Abstract
Highly fluorescent analogs of insulin and epidermal growth factor were prepared by the covalent attachment of these peptides to alpha-lactalbumin molecules that were highly substituted (i.e., seven to one) with rhodamine molecules. The alpha-lactalbumin was specifically linked to the lysine residue of insulin or to the alpha-amino group of epidermal growth factor. The insulin derivative retained 1.15% of its potency in stimulating glucose oxidation in fat cells but retained about 8.3% of its binding affinity toward receptors. The epidermal growth factor derivative was completely active in binding to fibroblast receptors and 40% as potent as the native hormone in stimulating DNA synthesis. These highly fluorescent derivatives were suitable for the specific visual labeling of receptor sites in viable cells and for measuring the lateral mobilities of the receptor-hormone complexes by fluorescent photobleaching recovery techniques. By these methods it was shown that the hormone-receptor complexes can move laterally in the plane of the plasma membrane with a diffusion coefficient of (3-5) X 10(-10) cm2/sec.
Full text
PDF

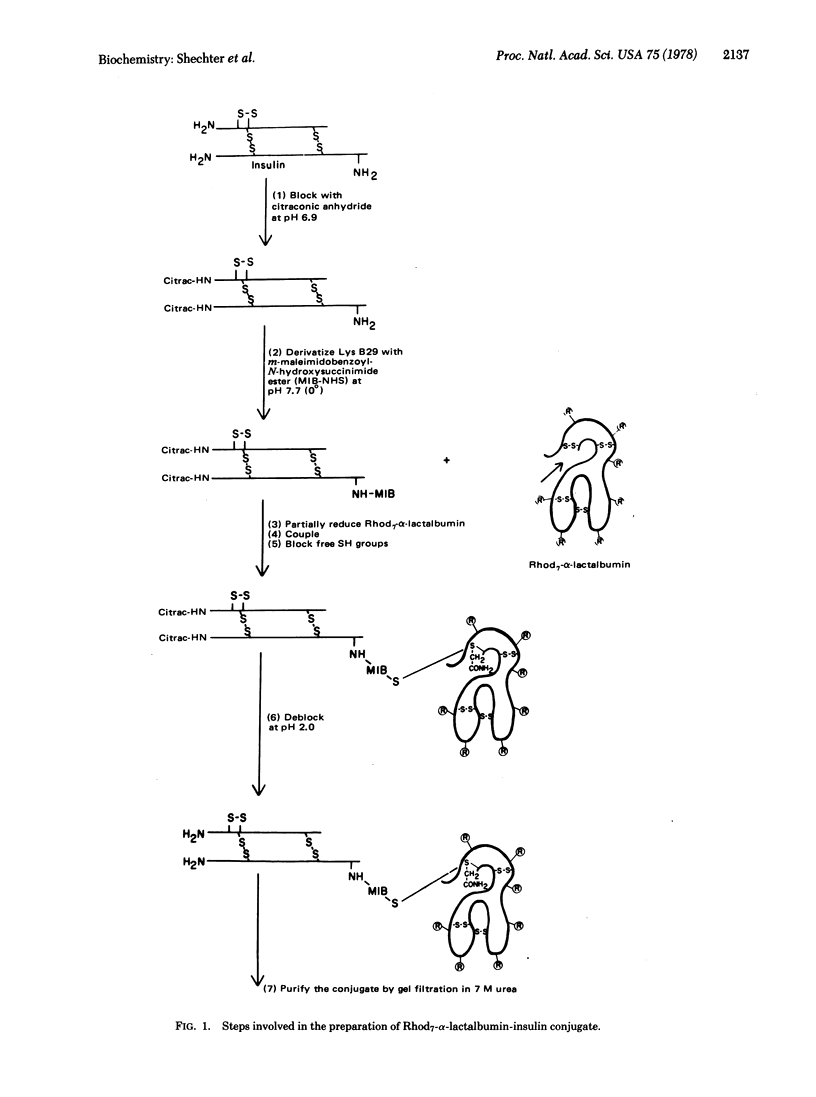
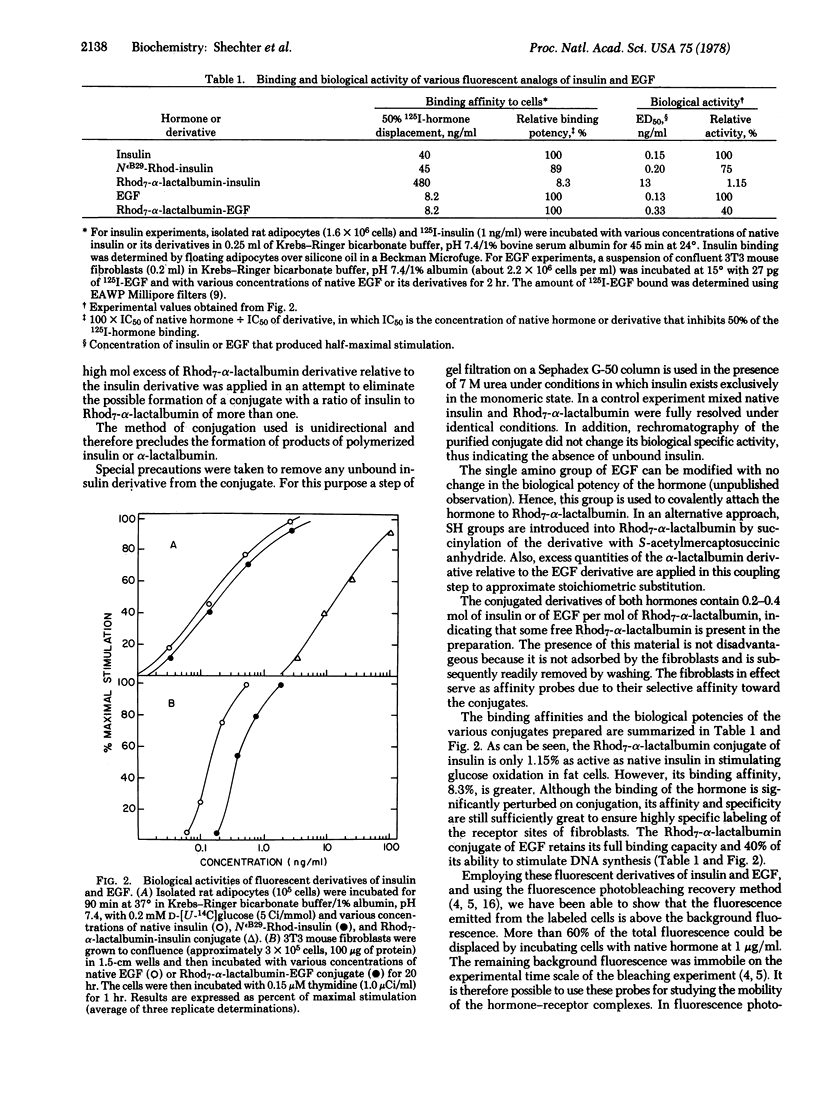
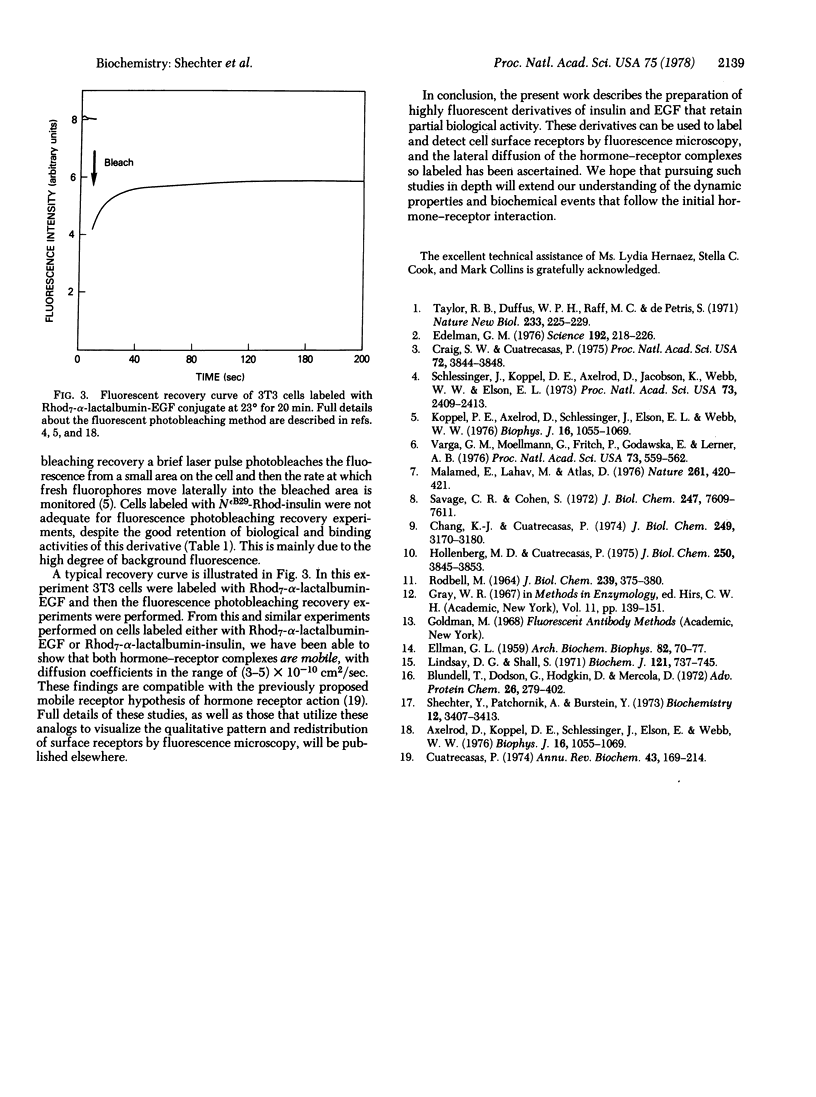
