Abstract
Objective
The calculation of spirometric Z-scores by Lambda-Mu-Sigma (LMS) rigorously accounts for age-related changes in lung function. Recently, the Global Lung Function Initiative (GLI) expanded the availability of LMS spirometric Z-scores to multiple ethnicities. Hence, in aging populations, the GLI provides an opportunity to rigorously evaluate ethnic differences in respiratory impairment, including spirometric airflow-limitation and restrictive-pattern
Methods
Using data from the Third National Health and Nutrition Examination Survey, including participants aged 40-80, we evaluated ethnic differences in GLI-defined respiratory impairment, including prevalence and associations with mortality and respiratory symptoms.
Results
Among 3,506 White-Americans, 1,860 African-Americans, and 1,749 Mexican-Americans, the prevalence of airflow-limitation was 15.1% (13.9, 16.4), 12.4% (10.7, 14.0), and 8.2% (6.7, 9.8), and of restrictive-pattern was 5.6% (4.6, 6.5), 8.0% (6.9, 9.0), and 5.7% (4.5, 6.9), respectively. Airflow-limitation was associated with mortality in White-Americans, African-Americans, and Mexican-Americans — adjusted hazard ratio (aHR) 1.66 (1.23, 2.25), 1.60 (1.09, 2.36), and 1.80 (1.17, 2.76), respectively, but associated with respiratory symptoms only in White-Americans — adjusted odds ratio (aOR) 2.15 (1.70, 2.73). Restrictive-pattern was associated with mortality but only in White-Americans and African-Americans — aHR 2.56 (1.84, 3.55) and 3.23 (2.06, 5.05), and associated with respiratory symptoms but only in White-Americans and Mexican-Americans — aOR 2.16 (1.51, 3.07) and 2.12 (1.45, 3.08), respectively.
Conclusion
In an aging population, we found ethnic differences in GLI-defined respiratory impairment, including prevalence and associations with health outcomes. In particular, African-Americans present a unique public health challenge, with high rates of respiratory impairment being associated with mortality but not respiratory symptoms.
Keywords: spirometry, mortality, respiratory symptoms, ethnicity
INTRODUCTION
Prior work suggests that ethnic differences exist in respiratory disease.1-3 For example, as reported by the Centers for Disease Control and Prevention (U.S.A.), prevalence rates for chronic bronchitis and emphysema are higher in White-Americans than in African-Americans or Hispanics.1,2 Similarly, age-adjusted death rates for chronic obstructive pulmonary disease (COPD), defined as chronic bronchitis or emphysema, are higher in White-Americans than in African-Americans or Hispanics.1,3 These epidemiologic data have limitations, however, for at least two reasons.1-7 First, confirmation of airway obstruction as a criterion for diagnosing COPD is underutilized in clinical practice — i.e., chronic bronchitis and emphysema may occur in the absence of airway obstruction, and vice-versa.4,5,7 Second, death certificates in patients with respiratory disease may misclassify the cause of death.6
Spirometry provides an objective evaluation of respiratory disease, including potential ethnic differences.8,9 In particular, respiratory disease is often established by a reduction in spirometric function, heretofore termed a respiratory impairment, and is subsequently categorized as airflow-limitation (airway obstruction) or restrictive-pattern.3,9,10 Importantly, because respiratory disease occurs more frequently in aging populations (≥40 years),1,2 the spirometric criteria that establish respiratory impairment must account for age-related reductions in lung function, as well as the age-related variability in spirometric performance.9-18
The Lambda-Mu-Sigma (LMS) method rigorously accounts for age-related changes in lung function by using Z-scores that incorporate the mean (Mu) — representing how spirometric measures change based on predictor variables (age and height); the coefficient-of-variation (Sigma) — representing the spread of reference values; and skewness (Lambda) — representing departure from normality.11,12 A Z-score of -1.64 defines the lower limit of normal as the 5th percentile of the distribution.11,12 Notably, using data from large reference populations of asymptomatic lifelong nonsmokers, the Global Lung Function Initiative (GLI) has recently published equations that expand the availability of LMS-calculated spirometric Z-scores, allowing respiratory impairment to be established across multiple ethnicities (see Methods).12
Whether ethnic differences exist in GLI-defined respiratory impairment has not yet been evaluated. Therefore, using GLI-based spirometric criteria and data from the Third National Health and Nutrition Examination Survey (NHANES III)19 — including participants aged 40-80 who were specifically identified as White-Americans, African-Americans, and Mexican-Americans — we calculated prevalence rates for airflow-limitation and restrictive-pattern, and their corresponding associations with 5-year all-cause mortality and respiratory symptoms. As a secondary aim, we also evaluated sex and smoking history as potential effect modifiers of the associations of interest. The results of the present study may inform public health policy and clinical practice regarding ethnic differences in respiratory impairment.
METHODS
Study population
NHANES III is a national probability sample of Americans aged 8-80, assembled in 1988-1994, with White-Americans, African-Americans, and Mexican-Americans representing the three largest ethnic groups. A separate Hispanic category was also identified but comprised only 2.4% of the NHANES III cohort.19 Given our specific aims, our analytical sample therefore included participants aged 40-80 who were White-Americans, African-Americans, or Mexican-Americans and who, at baseline, had no self-reported asthma and had completed at least two American Thoracic Society (ATS) acceptable spirometric maneuvers (the maximal exhalation maneuver continued for at least 6 seconds, with a minimum 2 second terminal plateau).19 We selected age ≥40 because respiratory impairment and its related mortality are unusual in younger persons.1,3 We excluded 569 participants who had self-reported asthma to focus on COPD as the cause of airflow-limitation (see on-line supplement, Table 1A, regarding frequency distributions of self-reported asthma, stratified by ethnicity and spirometric categories). Our final analytical sample included 7,115 participants.
