Abstract
Background
Pharmacokinetic (PK) parameters based on short sampling times (48 h or less) may contain inaccuracies due to their dependency on extrapolated values. This study was designed to measure PK parameters with greater accuracy in obese users of a low-dose oral contraceptive (OC), and to correlate drug levels with assessments of end-organ activity.
Study design
Obese (BMI ≥30 kg/m2), ovulatory, otherwise healthy, women (n = 32) received an OC containing 20 mcg ethinyl estradiol (EE)/100 mcg levonorgestrel (LNG) for two cycles. EE and LNG PK parameters were characterized for 168 h at the end of Cycle 1. During Cycle 2, biweekly outpatient visits were performed to assess cervical mucus, monitor ovarian activity with transvaginal ultrasound, and obtain serum samples to measure EE, LNG, estradiol (E2), and progesterone (P) levels. PK parameters were calculated and correlated with end-organ activity and compared against control samples obtained from normal and obese women sampled up to 48 h in a previous study. Standard determination of PK accuracy was performed; defined by the dependency on extrapolated values (‘excess’ area under the curve of 25% or less).
Results
The mean BMI was 39.4 kg/m2 (SD 6.6) with a range of 30–64 kg/m2. Key LNG PK parameters were as follows: clearance 0.52 L/h (SD 0.24), half-life 65 h (SD 40), AUC 232 h*ng/mL (SD 102) and time to reach steady-state 13.6 days (SD 8.4). The majority of subjects had increased ovarian activity with diameter of follicles ≥8 mm (n = 25) but only seven women had follicles ≥10 mm plus cervical mucus scores ≥5. Evidence of poor end-organ suppression did not correlate with the severity of the alterations in PK. As compared to historical normal and obese controls (48 h PK sampling), clearance, half-life, area under the curve (AUC) and time to reach steady-state were found to be significantly different (p ≤ 0.05) in obese women undergoing a longer duration of PK sampling (168 h). Longer sampling also improved PK accuracy for obese women (excess AUC 20%) as compared to both normal and obese controls undergoing shorter sampling times (48 h) with excess AUCs of 25% and 50%, respectively.
Conclusions
Obesity results in significant alterations in OC steroid PK parameters but the severity of these alterations did not correlate with end-organ suppression. A longer PK sampling interval (168 h vs. 48 h) improved the accuracy of PK testing.
1. Introduction
Controversy exists regarding whether obesity adversely impacts the effectiveness of oral contraceptives (OC) [1]. As obesity is related to both subfertility (secondary to anovulation) [2] and drug noncompliance (due to its association with low socioeconomic status) [3, 4], measuring the true impact of obesity on inherent drug efficacy is difficult. When calculated accurately, detailed pharmacokinetic (PK) parameters provide an invaluable tool for measuring altered drug therapeutics [5]. Carefully conducted PK studies in women of differing body weights could help to ensure efficacy in future contraceptive development.
Unfortunately, obtaining PK parameters is time-intensive for both the study participant and the investigator, as it requires closely spaced repetitive blood sampling. For this reason, studies often limit the duration of sampling to 24 h. Ideally, sampling should span over 4–5 half-lives of a drug [6, 7]. With a shorter sampling interval, estimates are used in combination with actual values to re-approximate the necessary time points for the area under the curve (AUC). The AUC is a measure of the systemic exposure of a drug and thus, the most important marker of drug efficacy. In combination with the known intra-subject variability common during evaluation of synthetic steroid hormones, PK parameters based on shorter sampling times may contain inaccuracies in the AUC due to their dependency on extrapolated values which, in turn, may mask important findings. Calculating an extrapolated AUC or ‘excess AUC’ can aid in determining the accuracy of AUC estimation as it identifies the amount of AUC dependent on extrapolated values rather than actual values [7]. Ideally, the percentage based on extrapolated values is less than 20–25%.
Prior OC PK studies in obese women have estimated PK parameters based on 24–48 h sampling or 1–2 half-lives [8, 9]. Although these studies suggest that differences exist between women of normal and obese BMI, confirmation of these differences using a PK study design with a longer sampling interval is needed to confirm these observations and to determine whether obesity impacts efficacy of hormonal contraceptive methods. This study was designed to measure PK parameters with greater accuracy than prior publications, and to correlate drug levels with assessments of end-organ activity.
