Abstract
We identified several diimidazoline mono- and diamides that were as potent as pentamidine against T. brucei rhodesiense in vitro. All of these were also less cytotoxic than pentamidine, but none was as effective as the latter in a T. brucei rhodesiense-infected mouse model. A single imidazoline may be sufficient for high antitrypanosomal activity provided that a second weak base functional group is present.
Keywords: diimidazoline, African trypanosomiasis, Trypanosoma brucei rhodesiense
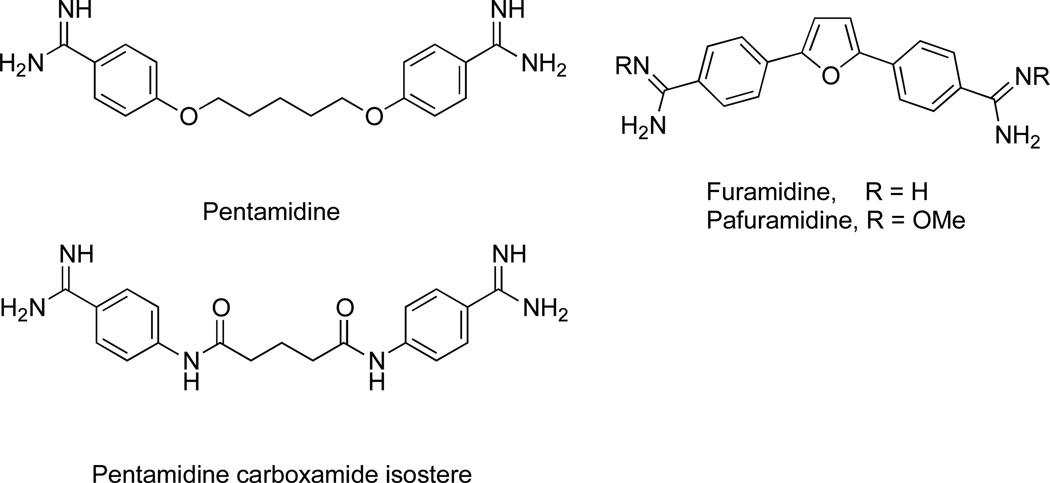
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a neglected vector borne protozoal disease. HAT exists as a chronic infection with Trypanosoma brucei gambiense or as an acute infection with T. brucei rhodesiense. In the first stage of HAT, parasites multiply within the hemolymphatic system. In the second encephalitic stage, parasites infect the central nervous system and the cerebrospinal fluid. Stage 1 disease can be treated with the diamidine pentamidine, whereas melarsoprol, eflornithine, and their combinations with nifurtimox are the only drugs effective against stage 2 disease, and all of these are poorly tolerated and require parenteral administration.1 For example, due to its toxicity and lack of oral bioavailability, pentamidine is usually administered only in hospital settings.2,3 Numerous analogs of pentamidine have been synthesized in order to increase the therapeutic index and provide the option of oral dosing.1–3 This work culminated in the identification of furamidine, a conformationally restricted analogue of pentamidine, and pafuramidine, the orally active dimethoxyamidine prodrug of furamidine (Figure 1). Pafuramidine advanced to phase III clinical trials, but these were suspended due to nephro- and hepatotoxicity.1 Despite this setback, efforts continue to identify a next-generation diamidine.1
Figure 1.
Antitrypanosomal diamidines
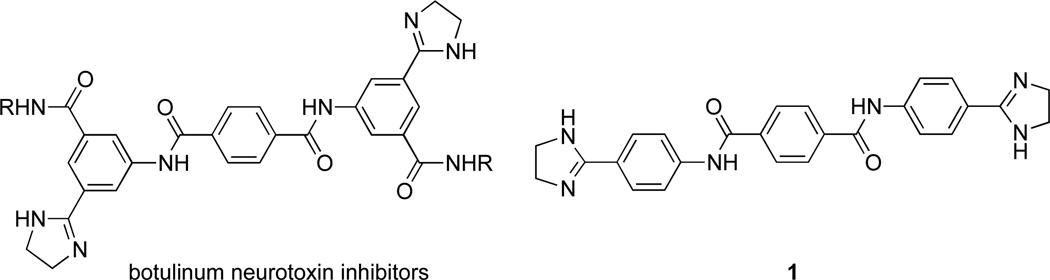
Our interest in diamidines arose from the potential of diimidazolines as inhibitors of botulinum neurotoxin.4 In this work, we prepared several diimidazoline terephthanilides as control compounds of our active botulinum neurotoxin inhibitors, and recognized the structural similarity of some of these (e.g. 1) (Figure 2) to that of pentamidine and other antitrypansomal diamidines (Figure 1). In this respect, the carboxamide isostere of pentamidine (Figure 1) is only slightly less potent and nearly two orders of magnitude less cytotoxic than pentamidine,5 suggesting that the carboxamide functional group may increase antitrypanosomal selectivity of diamidines and related compounds. Not surprisingly, 1 has been previously investigated for its antitrypanosomal effects6 where it was shown that this diimidazoline terephthanilide cured T. brucei brucei-infected mice at low doses. Thus, we set out to reinvestigate the activity of 1 against HAT and to begin to define its SAR.