The institutional review boards from the Veterans Affairs Connecticut Healthcare System and Yale University approved the study, granting exemption from participant consent because it involved de-identified data that were publicly available.
Clinical measures
NHANES III recorded all-cause mortality, ascertained from a public-use linked mortality file that contained information based on the National Death Index. 20 For the present study, mortality surveillance occurred over 5-years.
NHANES III also recorded respiratory symptoms at the baseline visit, including chronic bronchitis, wheezing, and dyspnea. Specifically, participants were classified as having respiratory symptoms if they answered “yes” to any of the following four questions:19 “Do you usually cough on most days for 3 consecutive months or more during the year?”; “Do you bring up phlegm on most days for 3 consecutive months or more during the year?”; “Have you had wheezing or whistling in your chest at any time in the past 12 months?”; or “Are you troubled by shortness of breath when hurrying on level ground or walking up a slight hill?”.
Other clinical data included age, sex, height, body mass index (BMI), ethnicity, health status, chronic conditions, and smoking history.21 Reduced health status was defined as a self-reported rating of “fair-to-poor.” Chronic conditions included self-reported, physician-diagnosed hypertension, COPD, diabetes, stroke, myocardial infarction, and heart failure. For a smoking history to be established, ≥10 pack-years of cigarette consumption was required. Participants were also classified as having high cardiovascular (CV) risk based on BMI ≥30 or having a history of hypertension, diabetes, stroke, myocardial infarction, or heart failure.
Spirometry
At the baseline visit, spirometry was performed using ATS protocols.19,21 The measures of interest included the forced vital capacity (FVC) and forced expiratory volume in 1-second (FEV1). Using the largest set of FEV1 and FVC values that were recorded in any of the ATS-acceptable spirometric maneuvers, the FEV1/FVC was also calculated.8,9,19,21 These spirometric measures were then expressed as Z-scores, using the GLI equations.12
As per recommendations from the ATS and European Respiratory Society, the diagnostic threshold for spirometric measures was set at the 5th percentile of distribution, defining the lower limit of normal (LLN).9 In addition, because a substantial proportion of participants had risk factors for respiratory impairment, including smoking history, respiratory symptoms, and high cardiovascular risk, the LLN at the 5th percentile was also deemed more clinically appropriate than the 2.5th percentile, which is otherwise recommended if screening only.9,11,12 In the present study, the LLN was thus defined by a Z-score of -1.64, corresponding to the 5th percentile of the distribution of Z-scores.10-18 The respiratory status of each participant was then categorized as normal spirometry (FEV1/FVC and FVC, both ≥LLN) or respiratory impairment, including airflow-limitation (FEV1/FVC <LLN) or restrictive-pattern (FEV1/FVC ≥LLN but FVC <LLN).
Statistical analysis
Baseline characteristics and the frequency distributions of respiratory impairment, respiratory symptoms, and death were first summarized as means and standard errors, or as counts and percentages. Ethnic differences were compared using the Rao-Scott chi-square test for categorical variables and least squares regression for continuous variables. P-values were adjusted for multiple comparisons using the false discovery rate procedure.22
The association between respiratory impairment and death was then evaluated using Cox regression models. The following covariates identified a priori as clinically plausible confounders were entered into the adjusted model (regardless of their level of statistical significance): age, sex, smoking history, high CV risk, and reduced health status. Airflow-limitation and restrictive-pattern were treated as nominal categories, with the reference group including participants who had normal spirometry. Each model’s goodness-of-fit was assessed by model-fitting procedures and by the analysis of residuals. The proportional hazards assumption was tested by using interaction terms for the time-to-event outcome and each variable in the multivariable model; the terms were retained if p<0.05 after adjusting for the multiplicity of comparisons. Higher-order effects were tested for the continuous covariates and included in the final model if they met a forward selection criterion of p<0.20.
Similarly, the association between respiratory impairment and respiratory symptoms at baseline was evaluated by calculating odds ratios, using logistic regression models. Covariates in the adjusted model were as described previously.
Lastly, potential effect modifiers of associations with health outcomes were assessed. In these analyses, interactions for each ethnic group were evaluated and involved “crossing” sex and smoking history, with airflow-limitation and restrictive-pattern. Hazard ratios for death and odds ratios for respiratory symptoms were estimated according to sex and smoking history, using separate regression models for each ethnic group and combinations of effect modifiers. In tests of potential effect modification, p values for interaction terms were not adjusted for the multiplicity of comparisons, because the clinical interest was toward avoiding Type II errors, rather than Type I errors. Covariates included age, sex, smoking history, high CV risk, and reduced health status, without the variable that was the effect modifier of interest.
SAS version 9.3 software (SAS Institute Inc. 2011; Cary, NC) was used in the analyses, with a p<0.05 (two-sided) denoting statistical significance. The analyses accounted for the complex study design to obtain accurate standard errors, but did not use sampling weights because post hoc deletions were made to the national NHANES III probability sample.