2. Materials and methods
A prospective cohort study was conducted at Oregon Health & Science University (OHSU) in Portland, Oregon, from January 2010 to June 2011. The OHSU Institutional Review Board and OHSU Clinical & Translational Research Institute (OCTRI) approved the study protocol, and all patients underwent informed written consent.
Thirty-two, otherwise healthy, obese (BMI ≥30 kg/m2) reproductive-aged (18–35 years old) women, not currently using hormonal contraception but seeking to initiate contraception with combination oral contraceptives, were recruited. Inclusion and exclusion criteria included regular menstrual cycles, not actively seeking weight gain or loss, no evidence of anemia (hematocrit ≥36%), no contraindications to hormonal contraception, no use of tobacco or drugs known to interfere with the metabolism of sex steroids, and no overt clinical features of or prior treatment for metabolic disorders (e.g., polycystic ovarian syndrome, diabetes).
In addition to baseline demographic information, several obesity biomarkers including weight, height, body composition measurements by air displacement plethysmorgraphy, and waist-to-hip ratios were collected. A blood sample was obtained to measure progesterone (P) levels during the luteal phase of the pretreatment cycle to confirm ovulation. A single value of ≥3 ng/mL was required for enrollment.
All qualifying study subjects were placed on a combination monophasic birth control pill containing 20 mcg ethinyl estradiol/100 mcg levonorgestrel (Aviane; Teva; Israel) at the onset of menses following the pre-treatment cycle. The medication was dosed in a cyclic fashion (21 days active pill with a 7-day hormone-free interval) for a total of two treatment cycles. Women were instructed to take the pill at 0900 AM daily. Self-reported compliance with the medication was recorded on a calendar (compliant cycle ≤2 late and/or missed pills) and confirmed based on OC serum trough levels (nonuser: all LNG values <0.16 ng/mL; inconsistent user: two or more values <1.0 ng/mL; consistent user: no more than one value ≥1.0 ng/mL)[4].
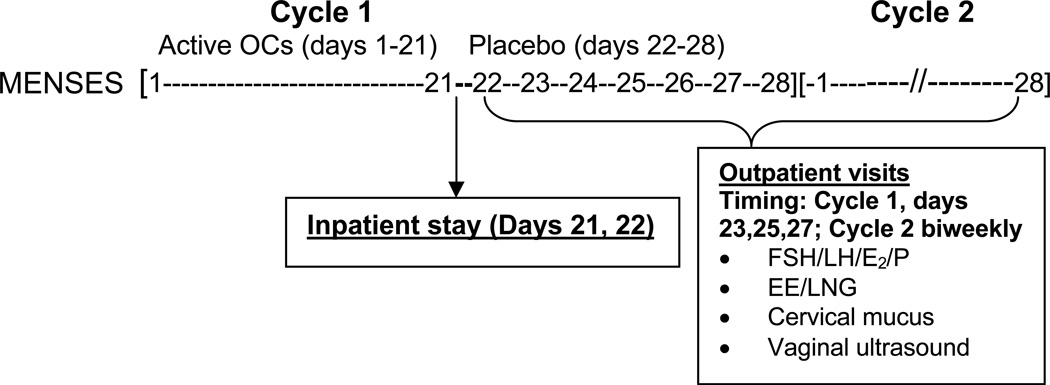
The study flow is illustrated in Fig. 1. To measure the PK profile during the hormone-free interval, subjects were admitted for one 48-h inpatient stay starting on day 21 of the first cycle of active pills through the first day of the 7-day hormone-free interval (Cycle 1, Day 21–22). An indwelling venous catheter was used to obtain serum samples during the inpatient stay to assay EE and LNG levels. LNG, EE, FSH, LH, estradiol (E2) and P were measured through the placebo week in Cycle 1 (Day 23, 25, 27) and then biweekly until the end of Cycle 2. At each of these visits, a vaginal probe ultrasound (GE LOGIQ 400 Proseries ultrasound, 7.5-MHZ; Fairfield, CT) was performed to measure size ovarian follicles. The total number of antral follicles (≥4 mm) was documented and the largest follicle on each ovary was measured in two dimensions. Cervical mucus was obtained at each visit from the endocervix using a Select endocervical aspirator (Select Medical Systems Inc., Williston, VT) and its quality scored according to World Health Organization guidelines (0–4 poor, 5–8 fair, 9–12 good) [10].