Figure 2.
Diimidazolines
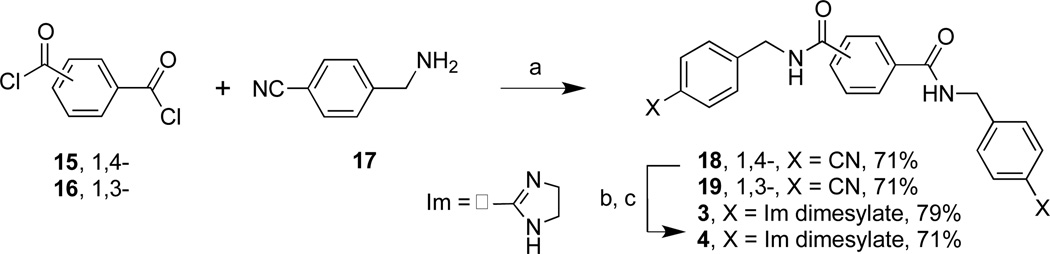
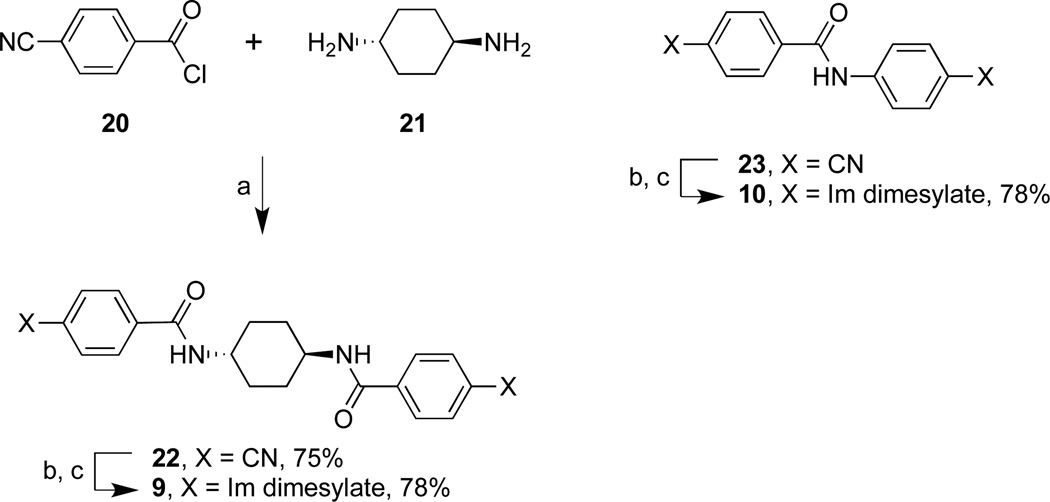
As shown in Schemes 1 and 2, 3, 4, 9, and 10 were obtained by the synthesis of their dinitrile precursors followed by treatment with ethylene diamine and sodium hydrosulfide7 in dimethylacetamide (DMA) to form the diimidazolines which were isolated as their dimesylate salts by treatment with methanesulfonic acid (MSA) (70–80% overall reaction yields).
Scheme 1.
Reagents and conditions: (a) TEA, DMA, rt, 24 h; (b) ethylene diamine, NaSH, DMA, 120 °C, 2 h; (c) MSA, CH3CN, 70 °C, 0.5 h.
Scheme 2.
Reagents and conditions: (a) TEA, CH2Cl2, rt, 24 h; (b) ethylene diamine, NaSH, DMA, 100 °C, 2–5 h; (c) MSA, CH3CN, 70 °C, 0.5 h.