RESULTS
Table 1 summarizes baseline characteristics according to ethnicity. Significant differences were as follows: White-Americans were older and had the highest rates of having a smoking history and (self-reported, physician-diagnosed) COPD or myocardial infarction, but the lowest rates of obesity (BMI ≥30) and reduced health status. African-Americans had the highest rate of CV risk, including hypertension. Mexican-Americans had the highest rates of diabetes and reduced health status, but the lowest rates of having a smoking history, hypertension, or stroke.
Table 1.
Baseline characteristics according to ethnicity
| Characteristic | White-Americans N =3,506 | African-Americans N=1,860 | Mexican-Americans N =1,749 | Adjusted p-value | ||
|---|---|---|---|---|---|---|
| W vs. A | W vs. M | A vs. M | ||||
| Age (years), mean (SE) | 60.7 (0.4) | 56.0 (0.5) | 56.0 (0.4) | < 0.001 | < 0.001 | 0.923 |
| Females, n (%) | 1830 (52.2) | 968 (52.0) | 847 (48.4) | 0.923 | 0.101 | 0.066 |
| BMI (kg/m2), mean (SE) | 27.2 (0.1) | 28.5 (0.2) | 28.6 (0.2) | <0.001 | < 0.001 | 0.905 |
| BMI ≥ 30, n (%) | 878 (25.0) | 636 (34.2) | 589 (33.9) | < 0.001 | < 0.001 | 0.905 |
| Smoking history, n (%) | ||||||
| < 10 pack-years | 1950 (56.1) | 1183 (64.9) | 1265 (73.2) | <0.001 | <0.001 | <0.001 |
| ≥ 10 pack-years | 1524 (43.9) | 641 (35.1) | 462 (26.8) | |||
| Chronic conditions, n (%) * | ||||||
| Hypertension | 1279 (36.5) | 859 (46.3) | 530 (30.5) | < 0.001 | 0.003 | < 0.001 |
| COPD † | 319 (9.1) | 93 (5.0) | 66 (3.8) | < 0.001 | < 0.001 | 0.139 |
| Diabetes mellitus | 299 (8.5) | 246 (13.2) | 312 (17.8) | < 0.001 | < 0.001 | < 0.001 |
| Myocardial infarction | 265 (7.6) | 96 (5.2) | 79 (4.7) | 0.002 | 0.001 | 0.506 |
| Stroke | 134 (3.8) | 80 (4.3) | 44 (2.5) | 0.430 | 0.014 | 0.004 |
| Heart failure | 127 (3.6) | 89 (4.8) | 105 (6.0) | 0.065 | < 0.001 | 0.165 |
| High CV risk, n (%) ‡ | 1885 (53.8) | 1233 (66.3) | 1021 (58.4) | < 0.001 | 0.020 | < 0.001 |
| Reduced health status, n (%) | 672 (19.2) | 609 (32.8) | 731 (41.8) | < 0.001 | < 0.001 | < 0.001 |
Abbreviations: A, African-Americans; BMI, body mass index (weight in kilograms divided by height in meters-squared); CV, cardiovascular; M, Mexican-Americans; NHANES III, Third National Health and Nutrition Examination Survey; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1-second; FVC, forced vital capacity; SE, standard error; W, White-Americans.
Self-reported, physician-diagnosed.
Included chronic bronchitis or emphysema.
Included the presence of any of the following: BMI ≥ 30, hypertension, diabetes, heart failure, stroke, or myocardial infarction.
Table 2 summarizes respiratory impairment, respiratory symptoms, and mortality according to ethnicity. Overall, the prevalence of airflow-limitation varied in a progression of White-Americans > African-Americans > Mexican-Americans, whereas restrictive-pattern varied in a progression of African-Americans > Mexican-Americans and White-Americans. Whereas airflow limitation exceeded the 5% prevalence level that is expected for a normal population (using our LLN threshold) across the three ethnicities, restrictive-pattern exceeded the 5% prevalence level only in African-Americans. The frequency of respiratory symptoms varied in a progression of White-Americans > African-Americans and Mexican-Americans. The frequency of deaths over 5-years varied in a progression of African-Americans and White-Americans > Mexican-Americans.
Table 2.
Spirometry and health outcomes according to ethnicity
| Characteristic | White-Americans N =3,506 | African-Americans N=1,860 | Mexican-Americans N =1,749 | Adjusted p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | Percent (95%CI) | n | Percent (95%CI) | n | Percent (95%CI) | W vs. A | W vs. M | A vs. M | |
| Spirometry * | |||||||||
| Normal | 2,778 | 79.3 (77.8, 80.7) | 1,482 | 79.7 (77.7, 81.7) | 1,504 | 86.0 (84.3, 87.8) | 0.780 | < 0.001 | < 0.001 |
| Airflow-limitation | 531 | 15.1 (13.9, 16.4) | 230 | 12.4 (10.7, 14.0) | 144 | 8.2 (6.7, 9.8) | 0.014 | < 0.001 | 0.002 |
| Restrictive-pattern | 196 | 5.6 (4.6, 6.5) | 148 | 8.0 (6.9, 9.0) | 100 | 5.7 (4.5, 6.9) | 0.002 | 0.863 | 0.016 |
| Health outcomes | |||||||||
| Respiratory symptoms † | 1,452 | 41.5 (39.2, 43.8) | 668 | 36.0 (33.5, 38.5) | 604 | 34.6 (31.9, 37.3) | < 0.001 | < 0.001 | 0.541 |
| Deaths, n (%) ‡ | 308 | 8.8 (7.6, 10.0) | 173 | 9.3 (8.0, 10.6) | 121 | 6.9 (5.4, 8.4) | 0.632 | 0.063 | 0.039 |
Abbreviations: A, African-Americans; CI, confidence interval; NHANES III, Third National Health and Nutrition Examination Survey; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1-second; FVC, forced vital capacity; LLN, lower limit of normal; M, Mexican-Americans; W, White-Americans.
