Abstract
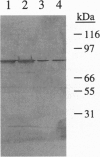
A novel vegetative insecticidal gene, vip3A(a), whose gene product shows activity against lepidopteran insect larvae including black cutworm (Agrotis ipsilon), fall armyworm (Spodoptera frugiperda), beet armyworm (Spodoptera exigua), tobacco budworm (Heliothis virescens), and corn earworm (Helicoverpa zea) has been isolated from Bacillus thuringiensis strain AB88. VIP3-insecticidal gene homologues have been detected in approximately 15% of Bacillus strains analyzed. The sequence of the vip3A(b) gene, a homologue of vip3A(a) isolated from B. thuringiensis strain AB424 is also reported. Vip3A(a) and (b) proteins confer upon Escherichia coli insecticidal activity against the lepidopteran insect larvae mentioned above. The sequence of the gene predicts a 791-amino acid (88.5 kDa) protein that contains no homology with known proteins. Vip3A insecticidal proteins are secreted without N-terminal processing. Unlike the B. thuringiensis 5-endotoxins, whose expression is restricted to sporulation, Vip3A insecticidal proteins are expressed in the vegetative stage of growth starting at mid-log phase as well as during sporulation. Vip3A represents a novel class of proteins insecticidal to lepidopteran insect larvae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown K. L., Whiteley H. R. Isolation of a Bacillus thuringiensis RNA polymerase capable of transcribing crystal protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4166–4170. doi: 10.1073/pnas.85.12.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. S., Cowles E. A., Pietrantonio P. V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecadet M. M., Chaufaux J., Ribier J., Lereclus D. Construction of Novel Bacillus thuringiensis Strains with Different Insecticidal Activities by Transduction and Transformation. Appl Environ Microbiol. 1992 Mar;58(3):840–849. doi: 10.1128/aem.58.3.840-849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Lory S. Determinants of extracellular protein secretion in gram-negative bacteria. J Bacteriol. 1992 Jun;174(11):3423–3428. doi: 10.1128/jb.174.11.3423-3428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh S. C., Stone T. B., Sims S. R., Hunst P. L., Greenplate J. T., Marrone P. G., Perlak F. J., Fischhoff D. A., Fuchs R. L. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J Invertebr Pathol. 1990 Sep;56(2):258–266. doi: 10.1016/0022-2011(90)90109-j. [DOI] [PubMed] [Google Scholar]
- Mettus A. M., Macaluso A. Expression of Bacillus thuringiensis delta-endotoxin genes during vegetative growth. Appl Environ Microbiol. 1990 Apr;56(4):1128–1134. doi: 10.1128/aem.56.4.1128-1134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993 Mar;57(1):50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Bron S., Van Ee J., Venema G. Construction and use of signal sequence selection vectors in Escherichia coli and Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):3321–3328. doi: 10.1128/jb.169.7.3321-3328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]