Abstract
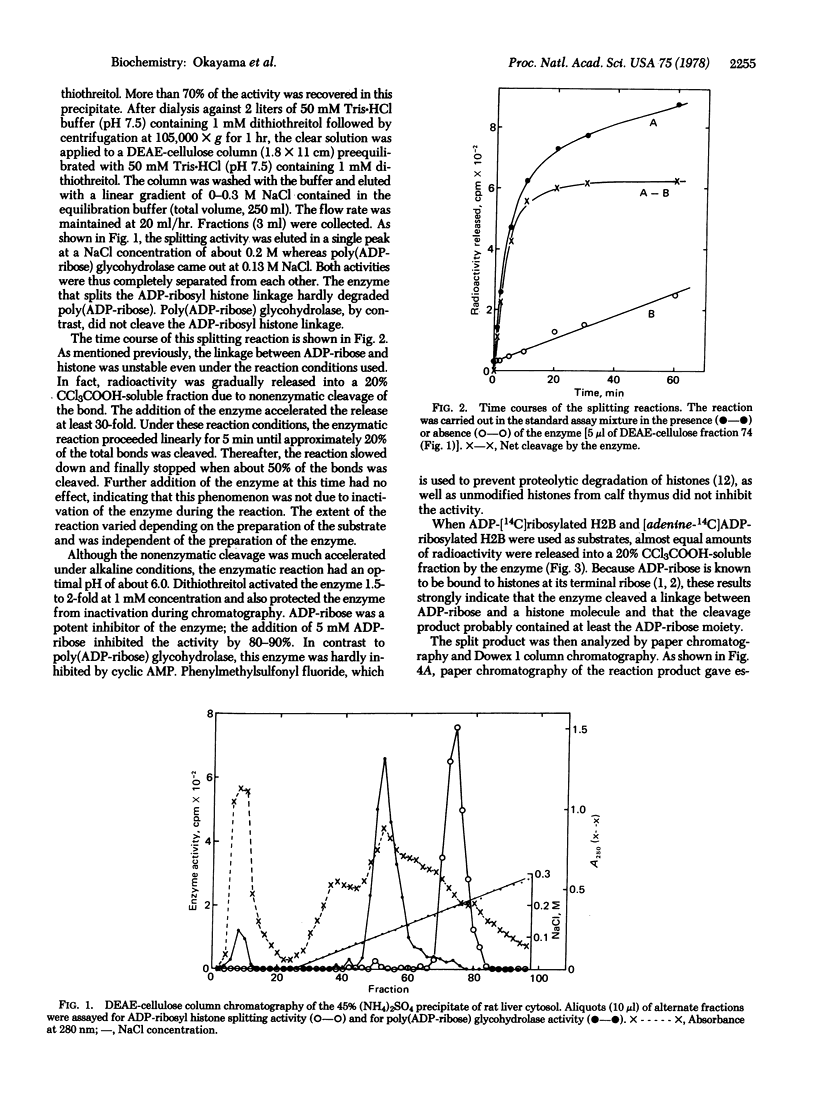
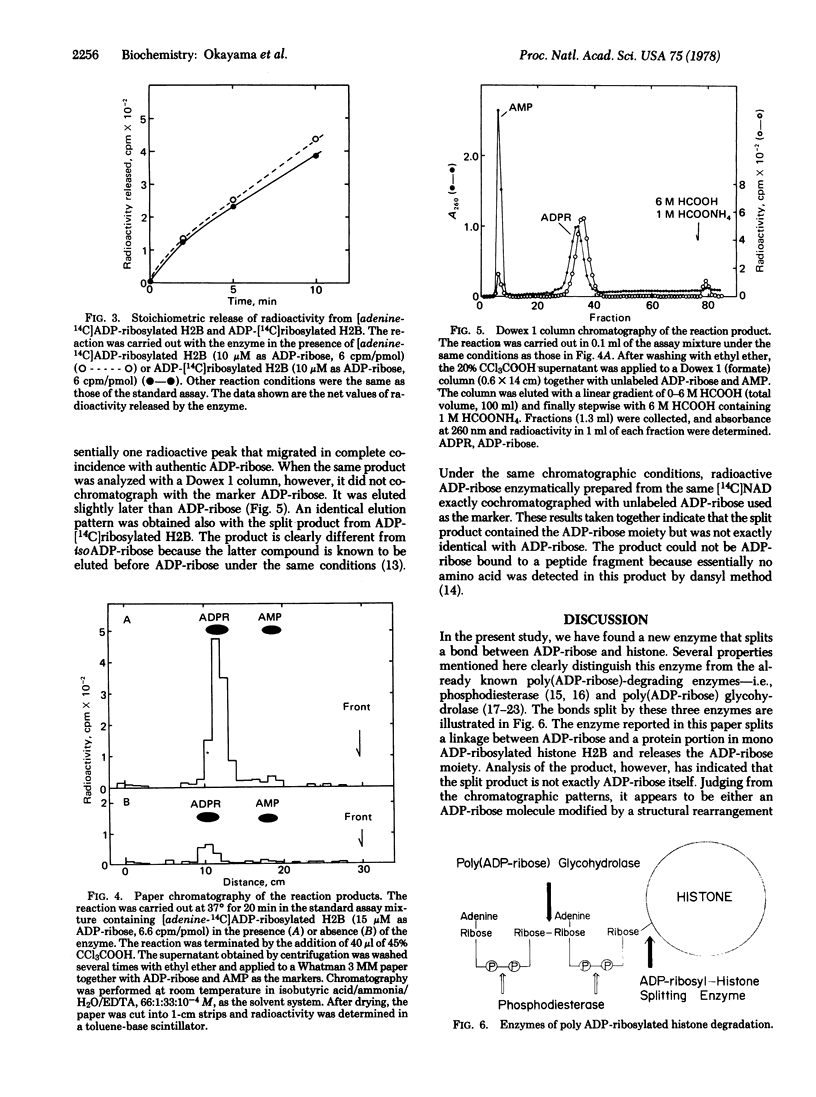
A novel enzyme that splits a bond between ADP-ribose and histone was discovered and partially purified from rat liver cytosol. The 105,000 X g supernatant of rat liver homogenate was precipitated by 45% saturated ammonium sulfate and then chromatographed on a DEAE-cellulose column. The enzyme activity was eluted in a single peak at about 0.2 M NaCl and clearly separated from poly(ADP-ribose) glycohydrolase which came out at 0.13 M NaCl. In contrast to the latter enzyme, this new enzyme catalyzed the spliting of a linkage between ADP-ribose and a protein portion in mono ADP-ribosylated histone H2B but little, if any, of the glycosidic ribosyl(1"-2') ribose bonds within poly(ADP-ribose). Analysis of the reaction product by paper chromatography and Dowex 1 column chromatography indicated that the split product contained the ADP-ribose moiety but was not exactly identical with ADP-ribose. Available evidence suggested that it was either an altered ADP-ribose molecule produced by a structural rearrangement or ADP-ribose itself linked to an unidentified compound. The enzyme had a pH optimum of about 6.0 and was inhibited by 80-90% in the presence of 5 mM ADP-ribose.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballal N. R., Goldberg D. A., Busch H. Dissociation and reconstitution of chromatin without appreciable degradation of the proteins. Biochem Biophys Res Commun. 1975 Feb 17;62(4):972–982. doi: 10.1016/0006-291x(75)90418-0. [DOI] [PubMed] [Google Scholar]
