Abstract
Genes within the major histocompatibility complex (MHC) are characterized by extensive polymorphism within species and also by a remarkable conservation of contemporary human allelic sequences in evolutionarily distant primates. Mechanisms proposed to account for strict nucleotide conservation in the context of highly variable genes include the suggestion that intergenic exchange generates repeated sets of MHC DRB polymorphisms [Gyllensten, U. B., Sundvall, M. & Erlich, H. A. (1991) Proc. Natl. Acad. Sci. USA 88, 3686-3690; Lundberg, A. S. & McDevitt, H. 0. (1992) Proc. Natl. Acad. Sci. USA 89, 6545-6549]. We analyzed over 50 primate MHC DRB sequences, and identified nucleotide elements within macaque and baboon DRB6-like sequences with deletions corresponding to specific exon 2 hypervariable regions, which encode a discrete alpha helical segment of the MHC antigen combining site. This precisely localized deletion provides direct evidence implicating segmental exchange of MHC-encoded DRB gene fragments as one of the evolutionary mechanisms both generating and maintaining MHC diversity. Intergenic exchange at this site may be fundamental to the diversification of immune protection in populations by permitting alteration in the specificity of the MHC that determines the repertoire of antigens bound.
Full text
PDF
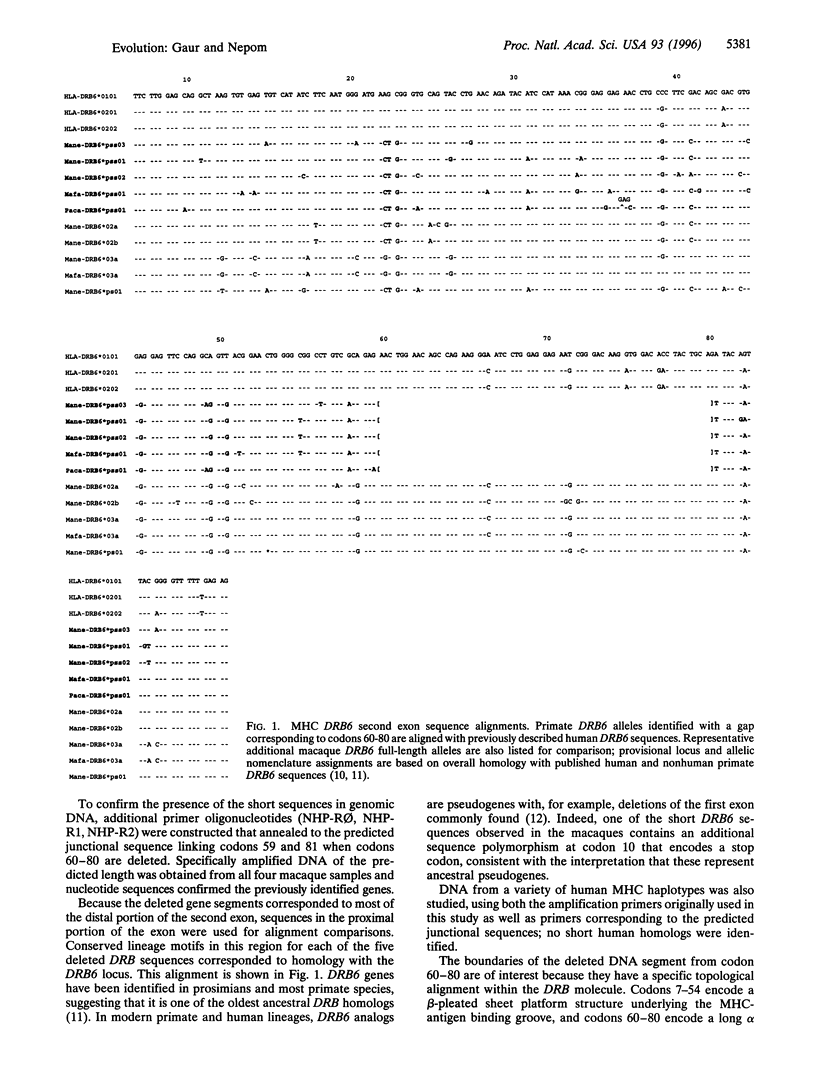


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chicz R. M., Urban R. G., Lane W. S., Gorga J. C., Stern L. J., Vignali D. A., Strominger J. L. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992 Aug 27;358(6389):764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- Corell A., Martin-Villa J. M., Morales P., de Juan M. D., Varela P., Vicario J. L., Martinez-Laso J., Arnaiz Villena A. Exon-2 nucleotide sequences, polymorphism and haplotype distribution of a new HLA-DRB gene: HLA-DRB sigma. Mol Immunol. 1991 Apr-May;28(4-5):533–543. doi: 10.1016/0161-5890(91)90168-j. [DOI] [PubMed] [Google Scholar]
- Fan W. M., Kasahara M., Gutknecht J., Klein D., Mayer W. E., Jonker M., Klein J. Shared class II MHC polymorphisms between humans and chimpanzees. Hum Immunol. 1989 Oct;26(2):107–121. doi: 10.1016/0198-8859(89)90096-7. [DOI] [PubMed] [Google Scholar]
- Figueroa F., O'hUigin C., Inoki H., Klein J. Primate DRB6 pseudogenes: clue to the evolutionary origin of the HLA-DR2 haplotype. Immunogenetics. 1991;34(5):324–337. doi: 10.1007/BF00211996. [DOI] [PubMed] [Google Scholar]
- Fogdell A., Olerup O. A novel DRB1 allele (DRB1*1415) formed by interallelic crossing over between the DRB1*1404 and the DRB1*0802 or 0804 alleles. Tissue Antigens. 1994 May;43(5):327–329. doi: 10.1111/j.1399-0039.1994.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Sundvall M., Erlich H. A. Allelic diversity is generated by intraexon sequence exchange at the DRB1 locus of primates. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3686–3690. doi: 10.1073/pnas.88.9.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllensten U., Sundvall M., Ezcurra I., Erlich H. A. Genetic diversity at class II DRB loci of the primate MHC. J Immunol. 1991 Jun 15;146(12):4368–4376. [PubMed] [Google Scholar]