Recorded at baseline: normal spirometry was defined by FEV1/FVC and FVC, both ≥LLN; airflow-limitation by FEV1/FVC <LLN; and restrictive-pattern by FEV1/FVC ≥LLN and FVC <LLN. Missing data: White-Americans (n=1); Mexican-Americans (n=1).
Recorded at baseline: chronic cough or sputum production, dyspnea-on-exertion, or wheezing. Missing data: White-Americans (n=6); Mexican-Americans (n=3); African-American (n=4).
Over 5-years of follow-up. Vital status was missing for 3 White-Americans and 2 Mexican-Americans.
Table 3 shows adjusted hazard ratios (aHR) for death, according to spirometric category and ethnicity. Relative to normal spirometry, airflow-limitation was associated with mortality in White-Americans, African-Americans, and Mexican-Americans — aHR 1.66 (1.23, 2.25), 1.60 (1.09, 2.36), and 1.80 (1.17, 2.76), respectively. Restrictive-pattern was associated with mortality in White-Americans and African-Americans — aHR 2.56 (1.84, 3.55) and 3.23 (2.06, 5.05), respectively. Mexican-Americans also had an increased aHR but this was not statistically significant — aHR 2.09 (0.89, 4.90). In these analyses of mortality, there were no significant interactions between respiratory impairment and ethnicity (i.e., hazard ratios were similar).
Table 3.
Hazard ratios for five-year mortality, according to spirometric category and stratified by ethnicity *
| Spirometric Category † | White-Americans N=3,467 | African-Americans N=1,821 | Mexican-Americans N=1,717 | |||
|---|---|---|---|---|---|---|
| Hazard Ratios for Five-Year Mortality (95% Confidence Interval) ठ| ||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Normal | 1.00 | |||||
| Airflow-limitation | 2.31 (1.75, 3.06) | 1.66 (1.23, 2.25) | 2.58 (1.91, 3.50) | 1.60 (1.09, 2.36) | 2.18 (1.39, 3.40) | 1.80 (1.17, 2.76) |
| Restrictive-pattern | 3.10 (2.30, 4.17) | 2.56 (1.84, 3.55) | 2.91 (1.88, 4.50) | 3.23 (2.06, 5.05) | 2.88 (1.34, 6.21) | 2.09 (0.89, 4.90) |
Missing data: White-Americans — 37 missing covariates, 2 missing mortality; African-American — 39 missing covariates; and Mexican-American — 30 missing covariates, 2 missing mortality.
See footnote to Table 2 for description of spirometric category.
Values were calculated using three separate Cox regression models for each of the ethnic groups. In the adjusted models, covariates included age, sex, smoking history, high cardiovascular risk, and health status. In White-Americans there was an age by time interaction added to the model, while in African-Americans age2, and age3 were also included.
Relative to White-Americans, there were no significant interactions in African-American x airflow-limitation (p=0.673), African-American x restrictive-pattern (p=0.973), Mexican-American x airflow-limitation (p=0.253), and Mexican-American x restrictive-pattern (p=0.189). These results suggest that the adjusted hazard ratios did not differ significantly by ethnicity.
Table 4 shows adjusted odds ratios (aOR) for respiratory symptoms, according to spirometric category and ethnicity. Relative to normal spirometry, airflow-limitation was associated with respiratory symptoms in White-Americans, but had only borderline statistical significance in African-Americans and was not associated in Mexican-Americans — aOR 2.15 (1.70, 2.73), 1.38 (0.99, 1.92), and 1.26 (0.90, 1.76), respectively. Restrictive-pattern was associated with respiratory symptoms in White-Americans and Mexican-Americans but not African-Americans — aOR 2.16 (1.51, 3.07), 2.12 (1.45, 3.08), and 1.08 (0.70, 1.67), respectively. In these analyses of respiratory symptoms, there were significant interactions between respiratory impairment and ethnicity, with African-Americans in particular having weak to no associations (i.e., odds ratios were significantly lower, relative to White-Americans).
Table 4.
Odds ratios for respiratory symptoms, according to spirometric category and stratified by ethnicity *
| Spirometric Category † | White-Americans N=3,465 | African-Americans N=1,821 | Mexican-Americans N=1,716 | |||
|---|---|---|---|---|---|---|
| Odds Ratios for Respiratory Symptoms (95% Confidence Interval) ठ| ||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Normal | 1.00 | |||||
| Airflow-limitation | 2.70 (2.20, 3.32) | 2.15 (1.70, 2.73) | 1.41 (1.02, 1.94) | 1.38 § (0.99, 1.92) | 1.25 (0.90, 1.72) | 1.26 § (0.90, 1.76) |
| Restrictive-pattern | 2.67 (1.90, 3.76) | 2.16 (1.51, 3.07) | 1.41 (0.96, 2.08) | 1.08 § (0.70, 1.67) | 2.60 (1.92, 3.52) | 2.12 (1.45, 3.08) |
Missing data: White-Americans — 37 missing covariates and 4 missing respiratory symptoms; African-Americans — 39 missing covariates, 4 missing respiratory symptoms; Mexican-Americans — 30 missing covariates, 3 missing respiratory symptoms.
See footnote to Table 2 for description of spirometric category.