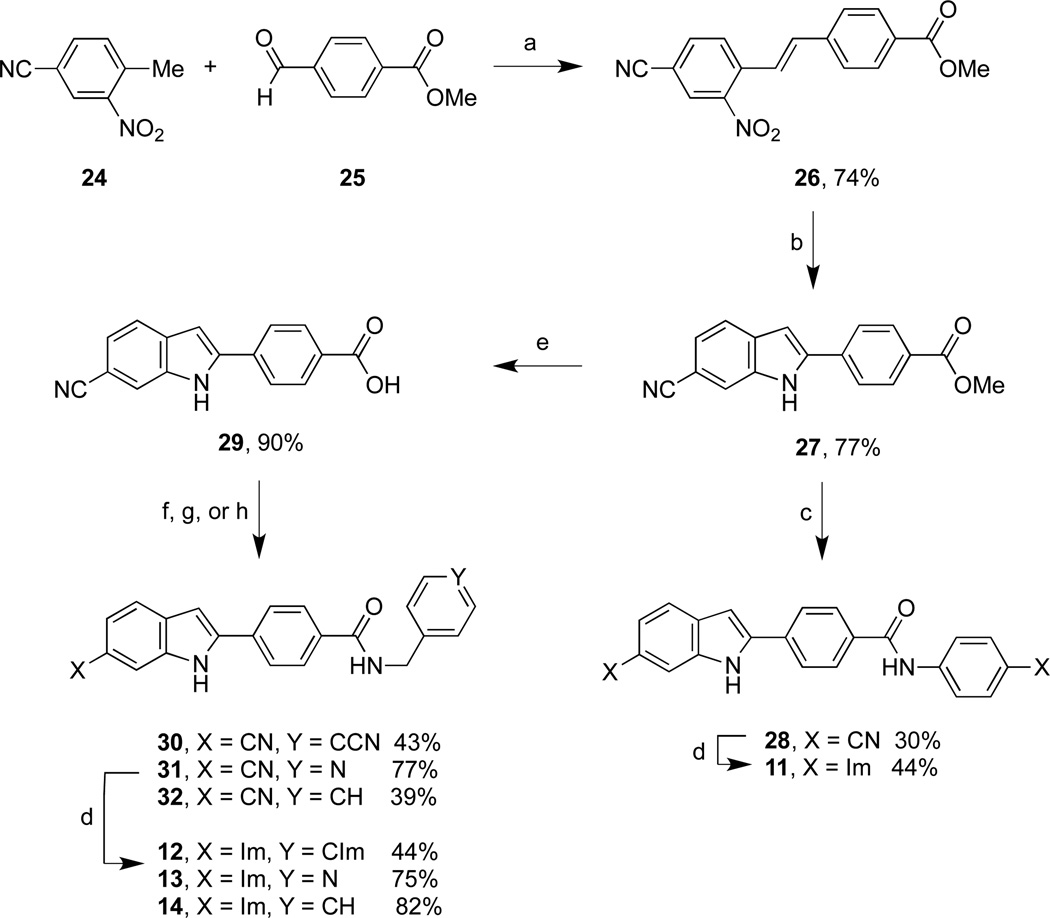
The synthesis of indole diimidazolines 11-13 and indole monoimidazoline 14 began with formation of nitrostilbene acid 26 from the commercially available precursors 24 and 25 (Scheme 3). The key step was reductive cyclization of 26 to indole nitrile ester 27 in hot triethylphosphite with a MoO2(acac)2 catalyst in a neat reaction. These reaction conditions advantageously combine features (refluxing triethylphosphite)8 and (triphenylphosphine and MoO2Cl2(dmf)2 in refluxing toluene)9 of similar reductive cyclization reactions. Nitrile ester 27 was directly converted10 into dinitrile amide 28. Hydrolysis of 27 afforded indole nitrile acid 29 that was readily converted into 30-32, the nitrile and dinitrile amide precursors of 12-14. Nitrile to imidazoline formation proceeded as already described. The remaining known diimidazolines 1, 2, and 5-811–14 (Table 1) were obtained by reaction sequences similar to those described in Schemes 1 and 2.15
Scheme 3.
Reagents and conditions: (a) 4-methylpiperidine, CH3CN, 90 °C, 48 h; (b) P(OEt)3, MoO2(acac)2, 130 °C, 2 h; (c) 4-aminobenzonitrile, Me3Al, PhMe, 75 °C, 17 h; (d) ethylene diamine, NaSH, DMA, 120 °C, 2 h; (e) NaOH, DMA:H2O (1:1), 70 °C, 4 h; then 1 M aq. HCl; (f-h) 4-(aminomethyl)benzonitrile, 4-(aminomethyl)pyridine, or benzylamine; HOBt, EDCI, TEA, DMA, rt, 24 h.
Table 1.
In vitro antitrypanosomal activity against the STIB900 strain of T. brucei rhodesiense and in vitro cytotoxicity against the L6 cell line.
 | |||||
|---|---|---|---|---|---|
| Compd | Subst. pattern |
X | Y |
T. b. rhodesiense IC50 (nM) |
L6 IC50 (nM) |
| 1a | 1,4- | NHCO | CONH | 4.7 | 110,000 |
| 2a | 1,3- | NHCO | CONH | 51 | 120,000 |
| 3a | 1,4- | CH2NHCO | CONHCH2 | 9,500 | 92,000 |
| 4a | 1,3- | CH2NHCO | CONHCH2 | 64,000 | >150,000 |
| 5a | 1,4- | CONH | CONH | 11 | 50,000 |
| 6a | 1,3- | CONH | CONH | 120 | 99,000 |
| 7a | 1,4- | CONH | NHCO | 120 | 92,000 |
| 8a | 1,4- | NHCO | 4-C6H4CONH | 19 | >150,000 |
| 9a | ----- | ----- | ----- | 690 | 130,000 |
| 10a | ----- | ----- | ----- | 86 | 68,000 |
| 11 | ----- | ----- | ----- | 2.2 | 19,000 |
| 12a | ----- | CIm | ----- | 1.5 | 41,000 |
| 13 | ----- | N | ----- | 23 | 89,000 |
| 14 | ----- | CH | ----- | 2,200 | 110,000 |
| melarsoprol | ----- | ----- | ----- | 3.8 | 19,000 |
| pentamidineb | ----- | ----- | ----- | 1.5 | 5,100 |
isolated and tested as the dimesylate salt
tested as the diisethionate salt
In vitro and in vivo assays with T. brucei rhodesiense STIB900 and in vitro cytotoxicity with the rat myoblast L6 cell line were performed as previously described.16,17 Target compound HAT activity data against T. brucei rhodesiense are shown in Tables 1 and 2. Diimidazoline prototype 1 was only slightly less potent against the STIB900 strain of T. brucei rhodesiense than were the control drugs malarsoprol and pentamidine. Diimidazoline 2, the meta analog of 1, was order of magnitude less potent than the latter, but was similarly cytotoxic. Compounds 3 and 4 demonstrate that insertion of a methylene between the aniline nitrogen atoms and distal phenyl rings of 1 and 2 decreases activity by two to three orders of magnitude. The IC50 values for 5-7, the three reversed amides of 1 and 2, show that at least one aniline nitrogen atom para to an 2-imidazoline substituent is required for high activity. Compound 8, the biphenyl analog of 1 was only slightly less potent than the prototype, but the resulting increase in molecular weight and aromatic ring count18 suggests that 8 offers no significant advantage over 1. Diimidazoline 10 illustrates that removing the central phenyl ring of 1 decreased activity by an order of magnitude. Interestingly, previous work19 demonstrated that the diamidine analog of 10 had no in vivo activity against HAT species. Comparing 7 to 9 indicates that replacing the central benzene ring with a cyclohexane decreased activity 6-fold and cytotoxicity 1.4-fold; thus there appears to be no benefit in increasing sp3 carbon count20 in this series of diimidazolines.