Fig. 1.
Study flow
2.1 Pharmacokinetics: timing of samples and assay characteristics
To determine the PK parameters of EE and LNG, serial serum samples were collected during the inpatient stay on Cycle 1, Day 21 at 0 (0900 AM; time of drug ingestion), and 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12 h, and continued on Cycle 1, Day 22 (first day of hormone-free interval in Cycle 1) at 0 (0900 AM), 4, 8, 12, 24 h. Single samples (drawn within 2–4 h of pill administration) were also obtained during Cycle 1 on Days 23, 25, 27 and then biweekly during Cycle 2. The Cycle 2 samples were used to compute the time to reach steady-state.
After separation of serum, samples were frozen at −80°C until assessed. LNG and EE levels were quantified in the laboratory of Dr. Frank Stanczyk at the University of Southern California by specific and sensitive radioimmunoassays (RIAs) as described previously [11]. Prior to RIA, each analyte was extracted with ethyl acetate:hexane (3:2) and subjected to Celite column partition chromatography to remove interfering steroids. Procedural losses were estimated by adding approximately 800 d.p.m. of titrated internal standard (3H-LNG or 3H-EE) to the serum prior to the extraction step. The losses ranged from 20–30% and the values were used to correct the RIA results. A highly specific antiserum was used in conjunction with an iodinated radioligand in each RIA. Separation of free from antiserum-bound LNG or EE was achieved by use of second antibody. The sensitivities of these RIAs are 0.05 ng/mL for LNG and 15 pg/mL for EE. Intra-assay and inter-assay coefficients of variation are 4.4% and 8.9% for LNG RIAs and 6.9% and 11% for EE RIAs, respectively.
2.2 Gonadotropins and ovarian hormones: assay characteristics
FSH, LH, E2 and P4 assays were performed at the Endocrine Technology Services Core Laboratory (ETSL) at the Oregon National Primate Research Center (ONPRC, Beaverton, Oregon) using an automated Immulite 2000 chemiluminescent immunoassay system (Siemens Healthcare Diagnostics, Deerfield, IL 60015, USA). The assay sensitivity of E2, P, FSH, and LH assays are 20 pg/mL, 0.2 ng/mL, 0.1 mIU/mL, and 0.1 mIU/mL respectively.
2.3 Pharmacokinetic parameter analysis
LNG and EE PK data were analyzed separately by noncompartmental methods using WinNonLin (v 5.2; Pharsight, Moutain View, CA). Maximum serum LNG and EE concentrations (Cmax) and time to maximum concentration (Tmax) were observed values. Area under the curve (AUC) was calculated from time zero to 168 h (AUC0–t) using the linear trapezoidal rule and then extrapolated to infinity (AUC0–∞) which provides a more accurate calculation of drug clearance [12]. AUC0–∞ is measured as a sum of AUC0–t and ‘excess area’ which is computed as a ratio or percentage of the last observed concentration (Clast) to terminal elimination rate constant (λz). Excess AUC was calculated and reported as a percentage. Drug half-life (t1/2), oral clearance (CL), and volume of distribution (VD) were estimated using standard pharmacokinetic calculations (t1/2= 0.693/λz, CL= dose/AUC0–∞; VD= CL//λz). The time to reach steady-state was estimated as 5*t1/2.
2.4 Comparisons of group and day differences
Descriptive statistics were generated for demographics, maximum serum FSH, LH, P, and E2 levels, maximum follicle diameter and cervical mucus scoring. PK parameters of individuals with and without a maximum follicular diameter ≥10 mm and cervical mucus scores ≥5 were compared.