Odds ratios were calculated using three separate logistic regression models for each ethnic group. In the adjusted models, covariates included age, sex, smoking history, high cardiovascular risk, and health status.
Relative to White-Americans, there were significant interactions in African-American x airflow-limitation (p=0.003), African-American x restrictive-pattern (p=0.012), and Mexican-American x airflow-limitation (p=0.001), but no significant interaction in Mexican-American x restrictive-pattern (p=0.669).
Effect modification by sex and smoking history of the association between respiratory impairment and mortality were not significant (data not shown). In contrast, and as shown in Figures 1 and 2, the aOR for respiratory symptoms was significantly modified in several situations: 1) sex, among African-Americans who had airflow-limitation — aOR 0.86 (0.55, 1.34) and 1.93 (1.18, 3.17), for female and male, respectively (p=0.010), and among African-Americans who had restrictive-pattern — aOR 0.83 (0.53, 1.31) and 1.66 (0.90, 3.07), for female and male, respectively (p=0.024); and 2) smoking history, among White-Americans who had airflow-limitation — aOR 1.48 (0.96, 2.30) and 2.57 (1.94, 3.41), for < and ≥10 pack-years, respectively (p=0.025).
Figure 1.
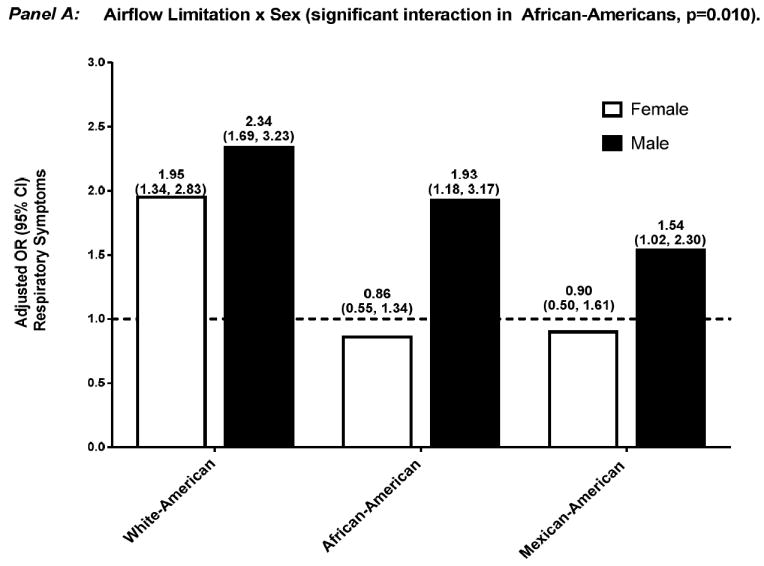
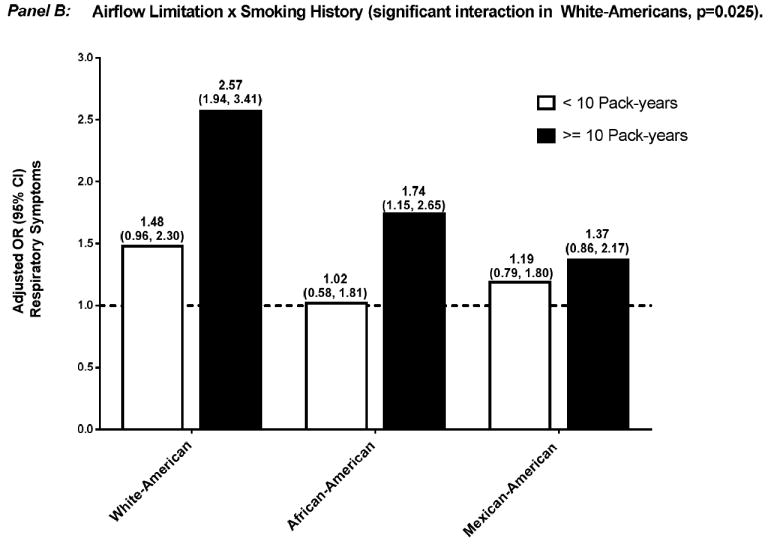
Adjusted OR (95% confidence interval) for respiratory symptoms among participants who had airflow-limitation, stratified by effect modifier — sex (Panel A) and smoking history (Panel B). Separate logistic regression models were used for each ethnic group and effect modifier combination, with normal spirometry as the reference group. Covariates included age, sex, smoking history, high CV risk, and reduced health status, without the variable that was the effect modifier of interest.
Abbreviations: CI, confidence interval; CV, cardiovascular; OR, odds ratio.
Figure 2.