Table 2.
Antitrypanosomal activity of selected compounds in the T. b. rhodesiense acute mouse model at doses of 4×50 mg/kga.
| Compd | Curesb | Mean Survival Daysc |
|---|---|---|
| Control | ------ | 6–10 |
| 1 | 2/4 | 39 |
| 2 | 4/4 | >60 |
| 5 | 4/4 | >60 |
| 6 | 4/4 | >60 |
| 7 | 4/4 | >60 |
| 8 | 0/4 | 11 |
| 10 | 2/4 | 34 |
| 12 | 0/4 | toxicd |
Administered ip on days 3–6 post-infection.
Cure is defined as survival for more than 60 days after infection without a parasitemia relapse.
Mean survival days is determined for mice with and without parasitemia relapse.
Mice died following first compound dose.
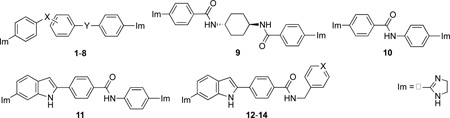
The remaining four compounds (11-14) are diimidazoline indoles, in which one of the anilide functional groups of 1 was replaced with a pyrrole substructure. Compounds 11-14 share some structural similarity with a previously reported21 set of biphenylbenzimidazole diamidines. Like 1 and pentamidine, diimidazoline indoles 11 and 12 had single digit nM IC50 values, but they were also the most cytotoxic target compounds. Target compound 12 reveals that insertion of a methylene between the aniline nitrogen atom and distal phenyl ring of 11 did not decrease activity; this contrasts to what was observed for 1 vs. 3 (vide supra). We also found that the nitrile and dinitrile precursors of target compounds 1-14 had T. brucei rhodesiense STIB900 IC50 values in the range of 10,000 to >150,000 nM demonstrating the importance of the 2-imidazoline substructure for HAT activity. However, comparing the relative activities of monoimidazolines 13 and 14 to their diimidazoline counterpart 12 reveals that only a single imidazoline is required for high activity provided that a second weak base functional group is present. With the exception of 11, 1-14 were significantly less cytotoxic than either melarsoprol or pentamidine, consistent with previous data demonstrating lower cytotoxicity for carboxamide analogs of pentamidine.5 Finally, there was no correlation between T. brucei rhodesiense STIB900 and L6 cytotoxicity IC50 values for 1-14, similar to what was previously observed for a series of adamantyl monoimidazolines.22
The ten target compounds with in vitro IC50 values < 150 nM against T. brucei rhodesiense STIB900 were administered as three consecutive 40 mg/kg ip doses to T. brucei rhodesiense-infected mice on days 1–3 post-infection. In this primary rodent model, all ten compounds were 100% curative. Next, eight of these23 were tested in a more stringent rodent model with a well-established infection. In this experiment, the compounds were administered as four consecutive 50 mg/kg ip doses to T. brucei rhodesiense-infected mice on days 3–6 post-infection (Table 2). Target compounds 2 and 5-7 were completely curative and 1 and 10 cured 2/4 infected animals. The partial curative efficacy of 1 contrasts with data from earlier experiments with this diimidazoline where it completely cured24 T. brucei brucei-infected mice at ip doses as low as 3 × 1 mg/kg.6 Only 8 and 12 were ineffective, and the latter was lethal; all four treated mice died after the first injection of this indole diimidazoline. In this respect, in vitro cytotoxicity seems to have been an inadequate predictor of in vivo toxicity, as 5 and 10 were only slightly less cytotoxic than 12, but they showed no in vivo toxicity. For the eight compounds tested in the 4 × 50 mg/kg experiment, there was no correlation between in vitro potency and in vivo efficacy. For comparison, in this same experimental format, administration of four 20 mg/kg doses of pentamidine and furamidine cured 2/4 and 3/4 of infected mice.16
In summary, we identified several diimidazoline mono- and diamides that were as potent as pentamidine against T. brucei rhodesiense in vitro, but none of these was as effective as pentamidine in a T. brucei rhodesiense-infected mouse model. Second, our data suggest that a single imidazoline may be sufficient for high antitrypanosomal activity provided that a second weak base functional group is present. Third, in vitro cytotoxicity assessment did not seem to be an inadequate predictor of in vivo toxicity for this series of compounds. Although mechanistic studies suggest that diamidines selectively accumulate in HAT species by way of the P2 nucleoside transporter and subsequently concentrate in the mitochondrion where they bind avidly to kinetoplast DNA,3 the promiscuity of dications such as diamidines is problematic.25,26 For example, diamidines are active against a wide range of pathogenic microbes and have been investigated as potential anticancer agents.2 Indeed, we note that 1, 2, and 5-8 had earlier been synthesized and tested for antitumor and antibacterial activities.12,13,27,28 Moreover, 1 is mutagenic at micromolar concentrations.29 Accordingly, future work will address the SAR of the imidazoline substructure6,17 with a goal to increase efficacy and selectivity against HAT species.