- Burzio L. O., Riquelme P. T., Ohtsuka E., Koide S. S. Evidence for two variants of poly(adenosine diphosphate ribose) glycohydrolase in rat testis. Arch Biochem Biophys. 1976 Mar;173(1):306–319. doi: 10.1016/0003-9861(76)90264-2. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Mizuno D., Sugimura T. Mode of action of rat liver phosphodiesterase on a polymer of phosphoribosyl adenosine monophosphate and related compounds. J Biol Chem. 1968 Dec 25;243(24):6325–6329. [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Miwa M., Nakatsugawa K., Hara K., Matsushima T., Sugimura T. Degradation of poly(adenosine diphosphate ribose) by homogenates of various normal tissues and tumors of rats. Arch Biochem Biophys. 1975 Mar;167(1):54–60. doi: 10.1016/0003-9861(75)90440-3. [DOI] [PubMed] [Google Scholar]
- Miwa M., Sugimura T. Splitting of the ribose-ribose linkage of poly(adenosine diphosphate-robose) by a calf thymus extract. J Biol Chem. 1971 Oct 25;246(20):6362–6364. [PubMed] [Google Scholar]
- Miwa M., Tanaka M., Matsushima T., Sugimura T. Purification and properties of glycohydrolase from calf thymus splitting ribose-ribose linkages of poly(adenosine diphosphate ribose). J Biol Chem. 1974 Jun 10;249(11):3475–3482. [PubMed] [Google Scholar]
- Miyakawa N., Ueda K., Hayaishi O. Association of poly ADP-ribose glycohydrolase with liver chromatin. Biochem Biophys Res Commun. 1972 Oct 6;49(1):239–245. doi: 10.1016/0006-291x(72)90035-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Honjo T., Hayaishi O. Enzymic adenosine diphosphate ribosylation of histone and poly adenosine diphosphate ribose synthesis in rat liver nuclei. J Biol Chem. 1968 Jul 10;243(13):3765–3767. [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Yoshihara K., Yamamura H., Takeda M., Hayaishi O. Enzymic adenosine diphosphoribosylation of nuclear proteins. Cold Spring Harb Symp Quant Biol. 1969;34:781–786. doi: 10.1101/sqb.1969.034.01.088. [DOI] [PubMed] [Google Scholar]
