Abstract
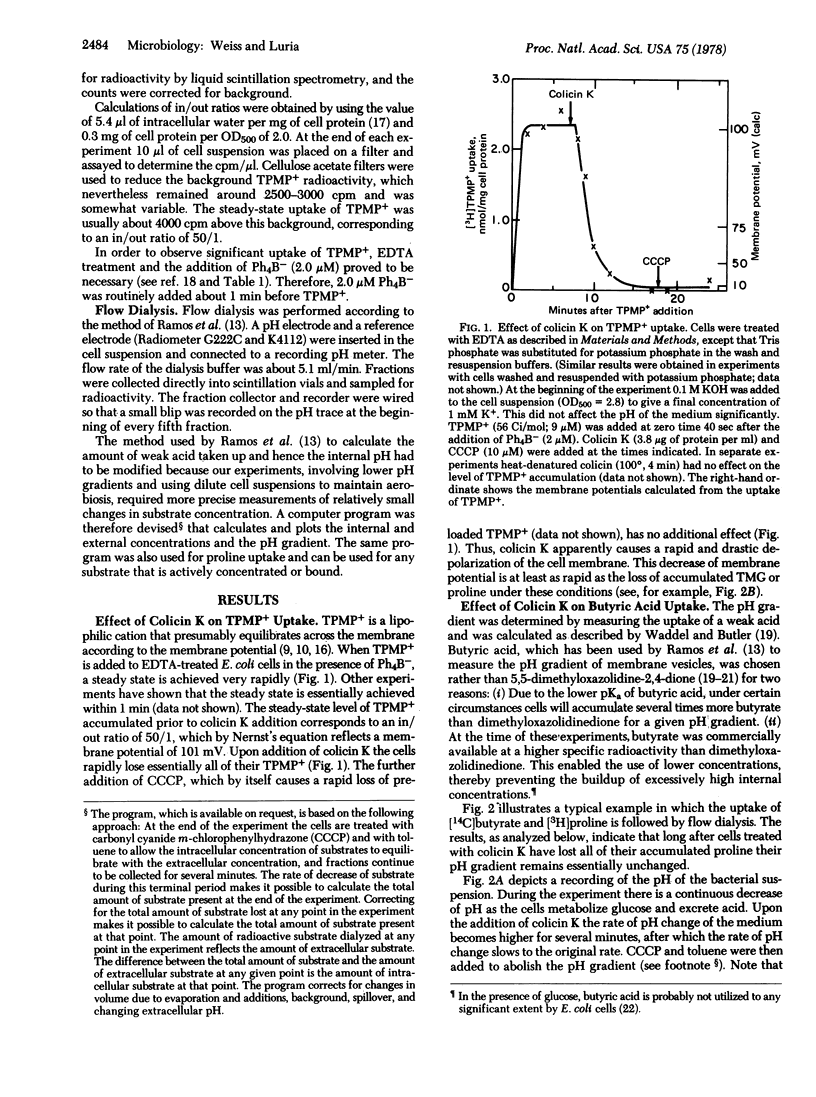
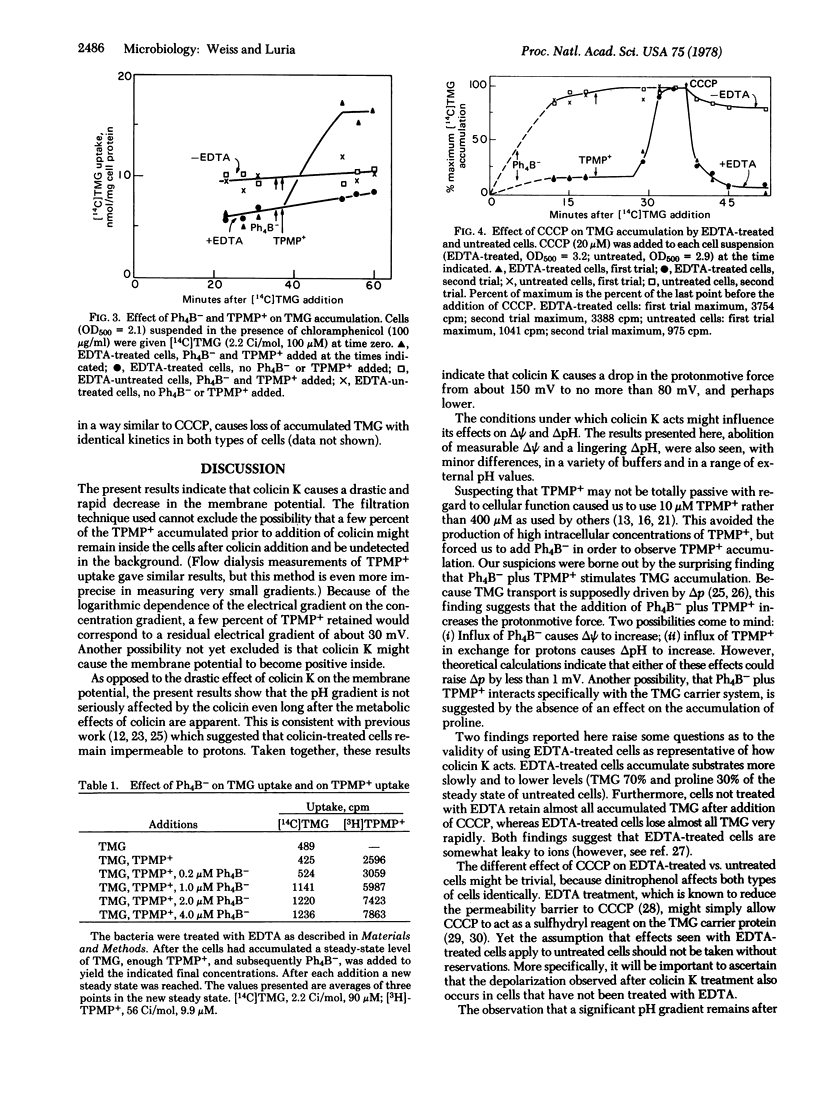
Colicin K causes a rapid and drastic reduction of the membrane potential of Escherichia coli cells, as measured by the uptake of the lipophilic cation triphenylmethylphosphonium. The colicin causes no major changes in the pH gradient, as measured by the uptake of butyric acid. The decrease in membrane potential following addition of colicin K to the cells is a prompt response that parallels or precedes known physiological effects such as efflux of accumulated substrates. Hence the loss of membrane potential qualifies as the primary action by which the colicin uncouples membrane-associated function from respiration. Certain peculiarities of bacterial cells pretreated with EDTA in their response to uncoupling agents and lipophilic ions are described.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- Cecchini G., Koch A. L. Effect of uncouplers on "downhill" beta-galactoside transport in energy-depleted cells of Escherichia coli. J Bacteriol. 1975 Jul;123(1):187–195. doi: 10.1128/jb.123.1.187-195.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg J. L., Wilson T. H. A protonmotive force as the source of energy for galactoside transport in energy depleted Escherichia coli. J Membr Biol. 1977 Mar 8;31(3):233–255. doi: 10.1007/BF01869407. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Helgerson S. L., Cramer W. A. Changes in E. coli cell envelope structure caused by uncouplers of active transport and colicin E1. J Supramol Struct. 1976;5(3):291–308. doi: 10.1002/jss.400050304. [DOI] [PubMed] [Google Scholar]