Supplementary Material
Acknowledgment
This investigation received financial support from the NIH (1U01AI082051-01) and the Swiss Tropical and Public Health Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Jacobs RT, Ding C. Ann. Rep. Med. Chem. 2010;45:277. [Google Scholar]
- 2.Soeiro MNC, De Souza EM, Stephens CE, Boykin DW. Expert Opin. Investig. Drugs. 2005;14:957. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]
- 3.Werbovetz K. Curr. Opin. Investig. Drugs. 2006;7:147. [PubMed] [Google Scholar]
- 4.Nuss JE, Dong Y, Wanner LM, Ruthel G, Wipf P, Gussio R, Vennerstrom JL, Bavari S, Burnett JC. ACS Med. Chem. Lett. 2010;1:301. doi: 10.1021/ml100056v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang TL, Vanden Eynde JJ, Mayence A, Collins MS, Cushion MT, Rattendi D, Londono I, Mazumder L, Bacchi CJ, Yarlett N. Bioorg. Med. Chem. Lett. 2009;19:5884. doi: 10.1016/j.bmcl.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan HC, Bacchi CJ, Nichol CA, Duch DS, Mullaney EA, Hutner SH. Am. J. Trop. Med. Hyg. 1984;33:845. doi: 10.4269/ajtmh.1984.33.845. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Wei HT, Li D, Zheng YG, Cai J, Ji M. Synth. Comm. 2008;38:3151. [Google Scholar]
- 8.Li B, Pai R, Cardinale SC, Butler MM, Peet NP, Moir DT, Bavari S, Bowlin TL. J. Med. Chem. 2010;53:2264. doi: 10.1021/jm901852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz R, Escribano J, Pedrosa MR, Aguado R, Arnáiz FJ. Adv. Synth. Catal. 2007;349:713. [Google Scholar]
- 10.Basha A, Lipton M, Weinreb SM. Tetrahedron Lett. 1977;18:4171. [Google Scholar]
- 11.Hirt R. Patentschrift (Switz.) CH 419145. 1967 [Google Scholar]
- 12.Hirt R, Fischer R. Patentschrift (Switz.) CH 459172. 1968 [Google Scholar]
- 13.Hirt R, Fischer R. Patentschrift (Switz.) CH 525896. 1972 [Google Scholar]
- 14.Wander A. GB 1007334. UK Patent. 1965
- 15.Melting points are uncorrected. 1H and 13C NMR spectra were recorded in DMSO-d6 on a 500 MHz spectrometer. All chemical shifts are reported in parts per million (ppm) and are relative to internal (CH3) 4Si (0 ppm) for 1H and 39.5 ppm for 13C NMR.N,N'-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]terephthalamide dimesylate (1). mp 179–181 °C. 1H NMR (60 °C) δ 2.34 (s, 6H), 4.01 (s, 8H), 7.97 (d, J = 8.3 Hz, 4H), 8.08 (d, J = 7.8 Hz, 4H), 8.15 (s, 4H), 10.30 (s, 4H), 10.76 (s, 2H); 13C NMR (60 °C) δ 44.33, 116.74, 120.09, 127.97, 129.43, 137.18, 144.53, 164.59, 165.34. Anal. Calcd for C28H32N6O8S2: C, 52.16; H, 5.00; N, 13.04. Found: C, 51.94; H, 5.02; N, 12.89.N,N'-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]isophthalamide dimesylate (2). mp 339–341 °C. 1H NMR δ 2.34 (s, 6H), 4.01 (s, 8H), 7.77 (t, J = 7.8 Hz, 1H), 7.99 (d, J = 8.3 Hz, 4H), 8.09 (d, J = 8.3 Hz, 4H), 8.23 (d, J = 7.8 Hz, 2H), 8.56 (s, 1H), 10.41 (s, 4H), 10.93 (s, 2H); 13C NMR δ 39.94, 44.50, 116.88, 120.16, 127.57, 129.12, 129.73, 131.53, 134.82, 144.79, 164.55, 165.82. Anal. Calcd for C28H32N6O8S2·0.5H2O: C, 51.44; H, 5.09; N, 12.86. Found: C, 51.43; H, 