- Okayama H., Ueda K., Hayaishi O. Purification of ADP-ribosylated nuclear proteins by covalent chromatography on dihydroxyboryl polyacrylamide beads and their characterization. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1111–1115. doi: 10.1073/pnas.75.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake H., Miwa M., Fujimura S., Sugimura T. Binding of ADP-ribose polymer with histone. J Biochem. 1969 Jan;65(1):145–146. [PubMed] [Google Scholar]
- Reeder R. H., Ueda K., Honjo T., Nishizuka Y., Hayaishi O. Studies on the polymer of adenosine diphosphate ribose. II. Characterization of the polymer. J Biol Chem. 1967 Jul 10;242(13):3172–3179. [PubMed] [Google Scholar]
- Shinshi H., Miwa M., Kato K., Noguchi M., Matsushima T., Sugimura T. A novel phosphodiesterase from cultured tobacco cells. Biochemistry. 1976 May 18;15(10):2185–2190. doi: 10.1021/bi00655a024. [DOI] [PubMed] [Google Scholar]
- Stone P. R., Whish W. J., Shall S. Poly (ADP-ribose) glycohydrolase in mouse fibroblast cells (LS cells). FEBS Lett. 1973 Nov 1;36(3):334–338. doi: 10.1016/0014-5793(73)80404-1. [DOI] [PubMed] [Google Scholar]
- Sugimura T., Yoshimura N., Miwa M., Nagai H., Nagao M. Studies on poly(adenosine diphosphate-ribose). XI. Purification of poly(adenosine diphosphate-ribose) on a hydroxylapatite column. Arch Biochem Biophys. 1971 Dec;147(2):660–665. doi: 10.1016/0003-9861(71)90425-5. [DOI] [PubMed] [Google Scholar]
- Ueda K., Oka J., Naruniya S., Miyakawa N., Hayaishi O. Poly ADP-ribose glycohydrolase from rat liver nuclei, a novel enzyme degrading the polymer. Biochem Biophys Res Commun. 1972 Jan 31;46(2):516–523. doi: 10.1016/s0006-291x(72)80169-4. [DOI] [PubMed] [Google Scholar]
- Wong N. C., Poirier G. G., Dixon G. H. Adenosine diphosphoribosylation of certain basic chromosomal proteins in isolated trout testis nuclei. Eur J Biochem. 1977 Jul 1;77(1):11–21. doi: 10.1111/j.1432-1033.1977.tb11635.x. [DOI] [PubMed] [Google Scholar]