2.5 Primary and secondary outcomes
The primary outcome was to confirm the PK parameters of EE and LNG in obese users using a prolonged sampling interval (168 h). As the pharmadynamic effects of OCs rely on the progestin component, additional analyses and comparisons focused on LNG. The accuracy of the LNG PK profile was determined by excess AUC; a PK profile with an excess AUC of ≤25% is considered highly accurate [7]. LNG PK parameters between subjects demonstrating increased end-organ activity were compared to those without (yes/no). Increased end-organ activity was defined as a follicle ≥10 mm, cervical mucus scores ≥5, E2 > 75 pg/mL, and/or P >3 ng/mL. Additionally, the LNG PK parameters based on a longer sampling interval were compared against control samples obtained from normal (mean BMI 21.9, SD 1.6) and obese (mean BMI 37.3, SD 6) women sampled up to 48 h in a previous study performed at our institution using the same methodology and OC formulation [9].
3. Results
Thirty-two subjects signed informed consent, met eligibility criteria and completed the PK sampling (1 subject discontinued Cycle 2, day 6). There were no serious adverse events or pregnancies during the study period. All participants were found to be compliant with study medication as determined by LNG trough levels (data not shown).
Composite demographic information is listed in Table 1. The average BMI was 39.4 kg/m2 (SD 6.6) with a range from 30 to 64 kg/m2. The majority of subjects identified themselves as non-Hispanic, Caucasian with an average age of 29 years old. Baseline characteristics of the normal and obese control groups have been previously published [9].
Table 1.
Composite demographics
| Current obese cohort |
Historical obese cohort [1] |
Historical normal BMI cohort [1] |
|
|---|---|---|---|
| Age (mean, SD) | 29 (SD 4.7) | 29.8 (SD 7.1) | 29.2 (SD 4.9) |
| Race (number) | |||
| Non-Hispanic, Caucasian | 29 | 9 | 8 |
| Other | 3 | 1 | 2 |
| Ever pregnant? (yes) | 15 | 5 | 2 |
| BMI (mean, SD) | 39.4 kg/m2 (SD 6.6) | 37.3 kg/m2 (SD 6) | 21.9 kg/m2 (SD 1.6) |
| BMI categories (number) | |||
| Normal (BMI < 25) | 10 | ||
| Obese Class 1 (BMI 30–34.9) | 6 | 5 | |
| Obese Class II (BMI 35–39.9) | 17 | 3 | |
| Obese Class III (BMI ≥ 40) | 9 | 2 | |
| % body fat (mean, SD) | 46.4 (SD 3.8) | 50.3 (SD 3.5) | 27.6 (SD 7.2) |
| Waist/hip ratio (mean, SD) | 0.85 (SD 0.06) | 0.91 (SD 0.09) | 0.83 (SD 0.06) |
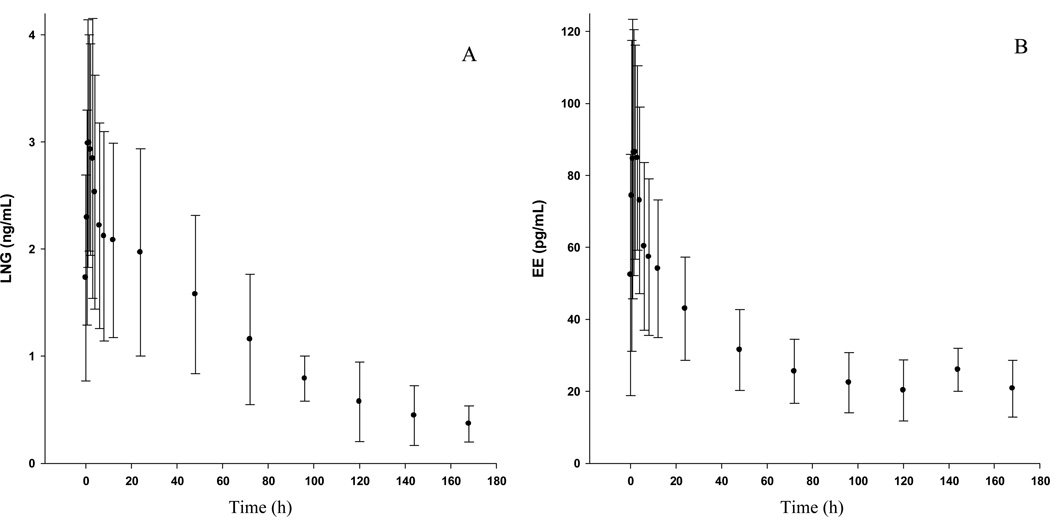
Detailed LNG and EE PK curves are illustrated in Fig. 2 and discrete PK parameters are reported in Table 2. Inter-individual variability in plasma concentrations were 24–64 % CV, consistent with the published literature [8, 9].