5.31; N, 12.57.N,N'-Bis[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]terephthalamide dimesylate (3). mp 306–308 °C. 1H NMR (60 °C) δ 2.36 (s, 6H), 4.01 (s, 8H), 4.61 (d, J = 4.9 Hz, 4H), 7.59 (d, J = 7.8 Hz, 4H), 7.91 (d, J = 7.8 Hz, 4H), 8.00 (s, 4H), 9.19 (brs, 2H), 10.40 (s, 4H); 13C NMR (60 °C) δ 42.57, 44.39, 120.57, 127.25, 127.87, 128.44, 136.52, 146.75, 165.00, 165.79. Anal. Calcd for C30H36N6O8S2: C, 53.56; H, 5.39; N, 12.49. Found: C, 53.49; H, 5.46; N, 12.35.N,N'-Bis[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]isophthalamide dimesylate (4). mp 219–221 °C. 1H NMR δ 2.35 (s, 6H), 4.01 (s, 8H), 4.60 (s, 4H), 7.51–7.69 (m, 5H), 7.92 (d, J = 7.4 Hz, 4H), 8.08 (d, J = 6.8 Hz, 2H), 8.45 (s, 1H), 9.34 (brs, 2H), 10.50 (s, 4H); 13C NMR (60 °C) δ 39.93, 42.74, 44.57, 120.76, 126.63, 128.07, 128.69, 128.81, 130.29, 134.49, 147.02, 164.99, 166.17. Anal. Calcd for C30H36N6O8S2: C, 53.56; H, 5.39; N, 12.49. Found: C, 53.12; H, 5.80; N, 12.19.N-[4-(4,5-Dihydro-1H-imidazol-2-yl)phenyl]-4-[[4-(4,5-dihydro-1H-imidazol-2-yl)benzoyl]amino]benzamide dimesylate (5). mp 277–279 °C. 1H NMR δ 2.34 (s, 6H), 4.01 (s, 4H), 4.06 (s, 4H), 7.96 (d, J = 8.8 Hz, 2H), 7.99 (d, J = 8.8 Hz, 2H), 8.05 (d, J = 8.8 Hz, 2H), 8.07 (d, J = 9.3 Hz, 2H), 8.10 (d, J = 8.3 Hz, 2H), 8.24 (d, J = 8.3 Hz, 2H), 10.37 (s, 2H), 10.66 (s, 1H), 10.69 (s, 2H), 10.81 (s, 1H); 13C NMR δ 39.95, 44.47, 44.76, 116.48, 119.84, 120.00, 125.04, 128.78, 128.80, 129.03, 129.45, 129.66, 139.74, 142.45, 145.11, 164.54, 164.57, 164.75, 165.66. Anal. Calcd for Anal. Calcd for C28H32N6O8S2: C, 52.16; H, 5.00; N, 13.04. Found: C, 52.43; H, 4.91; N, 12.92.N-[4-(4,5-Dihydro-1H-imidazol-2-yl)phenyl]-3-[[4-(4,5-dihydro-1H-imidazol-2-yl)benzoyl]amino]benzamide dimesylate (6). mp 145–147 °C. 1H NMR δ 2.34 (s, 6H), 4.00 (s, 4H), 4.05 (s, 4H), 7.58 (t, J = 7.8 Hz, 1H), 7.79 (d, J = 7.8 Hz, 1H), 8.02 (d, J = 8.8 Hz, 2H), 8.08 (d, J = 8.8 Hz, 3H), 8.17 (d, J = 8.3 Hz, 2H), 8.25 (d, J = 8.3 Hz, 2H), 8.41 (s, 1H), 10.53 (brs, 2H), 10.80 (s, 1H), 10.82 (s, 1H), 10.85 (brs, 2H); 13C NMR δ 39.96, 44.43, 44.71, 116.68, 120.04, 120.31, 123.48, 124.97, 128.69, 128.90, 129.08, 129.77, 135.10, 139.27, 139.70, 144.94, 164.44, 164.53, 166.29. Anal. Calcd for C28H32N6O8S2: C, 52.16; H, 5.00; N, 13.04. Found: C, 51.99; H, 4.85; N, 12.88.N,N’-1,4-Phenylenebis[4-(4,5-dihydro-1H-imidazol-2-yl)benzamide] dimesylate (7). mp 196– 198 °C. 1H NMR δ 2.34 (s, 6H), 4.06 (s, 8H), 7.80 (s, 4H), 8.09 (d, J = 8.3 Hz, 4H), 8.21 (d, J = 8.3 Hz, 4H), 10.54 (s, 2H), 10.68 (s, 4H); 13C NMR δ 39.96, 44.75, 120.98, 128.64, 128.74, 135.09, 140.14, 164.13, 164.59. Anal. Calcd for C28H32N6O8S2: C, 52.16; H, 5.00; N, 13.04. Found: C, 51.93; H, 5.25; N, 12.79.N,N'-Bis[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]biphenyl-4,4’-dicarboxamide dimesylate (8). mp 309–310 °C; 1H NMR δ 2.33 (s, 6H), 4.02 (s, 8H), 7.99 (t, J = 9 Hz, 4H), 8.10 (d, J = 9 Hz, 2H), 8.14 (dd, J = 9, 3 Hz, 2H), 10.39 (s, 4H), 10.81 (s, 2H); 13CNMR δ 39.91, 44.50, 