Abstract
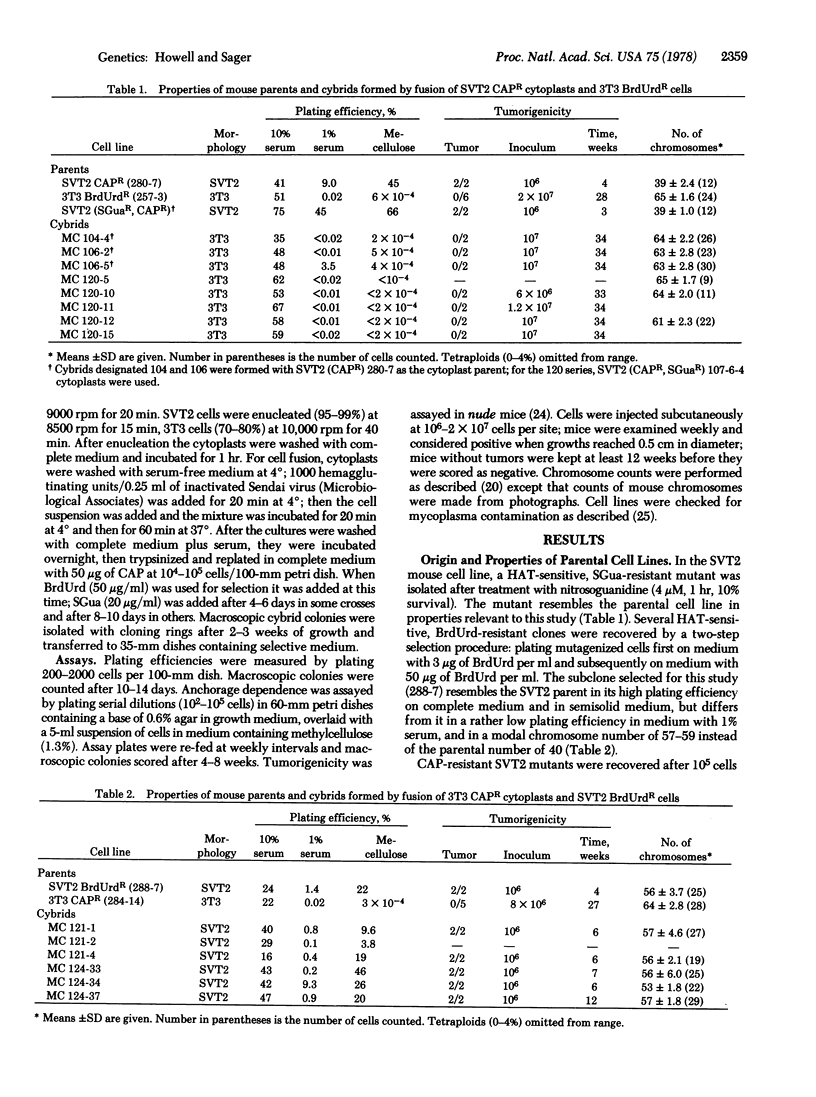
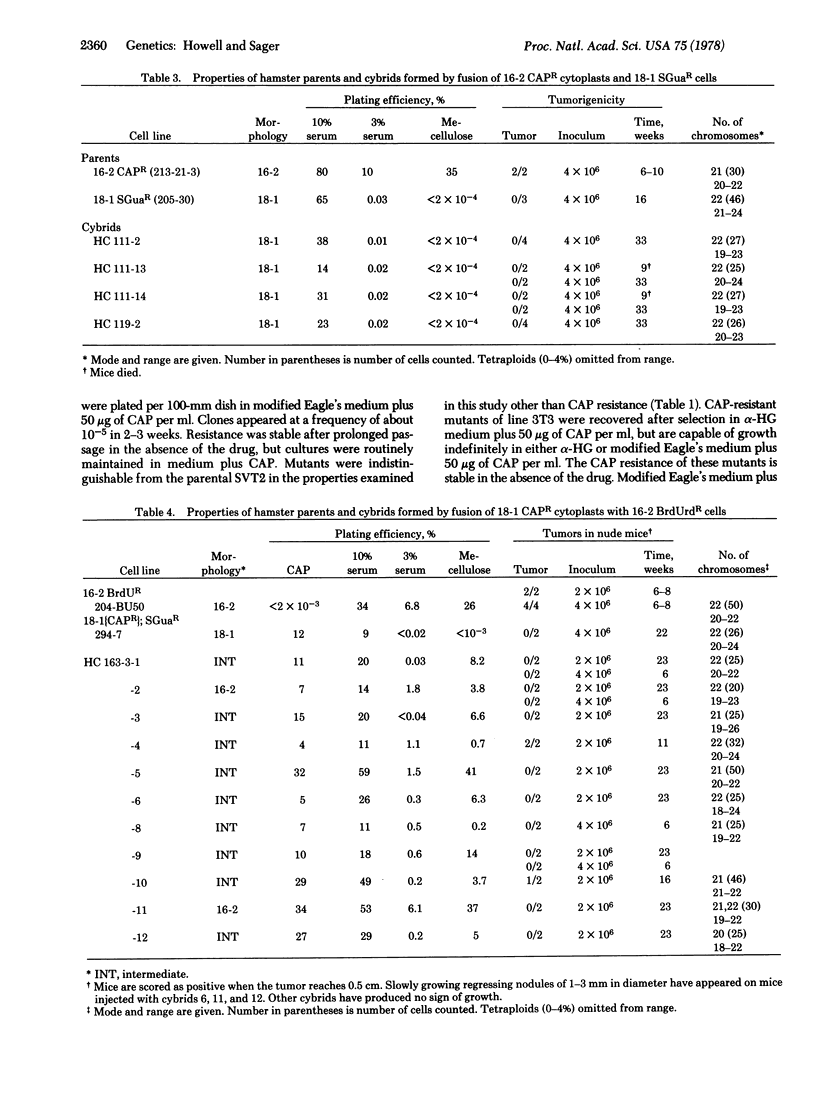
The effect of cytoplasm upon the expression of tumorigenicity was examined with a pair of mouse and a pair of Chinese hamster cell lines, in intraspecies cybrids formed by reciprocal fusions between either tumorigenic or nontumorigenic cells and cytoplasms derived from them. With the mouse cells, 3T3 and the simian virus 40-transformed line SVT2, the cybrid clones were tumorigenic when SVT2 cells were fused with 3T3 cytoplasts, but not in the reciprocal fusion. With the hamster cells, CHEF/18 and the spontaneous transformant CHEF/16, however, tumorigenicity was partially suppressed in cybrid clones formed by fusion of tumorigenic CHEF/16 cells with CHEF/18 cytoplasts; cybrids were nontumorigenic in the reciprocal fusion. Thus, cybrid analysis has shown that tumorigenicity is not cytoplasmically transmitted in these two cell pairs, but suppression of tumor-forming ability may be cytoplasmically transmitted in the hamster cybrids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEISSON J., SONNEBORN T. M. CYTOPLASMIC INHERITANCE OF THE ORGANIZATION OF THE CELL CORTEX IN PARAMECIUM AURELIA. Proc Natl Acad Sci U S A. 1965 Feb;53:275–282. doi: 10.1073/pnas.53.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUN E. H., VAUGHAN M. H., Jr, RICH A. THE ISOLATION AND CHARACTERIZATION OF DNA ASSOCIATED WITH CHLOROPLAST PREPARATIONS. J Mol Biol. 1963 Aug;7:130–141. doi: 10.1016/s0022-2836(63)80042-x. [DOI] [PubMed] [Google Scholar]
- Davies D. R., Lyall V. The assembly of a highly ordered component of the cell wall: the role of heritable factors and of physical structure. Mol Gen Genet. 1973 Jul 31;124(1):21–34. doi: 10.1007/BF00267161. [DOI] [PubMed] [Google Scholar]
- Heidemann S. R., Sander G., Kirschner M. W. Evidence for a functional role of RNA in centrioles. Cell. 1977 Mar;10(3):337–350. doi: 10.1016/0092-8674(77)90021-6. [DOI] [PubMed] [Google Scholar]
- Jonasson J., Harris H. The analysis of malignancy by cell fusion. VIII. Evidence for the intervention of an extra-chromosomal element. J Cell Sci. 1977 Apr;24:255–263. doi: 10.1242/jcs.24.1.255. [DOI] [PubMed] [Google Scholar]
- Jonasson J., Povey S., Harris H. The analysis of malignancy by cell fusion. VII. Cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci. 1977 Apr;24:217–254. doi: 10.1242/jcs.24.1.217. [DOI] [PubMed] [Google Scholar]