Fig.2.
Serum levels (mean ± standard deviation) of (A) levonorgestrel (LNG) and (B) ethinyl estradiol (EE).
Table 2.
Pharmacokinetic parameters (mean ± standard deviation) of LNG and EE in obese women
| LNG | EE | |
|---|---|---|
| Tmax (h) | 2.1 ± 1.2 | 1.8 ± 1.1 |
| Cmax (ng/mL) | 3.47 ± 1.13 | 0.108 ± 0.034 |
| AUC(0–∞) (h*ng/mL) | 232 ± 102 | 10.5 ± 4.3 |
| t1/2 (h) | 65 ± 40 | 171 ± 90 |
| Vd/F (L) | 45 ± 26 | 467 ± 156 |
| CL/F (L/h) | 0.52 ± 0.24 | 2.23 ± 0.91 |
End-organ activity is described in Table 3. Overall, 25 women had evidence of ovarian activity with follicles measuring ≥8 mm in diameter. Approximately half of this group had maximum follicular diameter measurements of ≥18 mm. In combination with cervical mucus scores, only seven women were found to have both a follicle ≥10 mm and a cervical mucus score ≥5. All of these women had E2 levels > 75 pg/mL but all P levels were < 3 ng/mL. PK parameters were no different among obese women with and without evidence of end-organ activity.
Table 3.
End-organ activity in obese women on OCs
| Number of subjects |
|
|---|---|
| Maximum follicular diameter* | |
| ≥8 mm | 25 |