119.17, 120.12, 127.19, 128.44, 128.88, 129.69, 133.88, 144.96, 164.55, 165.91. Anal. Calcd for C34H36N6O8S2: C, 56.65; H, 5.03; N, 11.66. Found: C, 56.65; H, 4.90; N, 11.75.trans-N,N'-1,4-Cyclohexanediylbis[4-(4,5-dihydro-1H-imidazol-2-yl)benzamide] dimesylate (9). mp > 320 °C; 1H NMR δ 1.44–1.56 (m, 4H), 1.88–2.00 (m, 4H), 2.32 (s, 6H), 3.76–3.86 (m, 2H), 4.04 (s, 8H), 8.02 (d, J = 9Hz, 4H), 8.08 (d, J = 9 Hz, 4H), 8.56 (d, J = 8 Hz, 2H), 10.62 (s, 4H); 13C NMR δ 31.20, 39.91, 44.84, 48.40, 124.50, 128.34, 128.78, 139.98, 164.49, 164.68. Anal. Calcd for C28H38N6O8S2·2H2O: C, 48.97; H, 6.16; N, 12.24. Found: C, 49.12; H, 6.04; N, 11.80.N-[4-(4,5-Dihydro-1H-imidazol-2-yl)phenyl]-4-(4,5-dihydro-1H-imidazol-2-yl)benzamide dimesylate (10). mp 256–258 °C. 1H NMR δ 2.34 (s, 6H), 4.01 (s, 4H), 4.05 (s, 4H), 7.99 (d, J = 8.7 Hz, 2H), 8.07 (d, J = 8.8 Hz, 2H), 8.10 (d, J = 8.3 Hz, 2H), 8.21 (d, J = 7.8 Hz, 2H), 10.52 (brs, 4H), 10.97 (s, 1H); 13C NMR δ 39.95, 44.53, 44.97, 117.13, 120.23, 125.57, 128.77, 128.84, 129.72, 139.28, 144.51, 164.43, 164.51, 165.13. Anal. Calcd for C21H27N5O7S2: C, 47.99; H, 5.18; N, 13.32. Found: C, 48.10; H, 4.92; N, 13.57.6-(4,5-Dihydro-1H-imidazol-2-yl)-2-[4-[[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]amino]carbonyl]phenyl]indole (11). mp 185–187 °C. 1H NMR δ 3.71 (s, 4H), 3.84 (s, 4H), 7.20 (s, 1H), 7.59 (d, J = 8.3 Hz, 1H), 7.70 (d, J = 8.3 Hz, 1H), 7.89 (d, J = 8.5 Hz, 2H), 7.95 (d, J = 8.5 Hz, 2H), 8.07 (s, 1H), 8.13 (s, 4H), 10.60 (s, 1H), 12.36 (s, 1H); 13C NMR δ 46.65, 48.26, 100.95, 112.11, 119.36, 119.83, 120.54, 123.40, 125.50, 128.35, 128.74, 131.82, 133.82, 134.69, 136.81, 140.34, 142.16, 163.68, 165.38, 165.48. Anal. Calcd for C27H24N6O: C, 72.30; H, 5.39; N, 18.74. Found: C, 71.90; H, 4.98; N, 18.70.6-(4,5-Dihydro-1H-imidazol-2-yl)-2-[4-[[[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]amino]carbonyl]phenyl]indole dimesylate (12). mp 177–179 °C. 1H NMR 2.34 (s, 6H), 4.01 (s, 4H), 4.03 (s, 4H), 4.62 (d, J = 5.7 Hz, 2H), 7.23 (d, J = 1.5 Hz, 1H), 7.57 (dd, J = 8.3, 1.5 Hz, 1H), 7.61 (d, J = 8.3 Hz, 2H), 7.80 (d, J = 8.3 Hz, 1H), 7.91 (d, J = 8.3 Hz, 2H), 8.05 (d, J = 8.8 Hz, 2H), 8.06 (s, 1H), 8.10 (d, J = 8.8 Hz, 2H), 9.28 (t, J = 5.7 Hz, 1H), 10.37 (s, 2H), 10.47 (s, 2H), 12.38 (s, 1H); 13C NMR δ 39.96, 42.69, 44.42, 44.55, 101.04, 112.81, 114.87, 120.74, 121.07, 125.81, 128.04, 128.26, 128.69, 133.14, 133.72, 133.94, 136.52, 141.80, 147.13, 165.00, 165.97, 166.06. Anal. Calcd for C30H34N6O7S2·H2O: C, 53.56; H, 5.39; N, 12.49. Found: C, 53.64; H, 4.93; N, 12.70.6-(4,5-Dihydro-1H-imidazol-2-yl)-2-[4-[[[(4-pyridyl)methyl]amino]carbonyl]phenyl]indole (13). mp 257–259 °C. 1H NMR δ 3.63 (s, 4H), 4.54 (d, J = 5.8 Hz, 2H), 7.09 (s, 1H), 7.34 (d, J = 4.4 Hz, 2H), 7.55 (d, J = 9.3 Hz, 1H), 7.57 (d, J = 9.3 Hz, 1H), 7.90 (s, 2H), 8.02 (s, 4H), 8.52 (d, J = 4.4 Hz, 2H), 9.19 (t, J = 5.9 Hz, 1H), 11.87 (s, 1H); 13C NMR δ 41.96, 49.90 (br), 100.51, 110.70, 119.30, 119.88, 122.33, 124.66, 125.11, 128.16, 130.18, 