- Hong J. S. An ecf mutation in Escherichia coli pleiotropically affecting energy coupling in active transport but not generation or maintenance of membrane potential. J Biol Chem. 1977 Dec 10;252(23):8582–8588. [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Kopecky A. L., Copeland D. P., Lusk J. E. Viability of Escherichia coli treated with colicin K. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4631–4634. doi: 10.1073/pnas.72.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Lusk J. E., Nelson D. L. Effects of colicins E1 and K on permeability to magnesium and cobaltous ions. J Bacteriol. 1972 Oct;112(1):148–160. doi: 10.1128/jb.112.1.148-160.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem. 1972 Sep 25;29(3):553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Plate C. A. Effects of temperature and of fatty acid substitutions on colicin K action. Antimicrob Agents Chemother. 1973 Jul;4(1):16–24. doi: 10.1128/aac.4.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate C. A., Suit J. L., Jetten A. M., Luria S. E. Effects of colicin K on a mutant of Escherichia coli deficient in Ca 2+, Mg 2+-activated adenosine triphosphatase. J Biol Chem. 1974 Oct 10;249(19):6138–6143. [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Kaback H. R. Membrane potential and active transport in membrane vesicles from Escherichia coli. Biochemistry. 1975 Dec 16;14(25):5451–5461. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt L. Mechanism of colicin action: early events. J Bacteriol. 1970 Dec;104(3):1236–1241. doi: 10.1128/jb.104.3.1236-1241.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]