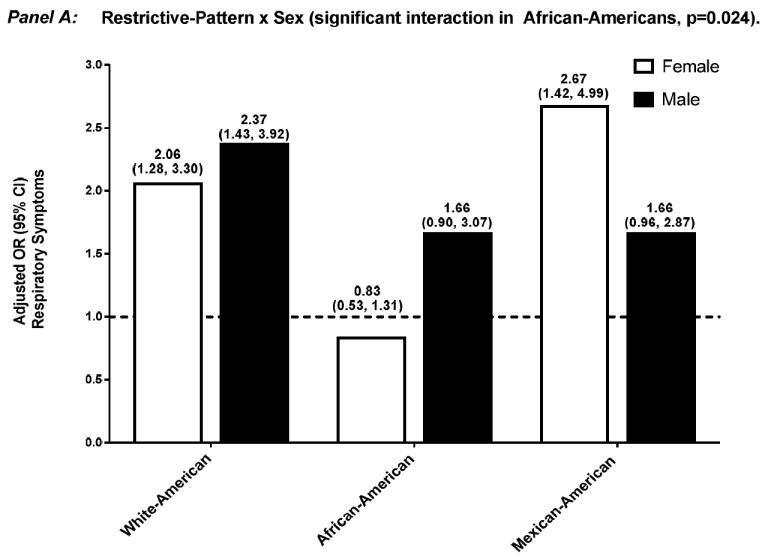
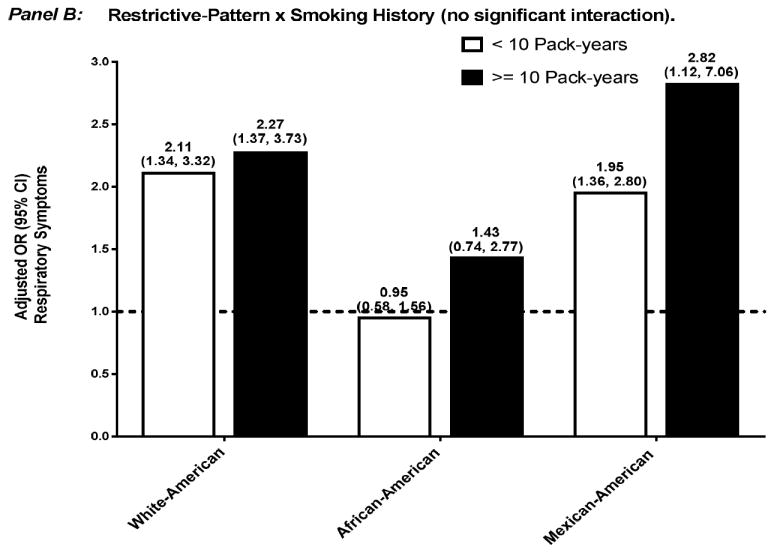
Adjusted OR (95% confidence interval) for respiratory symptoms among participants who had restrictive-pattern, stratified by effect modifier — sex (Panel A) and smoking history (Panel B). Separate logistic regression models were used for each ethnic group and effect modifier combination, with normal spirometry as the reference group. Covariates included age, sex, smoking history, high CV risk, and reduced health status, without the variable that was the effect modifier of interest.
Abbreviations: CI, confidence interval; CV, cardiovascular; OR, odds ratio.
DISCUSSION
In a large sample of persons aged 40-80, we found that GLI-defined respiratory impairment differed in: 1) prevalence: airflow-limitation was more common in White-Americans and African-Americans than Mexican-Americans, while restrictive-pattern was more common in African-Americans than White-Americans or Mexican-Americans; 2) all-cause mortality: airflow-limitation was associated with mortality in all three ethnic groups, while restrictive-pattern was associated with mortality only in White-Americans and African-Americans — furthermore, the magnitude of these associations did not differ by ethnicity; 3) respiratory symptoms: airflow-limitation was associated with respiratory symptoms but only in White-Americans, while restrictive-pattern was associated with respiratory symptoms but only in White-Americans and Mexican-Americans — furthermore, the magnitude of these associations differed by ethnicity, with African-Americans in particular having weak to no associations; and 4) effect modification: female sex decreased the association of airflow-limitation and restrictive-pattern with respiratory symptoms but only in African-Americans, while smoking history increased the association of airflow-limitation with respiratory symptoms but only in White-Americans — otherwise, there was no effect modification for the mortality outcome.
These results indicate that ethnic differences exist in GLI-defined respiratory impairment, including prevalence rates, associations with health outcomes, and effect modification. In particular, African-Americans present a unique public health challenge, with high rates of respiratory impairment being associated with mortality but not respiratory symptoms.
Our approach for evaluating ethnic differences in respiratory impairment has a strong mathematical and clinical rationale. As discussed earlier, GLI-defined respiratory impairment is based on LMS-calculated spirometric Z-scores that rigorously account for age-related changes in lung function, including variability in spirometric performance and skewness of reference data.11,12 As additional evidence supporting this approach in clinical practice, Z-scores are routinely used to diagnose osteoporosis (bone mineral density) and the LMS method is widely applied to construct growth charts.11,23 In the current context, ethnic differences were also evaluated based on associations between respiratory impairment and health outcomes. All-cause mortality is a definitive outcome that is resistant to miscoding and has been the primary endpoint in clinical trials.24 Respiratory symptoms, including dyspnea, chronic bronchitis, and wheezing, are often the bases for pursuing healthcare.24-26
Because we excluded participants who had self-reported asthma, the spirometric diagnosis of airflow-limitation in the present study was likely due to airway obstruction from COPD. Hence, our results suggest that White-Americans and African-Americans have significantly higher rates of spirometry-confirmed COPD than Mexican-Americans (Table 2). These higher rates may be due to a greater smoking exposure in White-Americans and African-Americans, along with a possible ethnic-specific protection in Mexican-Americans.27
Because a reduced FVC is associated with the metabolic syndrome, coronary heart disease, and sudden cardiac death,28-30 and because a reduced FVC is a required criterion for establishing restrictive-pattern,9 we postulate that a CV mechanism may have been an important contributor to restrictive-pattern in our study population, including its association with health outcomes.14,15,17,18 This is especially relevant to African-Americans. The latter group had the highest rates of CV risk and restrictive-pattern, whereas White-Americans and Mexican-Americans had lower rates of CV risk and restrictive-pattern (Tables 1 and 2, respectively).