- Hayashida H., Miyata T. Unusual evolutionary conservation and frequent DNA segment exchange in class I genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 May;80(9):2671–2675. doi: 10.1073/pnas.80.9.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Ancient interlocus exon exchange in the history of the HLA-A locus. Genetics. 1989 Jul;122(3):681–686. doi: 10.1093/genetics/122.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. L., Nei M. Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):958–962. doi: 10.1073/pnas.86.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., O'hUigin C. Class II B Mhc motifs in an evolutionary perspective. Immunol Rev. 1995 Feb;143:89–111. doi: 10.1111/j.1600-065x.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Klein J. Origin of major histocompatibility complex polymorphism: the trans-species hypothesis. Hum Immunol. 1987 Jul;19(3):155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Lundberg A. S., McDevitt H. O. Evolution of major histocompatibility complex class II allelic diversity: direct descent in mice and humans. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6545–6549. doi: 10.1073/pnas.89.14.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. G., Bodmer J. G. HLA class II nucleotide sequences, 1992. Tissue Antigens. 1992 Nov;40(5):229–243. doi: 10.1111/j.1399-0039.1992.tb02050.x. [DOI] [PubMed] [Google Scholar]
- McAdam S. N., Boyson J. E., Liu X., Garber T. L., Hughes A. L., Bontrop R. E., Watkins D. I. A uniquely high level of recombination at the HLA-B locus. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5893–5897. doi: 10.1073/pnas.91.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Ramachandran K., Flavell R. A. A potential donor gene for the bm1 gene conversion event in the C57BL mouse. Nature. 1983 Dec 22;306(5945):792–795. doi: 10.1038/306792a0. [DOI] [PubMed] [Google Scholar]
- Ohta N., Nishimura Y. K., Tanimoto K., Horiuchi Y., Abe C., Shiokawa Y., Abe T., Katagiri M., Yoshiki T., Sasazuki T. Association between HLA and Japanese patients with rheumatoid arthritis. Hum Immunol. 1982 Oct;5(2):123–132. doi: 10.1016/0198-8859(82)90057-x. [DOI] [PubMed] [Google Scholar]
- Sigurdardóttir S., Borsch C., Gustafsson K., Andersson L. Exon encoding the antigen-binding site of MHC class II beta-chains is divided into two subregions with different evolutionary histories. J Immunol. 1992 Feb 1;148(3):968–973. [PubMed] [Google Scholar]
- Tiercy J. M., Gebuhrer L., Betuel H., Mach B., Jeannet M. A new HLA-DR4 allele with a DR11 alpha-helix sequence. Tissue Antigens. 1993 Feb;41(2):97–101. doi: 10.1111/j.1399-0039.1993.tb01986.x. [DOI] [PubMed] [Google Scholar]