- Kelly F. Chromosome analysis of a simian virus 40-transformed mouse cell line and two variant sublines that are resistant to cytochalasin B1. Cancer Res. 1975 May;35(5):1210–1213. [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LUCK D. J., REICH E. DNA IN MITOCHONDRIA OF NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1964 Oct;52:931–938. doi: 10.1073/pnas.52.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS M. M., NASS S. INTRAMITOCHONDRIAL FIBERS WITH DNA CHARACTERISTICS. I. FIXATION AND ELECTRON STAINING REACTIONS. J Cell Biol. 1963 Dec;19:593–611. doi: 10.1083/jcb.19.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Jha K. K. Malignancy and transformation: expression in somatic cell hybrids and variants. Adv Cancer Res. 1977;25:53–93. doi: 10.1016/s0065-230x(08)60632-6. [DOI] [PubMed] [Google Scholar]
- Ruddle F. H., Creagan R. P. Parasexual approaches to the genetics of man. Annu Rev Genet. 1975;9:407–486. doi: 10.1146/annurev.ge.09.120175.002203. [DOI] [PubMed] [Google Scholar]
- SAGER R., ISHIDA M. R. CHLOROPLAST DNA IN CHLAMYDOMONAS. Proc Natl Acad Sci U S A. 1963 Oct;50:725–730. doi: 10.1073/pnas.50.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. A genetic map of non-Mandelian genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1970 Mar;65(3):593–600. doi: 10.1073/pnas.65.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R., Ramanis Z. Recombination of nonchromosomal genes in Chlamydomonas. Proc Natl Acad Sci U S A. 1965 May;53(5):1053–1061. doi: 10.1073/pnas.53.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J. A simple biochemical technique for the detection of mycoplasma contamination of cultured cells. Methods Cell Biol. 1975;10:277–290. doi: 10.1016/s0091-679x(08)60742-6. [DOI] [PubMed] [Google Scholar]
- Veomett G., Prescott D. M., Shay J., Porter K. R. Reconstruction of mammalian cells from nuclear and cytoplasmic components separated by treatment with cytochalasin B. Proc Natl Acad Sci U S A. 1974 May;71(5):1999–2002. doi: 10.1073/pnas.71.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veomett G., Shay J., Hough P. V., Prescott D. M. Large-scale enucleation of mammalian cells. Methods Cell Biol. 1976;13:1–6. doi: 10.1016/s0091-679x(08)61794-x. [DOI] [PubMed] [Google Scholar]