| ≥10 mm | 19 |
| ≥13 mm | 16 |
| ≥18 mm | 13 |
| Cervical mucus score | |
| 0–4 | 17 |
| 5–8 | 13 |
| 9–12 | 1 |
| Progesterone >3ng/mL | 0 |
Numbers are cumulative.
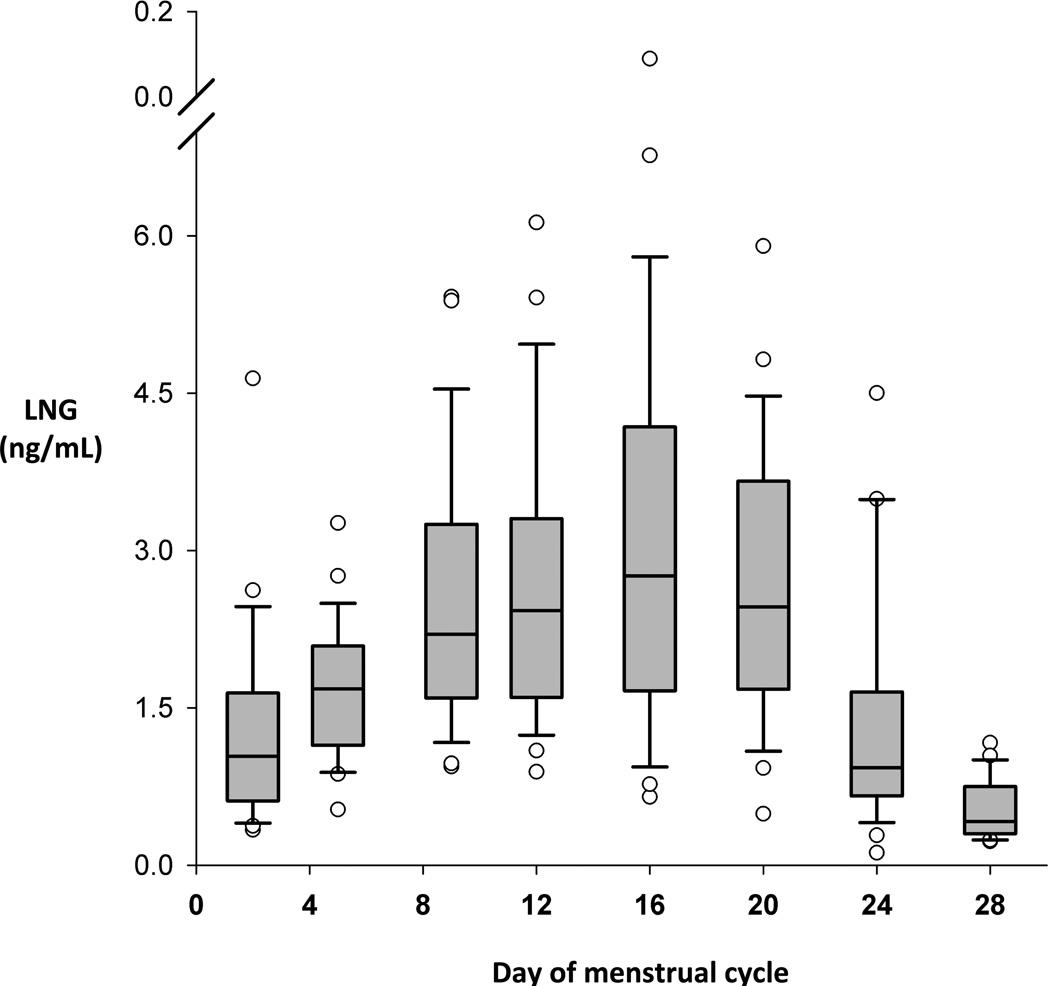
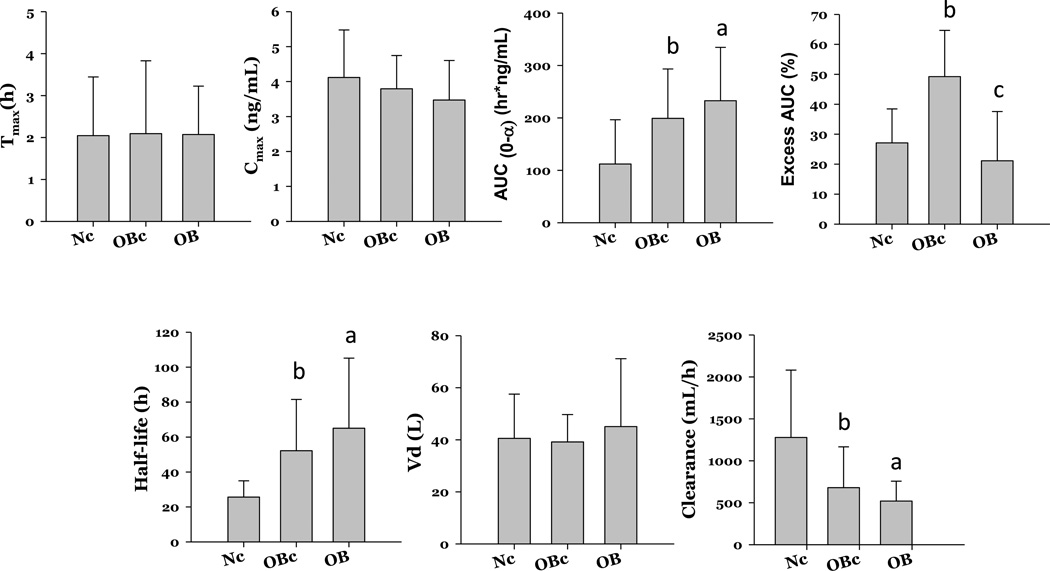
A direct comparison of mean PK parameters for LNG obtained over 48 h (historical normal and obese controls) [9] versus 168 h demonstrates very similar Tmax and Cmax serum levels (Fig. 3). The obese historical controls had a longer t1/2, a higher AUC, a lower CL and a longer time to reach steady-state than the historical normal BMI group [9]. The directionality of these differences between the current and historical groups is similar but more pronounced in the current obese group utilizing longer PK sampling times (Fig. 4). The mean time to reach steady-state was longest for the current obese group [13.6 days (SD 8.4); median 11.9, range 4.1–52.7], as compared to the historical controls [obese: 10.9 days (6.1); normal 5.3 days (1.9)] (Fig. 4). ‘Excess’ AUC or dependency on extrapolated values was 20% in obese women with longer PK sampling, 25% in normal controls, and 50% in obese controls.