The present study also demonstrated a differential impact of ethnicity on associations with death and respiratory symptoms (Tables 3 and 4, respectively). For example, in adjusted analyses, we found that White-Americans who had airflow-limitation or restrictive-pattern had a significant increase in the risk of death and odds of having respiratory symptoms. In contrast, African-Americans who had restrictive-pattern had a significant increase in the risk of death but not in the odds of having respiratory symptoms. Similarly, Mexican-Americans who had airflow-limitation had a significant increase in the risk of death but not in the odds of having respiratory symptoms. Lastly, ethnic differences on associations with respiratory symptoms, but not death, were also seen regarding effect modification by female sex, being significant only in African-Americans, and by smoking history, being significant only in White-Americans (Figures 1 and 2).
The differential impact of ethnicity on associations with respiratory symptoms may affect prevalence rates for respiratory disease. In particular, we found that the rate of airflow-limitation (spirometry-confirmed COPD) relative to self-reported, physician-diagnosed COPD was higher in White-Americans (15.1% vs. 9.1%, respectively), yet more than doubled in African-Americans (12.4% vs. 5.0%, respectively) and Mexican-Americans (8.2% vs. 3.8%, respectively) (Tables 1 and 2). We postulate that the lower rates of self-reported, physician-diagnosed COPD occurred because spirometry is not routinely applied in clinical practice, or participants may not have sought medical care, and because respiratory symptoms are neither sufficient nor necessary to establish clinically-meaningful respiratory disease.5,7,16 Prior work has shown, for example, that 31% of participants who had moderate-to-severe spirometry-confirmed COPD (based on FEV1 Z-scores) had no respiratory symptoms.16 In addition, the designation of self-reported, physician-diagnosed COPD may have limited diagnostic accuracy. In the present study, more than half of the participants who had self-reported, physician-diagnosed COPD had normal spirometry (see on-line supplement, Table 2A).
In light of the above discussion, future work should evaluate additional health outcomes, including other verbal descriptors of dyspnea, measures of health related quality of life, and exercise capacity (6-minute walk test), across multiple ethnicities.31,32 Moreover, because preventive healthcare is available (smoking cessation, vaccinations, CV risk modification, and reduction of indoor and outdoor air pollutants), health outcomes should also be evaluated in persons aged ≥40 who at baseline have a spirometric respiratory impairment but no respiratory symptoms (as defined in the present study).31,32 The latter assessment has precedence, given that the Framingham Risk Score is currently recommended in all asymptomatic persons aged ≥40, including those without a clinical history of coronary heart disease.33
Although the present study used rigorous spirometric criteria, at least three potential limitations are noted. First, NHANES III does not provide sufficient data to confirm the pathophysiology of respiratory impairment. For example, in addition to COPD, airflow-limitation could be due to asthma, given that spirometry in NHANES III was not specifically obtained after a bronchodilator, that self-reported asthma (a key exclusion criterion) may have been underreported by participants, and that longstanding asthma may lead to irreversible airflow-limitation.34 Similarly, restrictive-pattern as a basis for establishing pulmonary restriction requires confirmation by a reduced total lung capacity (TLC), and may have included several non-CV etiologies.9,10 Second, self-reported ethnicity may not be entirely accurate, potentially leading to misclassification in the ethnic-specific reference equations that were used to calculate spirometric Z-scores.12 Moreover, because pulmonary function like many clinical phenomenon occurs along a continuum, spirometric Z-scores that are just above or below the LLN may misclassify normal spirometry and respiratory impairment, respectively. A potential related limitation is that establishing the LLN at the 5th percentile, rather than the 2.5th percentile (see methods),9,11,12 may increase false-positive designations for respiratory impairment. Nonetheless, the 2.5th percentile threshold may substantially increase false-negative designations, particularly given that NHANES III participants aged 40-80 frequently had a smoking history, respiratory symptoms, and high cardiovascular risk, Lastly, the present study may have been limited by differences in cultural, geographic, and socioeconomic factors between and within the three ethnic categories.35 In particular, differences in sedentary behavior could alter the association between respiratory impairment and symptoms (exertional dyspnea). To address these limitations, future studies should include comprehensive tests of cardiopulmonary function, including alternative diagnostic thresholds for respiratory impairment, and an assessment of genetic, cultural, geographic, and socioeconomic factors.
Conclusion
In a large sample of persons aged 40-80, we found that significant ethnic differences existed in GLI-defined respiratory impairment, including prevalence rates, associations with health outcomes, and the presence of effect modifiers. In particular, African-Americans present a unique public health challenge, with high rates of airflow-limitation and restrictive-pattern being associated with increased mortality but not respiratory symptoms. These results may inform future research and public health policy regarding ethnic differences in respiratory impairment.
Supplementary Material
Acknowledgments
The study was conducted at the VA Clinical Epidemiology Research Center and the Yale Claude D. Pepper Older Americans Independence Center (P30AG02134). Dr. Vaz Fragoso is a recipient of a Career Development Award from the Department of Veterans Affairs and an R03 award from the National Institute on Aging (R03AG037051). Dr. Gill is the recipient of K24AG021507 and K07AG043587 from the National Institute on Aging. Dr. Concato is supported by the Department of Veterans Affairs Cooperative Studies Program.
Footnotes
Dr. Vaz Fragoso had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to study concept and design, to data acquisition, analysis and interpretation, and to drafting the submitted article.
We report no conflicts of interest.
