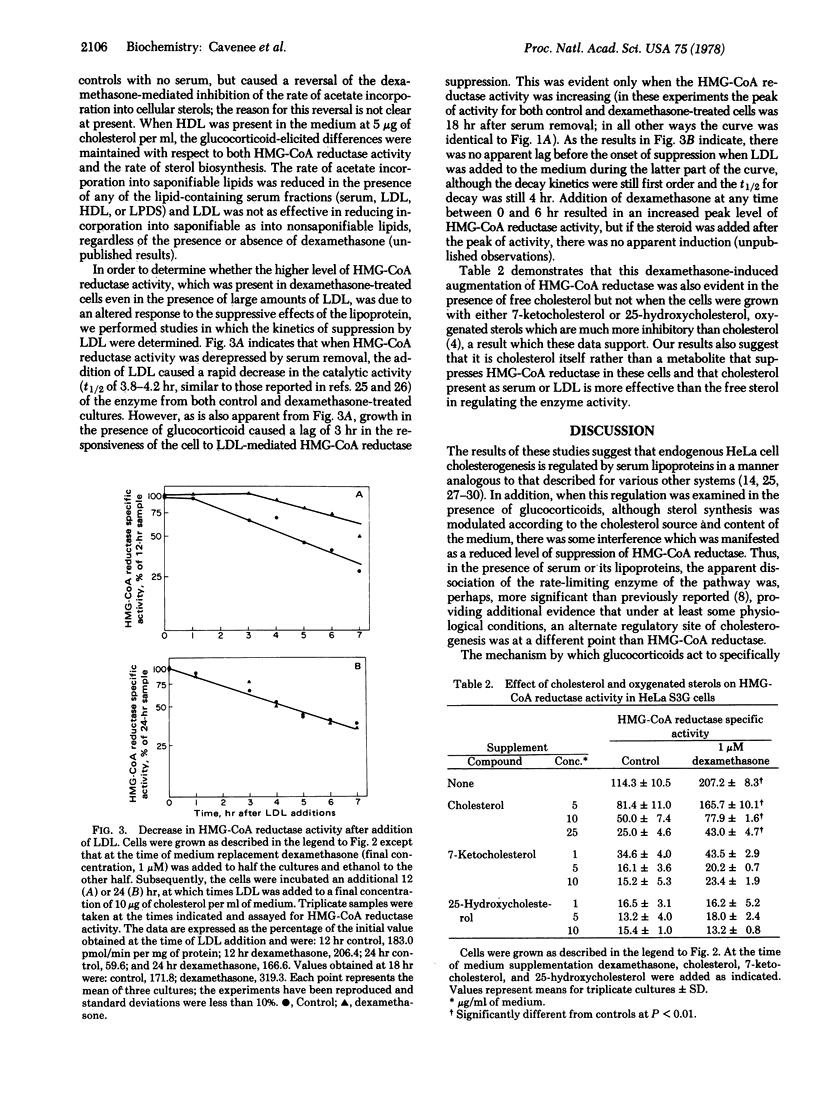
Depression of the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase [mevalonate:NADP+ oxidoreductase (CoA-acylating); EC 1.1.1.34] was elicited by the removal of serum from the growth medium of HeLa S3G cells with a concomitant expected increase in cellular sterol biosynthesis; if dexamethasone (9α-fluoro-11β,17α,21-trihydroxy-16α-methyl-1, 4-pregnadiene-3,20-dione) was present in the serumless medium, there was an augmentation of HMG-CoA reductase activity but a suppression of sterol biosynthesis. When human serum, human low density lipoprotein, or calf serum was present in the medium, there was a reduction of both the enzyme activity and sterol biosynthesis, but the presence of dexamethasone resulted in an increase in HMG-CoA reductase activity as compared to the controls containing human serum, low density lipoprotein, or calf serum alone. In contrast, either low density lipoprotein or whole serum supplementation eliminated the differences in acetate incorporation into sterols between glucocorticoid-treated and untreated cells. Human high density lipoproteins had little effect on the enzyme activity and abolished the difference in sterol biosynthesis only at relatively high concentrations. Addition of low density lipoproteins to cells after preincubation in serumless medium elicited the same rate of decay of HMG-CoA reductase (t1/2 3.8-4.2 hr) regardless of the presence of glucocorticoids in the medium, but there was an exaggerated lag before the onset of suppression in the hormone-treated cells. If free cholesterol was present in the medium, the dexamethasone augmentation of HMG-CoA reductase was maintained, but the addition of either 7-ketocholesterol or 25-hydroxycholesterol abolished the difference between glucocorticoid-treated and control cells. These observations suggest that, under certain physiological conditions, HMG-CoA reductase activity no longer accurately reflects cellular sterol biosynthesis.