Fig.3.
Time to reach steady-state for LNG. Medians with interquartile range (95% confidence intervals). Outliers are indicated by open circles.
Fig 4.
Comparison of LNG PK parameters between current obese cohort (OB) and historical obese (OBc) and normal (Nc) BMI controls. ap value of ≤.05 between Nc and OB; bp value of ≤.05 between Nc and OBc; cp value of ≤.05 between OB and OBc.
4. Discussion
Prior studies have demonstrated only slight differences in the PK parameters between normal and obese OCs users [8, 9]. Confirmation of these differences is critical to understanding if potential biologic mechanisms exist regarding contraceptive failures due to body weight and if these changes might predict failure. When PK differences are small or the assay variability is substantial as with contraceptive steroids, more accurate PK sampling can aid in better estimations of PK parameters [7]. In the present study, we have confirmed that obesity alters PK parameters of contraceptive steroids, and the effect on PK indices critical for drug therapeutics, t1/2, CL and AUC, is more pronounced than originally documented [8,9]. Unfortunately, we were not able to detect differences in PK profiles between obese women with and without end-organ activity but the majority of our obese cohort had evidence of poor end-organ suppression although no overt evidence of ovulation. While this finding does not prove decreased contraceptive efficacy in obese women, it is particularly worrisome in a population that may have greater difficulties with consistent OC use [3].
This study represents the most accurate PK profiles of obese OC users yet to be published. We employed a longer sampling scheme, 168 h, as compared to prior studies which were limited to 24–48 h [8, 9]. With shorter sampling times, estimates are used to re-approximate the necessary time points to determine AUC. The PK parameter, AUC0–∞, is an important indicator of drug exposure [13], and calculations of other PK parameters (Vd and CL) are dependent on this estimated value. Therefore, accurate estimation of AUC0–∞ is critical in characterizing PK behavior of any drug. As AUC0–∞ estimation involves extrapolation to infinity which is computed using the elimination rate constant (λz), longer sampling schemes reaching up to 3–5 half-lives yield a more accurate λz value which in turn increases the accuracy of PK parameters reliant on the AUC. A dependency on extrapolated values to determine AUC or an ‘excess AUC’ below 20–25% can be helpful in determining the accuracy of the AUC estimation [7]. As obesity significantly impacts t1/2 and CL, the length of sampling time appears to be critically important to calculating PK parameters for an obese individual (excess AUC: current obese cohort 20% versus historical control 50%). Whereas a normal BMI group maintains a low excess AUC (25%) even with a shorter sampling time. We were also able to confirm treatment compliance through study diaries and OC serum levels. Our study population also represented a broader range of obese BMI (30–63 kg/m2) than previous publications [8, 9].
Our current study also includes a broader range of obese BMI than our historical obese control group which could potentially exaggerate the differences found. However, truncating our current sampling to 48 h and recalculating our PK parameters demonstrates very similar findings to our previous publication [9]. Furthermore, both in this and our previous study, the degree or magnitude of obesity does not appear to correlate linearly with the amount of PK alteration. Although we have proven that obesity does affect PK parameters, we have not yet determined what level of obesity dictates the magnitude of change. Additionally, the severity of change in PK parameters did not correlate with end-organ activity. Although the majority of our subjects had end-organ activity, our ability to detect evidence of ovulation in this activity, like follicular rupture, rise in P, or favorable changes in cervical mucus, may have been limited by our bi-weekly monitoring.