132.86, 134.79, 137.02, 138.67, 148.78, 149.73, 164.82, 166.15. Anal. Calcd for C24H21N5O: C, 72.89; H, 5.35; N, 17.71. Found: C, 72.40; H, 5.40; N, 17.57.6-(4,5-Dihydro-1H-imidazol-2-yl)-2-[4-[[(benzyl)amino]carbonyl]phenyl]indole (14). mp 224– 226 °C. 1H NMR δ4.52 (d, J = 6.3 Hz, 2H), 7.08 (s, 1H), 7.21–7.29 (m, 1H), 7.30–7.40 (m, 4H), 7.54 (d, J = 8.8 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.90 (s, 1H), 8.01 (brs, 4H), 9.10 (t, J = 6.1 Hz, 1H), 11.88 (s, 1H); 13C NMR δ 42.82, 49.64 (br), 100.43, 110.76, 119.29, 119.88, 124.38, 125.09, 126.93, 127.42, 128.12, 128.47, 130.25, 133.22, 134.59, 136.99, 138.80, 139.87, 164.86, 165.83. Anal. Calcd for C25H22N4O·H2O: C, 72.80; H, 5.86; N, 13.58. Found: C, 73.09; H, 5.51; N, 13.20.
- 16.Wenzler T, Boykin DW, Ismail MA, Hall JE, Tidwell RR, Brun R. Antimicrob. Agents Chemother. 2009;53:4185. doi: 10.1128/AAC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakunova SM, Bakunov SA, Patrick DA, Kumar EVKS, Ohemeng KA, Bridges AS, Wenzler T, Barszcz T, Kilgore Jones S, Werbovetz KA, Brun R, Tidwell RR. J. Med. Chem. 2009;52:2016. doi: 10.1021/jm801547t. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie TJ, MacDonald SJF. Drug Discov. Today. 2009;14:1011. doi: 10.1016/j.drudis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Ashley JN, Barber HJ, Ewins AJ, Newbery G, Self ADH. J. Chem. Soc. 1942:103. [Google Scholar]
- 20.Lovering F, Bikker J, Humblet C. J. Med. Chem. 2009;52:6752. doi: 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- 21.Ismail MA, Batista-Parra A, Miao Y, Wilson WD, Wenzler T, Brun R, Boykin DW. Bioorg. Med. Chem. 2005;13:6718. doi: 10.1016/j.bmc.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Koperniku A, Papanastasiou I, Foscolos GB, Tsotinis A, Taylor MC, Kelly JM. Med. Chem. Commun. 2013;4:856. [Google Scholar]
- 23.Insufficient quantities of 11 and 13 were available for the 4 × 50 mg/kg ip in vivo experiment.
- 24.The higher in vivo efficacy of TP51 against T. brucei brucei reported by Nathan et al. (Ref 6) may be due to infection with a different trypanosome species and that cures were defined as infected animals that survived for more than 30 days beyond controls, whereas in our experiment, cures were defined as survival for more than 60 days post-infection without a parasitemia relapse.
- 25.Patrick DA, Ismail MA, Arafa RK, Wenzler T, Zhu X, Pandharkar T, Jones SK, Werbovetz KA, Brun R, Boykin DW, Tidwell RR. J. Med. Chem. 2013;56:5473. doi: 10.1021/jm400508e. [DOI] [PubMed] [Google Scholar]
- 26.Peters JU, Schnider P, Mattei P, Kansy M. ChemMedChem. 2009;4:680. doi: 10.1002/cmdc.200800411. [DOI] [PubMed] [Google Scholar]
- 27.Pine MJ. Biochem. Pharmacol. 1968;17:75. doi: 10.1016/0006-2952(68)90160-3. [DOI] [PubMed] [Google Scholar]
- 28.Hirt R. Int. Cong. Chemother. Proc. 1964;2:1055. [Google Scholar]
- 29.Ferguson LR, Sundberg RJ. Antimicrob. Agents Chemother. 1991;35:2318. doi: 10.1128/aac.35.11.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.