Keywords: enzyme regulation, glucocorticoids, cholesterogenesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigan J., Bhathena S. J., Schreiner M. E. Control of sterol synthesis and of hydroxymethylglutaryl CoA reductase in skin fibroblasts grown from patients with homozygous type II hyperlipoproteinemia. J Lipid Res. 1975 Mar;16(2):151–154. [PubMed] [Google Scholar]
- Bates S. R., Rothblat G. H. Regulation of cellular sterol flux and synthesis by human serum lipoproteins. Biochim Biophys Acta. 1974 Jul 26;360(1):38–55. doi: 10.1016/0005-2760(74)90178-7. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Cavenee W. K., Melnykovych G. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in HeLa cells by glucocorticoids. J Biol Chem. 1977 May 25;252(10):3272–3276. [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Dietschy J. M., Brown M. S. Effect of alterations of the specific activity of the intracellular acetyl CoA pool on apparent rates of hepatic cholesterogenesis. J Lipid Res. 1974 Sep;15(5):508–516. [PubMed] [Google Scholar]
- Dugan R. E., Slakey L. L., Briedis A. V., Porter J. W. Factors affecting the diurnal variation in the level of -hydroxy- -methylglutaryl coenzyme A reductase and cholesterol-synthesizing activity in rat liver. Arch Biochem Biophys. 1972 Sep;152(1):21–27. doi: 10.1016/0003-9861(72)90188-9. [DOI] [PubMed] [Google Scholar]
- Edwards P. A. Effect of adrenalectomy and hypophysectomy on the circadian rhythm of -hydroxy- -methylglutaryl coenzyme A reductase activity in rat liver. J Biol Chem. 1973 Apr 25;248(8):2912–2917. [PubMed] [Google Scholar]
- Edwards P. A., Gould R. G. Turnover rate of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase as determined by use of cycloheximide. J Biol Chem. 1972 Mar 10;247(5):1520–1524. [PubMed] [Google Scholar]
- Hatch F. T. Practical methods for plasma lipoprotein analysis. Adv Lipid Res. 1968;6:1–68. [PubMed] [Google Scholar]
- Ishikawa T. T., MacGee J., Morrison J. A., Glueck C. J. Quantitative analysis of cholesterol in 5 to 20 microliter of plasma. J Lipid Res. 1974 May;15(3):286–291. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J Biol Chem. 1974 Oct 10;249(19):6057–6061. [PubMed] [Google Scholar]
- Kirsten E. S., Watson J. A. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in hepatoma tissue culture cells by serum lipoproteins. J Biol Chem. 1974 Oct 10;249(19):6104–6109. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linn T. C. The effect of cholesterol feeding and fasting upon beta-hydroxy-beta-methylglutaryl coenzyme A reductase. J Biol Chem. 1967 Mar 10;242(5):990–993. [PubMed] [Google Scholar]
- Melnykovych G., Matthews E., Gray S., Lopez I. Inhibition of cholesterol biosynthesis in HeLa cells by glucocorticoids. Biochem Biophys Res Commun. 1976 Jul 26;71(2):506–512. doi: 10.1016/0006-291x(76)90816-0. [DOI] [PubMed] [Google Scholar]
- Mitropoulos K. A., Balasubramaniam S. The role of glucocorticoids in the regulation of the diurnal rhythm of hepatic beta-hydroxy-beta-methylglutaryl-coenzyme A reductase and cholesterol 7 alpha-hydroxylase. Biochem J. 1976 Oct 15;160(1):49–55. doi: 10.1042/bj1600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepokroeff C. M., Lakshmanan M. R., Ness G. C., Dugan R. E., Porter J. W. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974 Feb;160(2):387–396. doi: 10.1016/0003-9861(74)90412-3. [DOI] [PubMed] [Google Scholar]
- Nervi F. O., Carrella M., Dietschy J. M. Dissociation of beta-hydroxy-beta-methylglutaryl-CoA reductase activity from the overall rate of cholesterol synthesis in the liver following the intravenous administration of lipid. J Biol Chem. 1976 Jun 25;251(12):3831–3833. [PubMed] [Google Scholar]
- Noble R. P. Electrophoretic separation of plasma lipoproteins in agarose gel. J Lipid Res. 1968 Nov;9(6):693–700. [PubMed] [Google Scholar]
- Phillips G. B. Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1729–1733. doi: 10.1073/pnas.74.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert M. M., Gray S. L., Melnykovych G. Growth characteristics of two HeLa S3 strains possessing low level glucocorticoid-inducible and high level suppressible alkaline phosphatase. Exp Cell Res. 1975 Aug;94(1):56–62. doi: 10.1016/0014-4827(75)90530-3. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Hennessy S. W. Cholesterol biosynthesis and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase in cultured glial and neuronal cells. Regulation by lipoprotein and by certain free sterols. Biochim Biophys Acta. 1977 Mar 25;486(3):408–420. doi: 10.1016/0005-2760(77)90090-x. [DOI] [PubMed] [Google Scholar]
- Weinstein D. B., Carew T. E., Steinberg D. Uptake and degradation of low density lipoprotein by swine arterial smoot muscle cells with inhibition of cholesterol biosynthesis. Biochim Biophys Acta. 1976 Mar 26;424(3):404–421. doi: 10.1016/0005-2760(76)90030-8. [DOI] [PubMed] [Google Scholar]