As with our previous study [9], we continue to find that obese women have significant alterations in their t1/2 and drug CL but not their drug distribution or Vd. A longer t1/2 translates into a longer time to achieve steady-state and also the threshold for ovulation inhibition at the time of OC initiation or after the 7-day hormone-free interval, creating a ‘window of opportunity’ for failure. While the translation of these PK findings into actual clinical evidence of failure (i.e., pregnancy) has not been sufficiently examined in OC users, studies of emergency contraception where actual exposure to sexual activity is documented provide some additional insights. Recently, LNG-based emergency contraceptives have been shown to be less effective in obese women as compared to women of normal BMI (OR4.41, 95% CI 2.05–9.44) [14]. It is interesting to consider that these emergency therapies are single-dose treatments, reliant on achieving a certain peak level, which is determined by drug clearance at a critical time shortly prior to ovulation with one dose of the drug.
We have clearly determined that obesity causes changes in the PK parameters of OCs and that these changes are due to alterations in drug clearance. Drug clearance is a key determinant of drug exposure and thus drug therapeutics. Currently, we have no way to predict who is at greatest risk for OC failure as, obviously, these changes do not translate into a 1:1 risk of failure and the greatest alterations in PK do not correlate with the magnitude of obesity end-organ activity. Further work should be focused on defining the particular phenotypes that might be at greatest risk, and in the development of useful office-based strategies to screen users.
Acknowledgements
The authors would like to thank the Women’s Health Research Unit, the Department of Obstetrics & Gynecology, and the Oregon Clinical & Translational Research Institute at Oregon Health & Science University
Financial Support: The authors acknowledge the grant support from National Institutes of Health (R01 HD061582-01 NICHD), the OHSU Oregon Clinical & Translational Research Institute (NIH NCRR 1 UL1 RR024120), and the Bioanalytical Shared Resource /Pharmacokinetics Core at OHSU
References
- 1.Edelman A. Society of Family Planning Clinical Guidelines: Contraceptive Considerations in Obese Women. Contraception. 2009;80:583–590. doi: 10.1016/j.contraception.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 2.The practice committee for American Society for Reproductive Medicine. Obesity and reproduction: an educational bulletin. Fertil Steril. 2008;90:S21–S29. doi: 10.1016/j.fertnstert.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012;85:465–469. doi: 10.1016/j.contraception.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Westhoff CL, Torgal AH, Mayeda ER, et al. Ovarian suppression in normal-weight and obese women during oral contraceptive use: a randomized controlled trial. Obstet Gynecol. 2010;116:275–283. doi: 10.1097/AOG.0b013e3181e79440. [DOI] [PubMed] [Google Scholar]
- 5.Nicolau DP. Predicting antibacterial response from pharmacodynamic and pharmacokinetic profiles. Infection. 2001;29(suppl 2):11–15. [PubMed] [Google Scholar]
- 6.Kuhnz WBH, Zimmerman H. Pharmacokinetics of exogenous natural and synthetic estrogens and antiestrogens. In: M Oettel ES, editor. Estrogens and Antiestrogens II: Pharmacology and clinical application of estrogens and antiestrogens. NY: Springer; 1999. pp. 261–322. [Google Scholar]
- 7.Fotherby K. Pharmacokinetics of gestagens: some problems. Am J Obstet Gynecol. 1990;163:323–328. doi: 10.1016/0002-9378(90)90576-s. [DOI] [PubMed] [Google Scholar]
- 8.Westhoff CL, Torgal AH, Mayeda ER, Pike MC, Stanczyk FZ. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474–480. doi: 10.1016/j.contraception.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman AB, Carlson NE, Cherala G, et al. Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception. 2009;80:119–127. doi: 10.1016/j.contraception.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO laboratory manual for the examination of human sperm and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 11.Stanczyk FZ, Hiroi M, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR., Jr Radioimmunoassay of serum d-norgestrel in women following oral and intravaginal administration. Contraception. 1975;12:279–298. doi: 10.1016/0010-7824(75)90088-8. [DOI] [PubMed] [Google Scholar]
- 12.Rowland M, Tozer TN. Clinical pharmacokinetics: concepts and applications. Philadelphia: Lea & Febiger; 1980. [Google Scholar]
- 13.Administration FDA, editor. Guidance for Industry: exposure-response relationships - study design, data analysis, and regulatory applications. Washington, DC: US Department of Health and Human Services; 2003. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf. [Google Scholar]
- 14.Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84:363–367. doi: 10.1016/j.contraception.2011.02.009. [DOI] [PubMed] [Google Scholar]