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. J., Cuatrecasas P. Adenosine triphosphate-dependent inhibition of insulin-stimulated glucose transport in fat cells. Possible role of membrane phosphorylation. J Biol Chem. 1974 May 25;249(10):3170–3180. [PubMed] [Google Scholar]
- Craig S. W., Cuatrecasas P. Mobility of cholera toxin receptors on rat lymphocyte membranes. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3844–3848. doi: 10.1073/pnas.72.10.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Lindsay D. G., Shall S. The acetylation of insulin. Biochem J. 1971 Mar;121(5):737–745. doi: 10.1042/bj1210737a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed E., Lahav M., Atlas D. Direct localisation of beta-adrenoceptor sites in rat cerebellum by a new fluorescent analogue of propranolol. Nature. 1976 Jun 3;261(5559):420–422. doi: 10.1038/261420a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schechter Y., Patchornik A., Burstein Y. Selective reduction of cystine 1-8 in alpha-lactalbumin. Biochemistry. 1973 Aug 28;12(18):3407–3413. doi: 10.1021/bi00742a007. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J. M., Moellmann G., Fritsch P., Godawska E., Lerner A. B. Association of cell surface receptors for melanotropin with the Golgi region in mouse melanoma cells. Proc Natl Acad Sci U S A. 1976 Feb;73(2):559–562. doi: 10.1073/pnas.73.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]