References
- 1.Chronic Obstructive Pulmonary Disease. [03/05/2013];American Lung Association State of Lung Disease in Diverse Communities. 2010 :35–40. Available at: http://www.lung.org/assets/documents/publications/solddc-chapters/copd.pdf.
- 2.Centers for Disease Control and Prevention. National Center for Health Statistics: National Health Interview Survey Raw Data, 2008. Analysis performed by American Lung Association Research and Program Services using SPSS and SUDAAN software [Google Scholar]
- 3.Centers for Disease Control and Prevention. National Center for Health Statistics. 2009 CDC Wonder On-line Database, compiled from Compressed Mortality File 1999-2006 Series 20 No 2L. [Google Scholar]
- 4.Miniati M, Monti S, Stolk J, et al. Value of chest radiography in phenotyping chronic obstructive pulmonary disease. Eur Respir J. 2008;31:509–14. doi: 10.1183/09031936.00095607. [DOI] [PubMed] [Google Scholar]
- 5.Peto R, Speizer FE, Cochrane AL, et al. The relevance in adults of airflow obstruction, but not of mucous hypersecretion, to mortality from chronic lung disease. Am Rev Respir Dis. 1983;128:491–500. doi: 10.1164/arrd.1983.128.3.491. [DOI] [PubMed] [Google Scholar]
- 6.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–6. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 8.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 10.Vaz Fragoso CA, Gill T. Respiratory Impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol Med Sci. 2012;67:264–75. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–60. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95 year age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz Fragoso CA, Concato J, McAvay G, et al. The ratio of the forced expiratory volume in 1-second to forced vital capacity in establishing chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:446–51. doi: 10.1164/rccm.200909-1366OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaz Fragoso CA, Gill TM, McAvay G, et al. Evaluating respiratory impairment in middle-aged persons using lambda-mu-sigma derived Z-scores. Respir Care. 2011;56(11):1771–77. doi: 10.4187/respcare.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaz Fragoso CA, Gill TM, McAvay G, et al. Respiratory impairment and mortality in older persons: a novel spirometric approach. J Investig Med. 2011;59(7):1089–95. doi: 10.231/JIM.0b013e31822bb213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaz Fragoso CA, Concato J, McAvay G, et al. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc. 2011;59:1847–54. doi: 10.1111/j.1532-5415.2011.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaz Fragoso CA, Enright PL, McAvay G, Van Ness P, Gill T. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaz Fragoso CA, Gill TM, McAvay G, et al. Respiratory impairment in older persons: when less means more. Am J Med. 2013;126:49–57. doi: 10.1016/j.amjmed.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services. National Center for Health Statistics. Third National Health and Nutrition Examination Survey,1988-94, NHANES III Laboratory Data File (CD-ROM), Public Use Data File Documentation Number 76200. Hyattsville, Maryland: Centers for Disease Control and Prevention, 1996; Available from National Technical Information Service Springfield, VA. [Google Scholar]
- 20.Wheatcroft G, Cox CS, Lochner KA. National Center for Health Statistics; Hyattsville, Maryland: 2007. Comparative analysis of the NHANES III public-use and restricted-use linked mortality files. http://www.cdc.gov/nchs/data/datalinkage/h3_mort_compare_2007_final.pdf. [Google Scholar]
- 21.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:1202–18. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;51:289–300. [Google Scholar]
- 23.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–97. doi: 10.1001/jama.288.15.1889. [DOI] [PubMed] [Google Scholar]
- 24.Gross NJ. Chronic obstructive pulmonary disease outcome measurements. Proc Am Thorac Soc. 2005;2(4):267–71. doi: 10.1513/pats.200504-036SR. [DOI] [PubMed] [Google Scholar]
- 25.Cherry DK, Burt CW, Woodwell DA. National ambulatory medical care survey: 1999 summary. Advanced Data from Vital and Health Statistics. 2001 Jul 17;322:1–36. [Google Scholar]
- 26.Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2007;147(9):633–8. [PubMed] [Google Scholar]
- 27.Bruse S, Sood A, Petersen H, et al. New Mexican Hispanic smokers have lower odds of chronic obstructive pulmonary disease and less decline in lung function than non-Hispanic whites. Am J Respir Crit Care Med. 2011;184:1254–60. doi: 10.1164/rccm.201103-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman GD, Klatsky AL, Siegelaub AB. Lung function and risk of myocardial infarction and sudden cardiac death. NEJM. 1976;294:1071–5. doi: 10.1056/NEJM197605132942001. [DOI] [PubMed] [Google Scholar]
- 29.Lee HM, Le H, Lee BT, et al. Forced vital capacity paired with Framingham risk score for prediction of all-cause mortality. Eur Respir J. 2010;36:1002–6. doi: 10.1183/09031936.00042410. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima K, Kubouchi Y, Muneyuki T, et al. A possible association between suspected restrictive pattern as assessed by ordinary pulmonary function test and the metabolic syndrome. Chest. 2008;134:712–8. doi: 10.1378/chest.07-3003. [DOI] [PubMed] [Google Scholar]
- 31.Parshall MB, Schwartzstein RM, Adams L, et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–52. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han MK, Curran-Everett D, Dransfield MT, et al. Racial differences in quality of life in patients with COPD. Chest. 2011;140(5):1169–76. doi: 10.1378/chest.10-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128:1239–44. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 35.Braun L, Wolfgang M, Dickersin K. Defining race/ethnicity and explaining difference in research studies on lung function. Eur Respir J. 2012 doi: 10.1183/09031936.0009161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


