Abstract
Little is known about the contribution of diet components independent of body composition to persistent fatigue in breast cancer survivors. Therefore, our study aim was to determine the associations among dietary intake and fatigue in relation to and independent of adiposity and physical activity (PA) in breast cancer survivors. Baseline data from 42 breast cancer survivors enrolled in a randomized exercise trial were analyzed: fatigue (FACT-F), diet components (3-day diet record), body mass index, percent body fat (DXA), and PA (accelerometer). The mean age was 54±9 years with an average BMI of 30.5±8.1 kg/m2. Fatigue was positively associated with % of kcal/day fat intake (r=.31, p<.05) and inversely related to fibre g/day (r=.38, p<.05) and carbohydrate g/day intake (r=.31, p<.05). Mean fatigue was greater for participants eating <25 g/day of fibre compared with >25 g/day of fibre (15.7±10.8 versus 6.4±3.7, p<.005). No significant associations were noted between fatigue and PA or body composition. Diets high in fibre and low in fat are associated with reduced fatigue in breast cancer survivors. The difference in fatigue for low versus high fibre diets exceeded the minimal clinically important difference of 3 units. Prospective studies evaluating the effect of changing diet on fatigue in breast cancer survivors are warranted.
Introduction
Breast cancer is the most commonly diagnosed cancer among women, worldwide. In Europe, breast cancer incidence is estimated to be as high as 13.5% of all cancer diagnoses in women (Ferlay et al., 2007). The highest rates of breast cancer incidence occur in North America (Tyczynski et al., 2002) with 26% of new cancer diagnoses in US women being breast (Jemal et al., 2008). However, when age-standardized per 100,000, incidence and mortality rates are highest within Western Europe (Jemal et al., 2011). With improved detection and treatment, the majority of women are surviving but many breast cancer survivors experience deficits in their health-related quality of life compared to the general female population of similar age (Lee et al., 2011). The association between mood and sleep disturbances with depression and fatigue is an important aspect of quality of life in breast cancer survivors. Importantly, fatigue is recognized as an independent symptom and a cause of mood disturbances and depression in this cohort (Bower et al., 2000). Notably, fatigue is a significant issue for women attempting to thrive post-treatment with as many as 70% of breast cancer survivors having levels that reduce their quality of life up to five years after completing treatment (Bower et al., 2000, Minton and Stone, 2008).
The National Comprehensive Cancer Network defines cancer-related fatigue as a “distressing persistent, subjective sense of physical, emotional and /or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning” (NCCN, 2011). In a longitudinal study of breast cancer survivors 5-10 years after diagnosis, Bower and associates demonstrated that in addition to cancer treatment, several non-modifiable predictive factors of fatigue include genetics, family history, and pre-cancer depression and fatigue (Bower et al., 2006). De Jong (de Jong, 2011) addressed the complexity of cancer-related fatigue in 2011 and suggested the need for further research to better understand coping strategies for self-management of fatigue. Moreover, the negative impact of fatigue after breast cancer is a contemporary concern internationally as exemplified by a recent call for European employment policy changes to assist breast cancer survivors struggling with fatigue (Banning, 2011).
Fortunately, several non-pharmacological modifiable factors may be targets for interventions to reduce fatigue in breast cancer survivors including weight status (specifically adiposity), and as determinants of weight status, physical activity and dietary intake. Higher levels of fat mass have been implicated in the occurrence of fatigue in the older adult female population (Resnick et al., 2006, Valentine et al., 2009, Valentine et al., 2011) and in breast cancer survivors (Irwin et al., 2009). For example, a body mass index (BMI) greater than 30 kg/m2 was associated with fatigue in breast cancer survivors (Reinertsen et al., 2010).
Of the two determinants of weight status, physical activity has been most explored as a lifestyle behaviour to influence health status in breast cancer survivors, including fatigue. Physical activity is often recommended for cancer survivors as it can improve body image and decrease depression and anxiety as well as improve physical activity tolerance (NCCN, 2011). We and others have determined that physical activity favourably impacts body composition, psycho-social health, including fatigue and health related quality of life in breast cancer survivors (Irwin et al., 2009, Wanchai et al., 2011, Rogers et al., 2009b).
In contrast to physical activity, the associations between diet components and fatigue independent of adiposity have not been well-studied. With specific reference to cancer of the breast, dietary intake has been most explored with regard to risk of initial diagnosis and reoccurrence rather than fatigue (Tramm et al., 2011, Jevtic et al., 2010, Hanf and Gonder, 2005, Duncan, 2004). Moreover, the disease and treatment of most cancer types often result in cachexia (Donohoe et al., 2011) while, in contrast, weight gain frequently occurs during breast cancer treatment (Rock and Demark-Wahnefried, 2002). In addition, nutrition intervention to increase energy ( calorific) intake and correct electrolyte imbalance is a common recommendation for patients undergoing active cancer treatment; however, less is known about how diet intake, specifically diet quality, affects fatigue during post-treatment and under calorifically sufficient conditions. For example, Porock and colleagues suggest that nutritional intake in cancer survivors affects their perceived fatigue levels, but only when consuming less than sufficient calories (Porock et al., 2005). Minimal research has been conducted to explore the link between dietary intake and fatigue, independent of weight status in breast cancer survivors without cachexia.
The association between dietary intake and fatigue, independent of adiposity and physical activity, warrants further evaluation in breast cancer survivors. Although not well characterized, current evidence suggests that post-treatment fatigue experienced by breast cancer survivors may be reduced by changes in lifestyle behaviours. Recent cross-sectional evidence suggests that a cancer diagnosis encourages positive behaviour changes with ~30% reporting greater physical activity and ~40% changing dietary intake to reduce fat intake, increase fibre and fruit and vegetable consumption with the latter being associated with reductions in fatigue (Alfano et al., 2009). Similar to other cohorts in the population, health behaviours tend to cluster (i.e. individuals with better nutritional habits also have higher levels of physical activity and do not smoke). Therefore, the primary aim of this study was to determine the associations among dietary intake and fatigue in relation to and independent of adiposity and physical activity in breast cancer survivors varying in age and weight status. Such information is needed to improve current lifestyle interventions for fatigue, especially related to diet components.
Methods & Materials
Data and Participants
A post-hoc analysis of baseline data obtained from a randomized control trial (RCT) conducted to assess the efficacy of a physical activity behaviour change intervention compared to usual care was performed (Rogers et al., 2009b). Detailed information about the design, setting, and participants for the original RCT was previously reported (Rogers et al., 2009a, Rogers et al., 2009b). In brief, participants were female between 18-70 years of age, English speaking, and currently receiving adjuvant breast cancer treatment (aromatase inhibitor or oestrogen receptor modulator). Importantly, breast cancer survivors with BMI classifications of normal, overweight or obese were allowed to participate and dietary intake did not impact enrolment status; however, due to the physical activity intervention, breast cancer survivors had to report participating ≤ 60 minutes of vigorous physical activity or ≤ 150 minutes of moderate plus vigorous exercise per week for the past month although daily and recreational physical activity was allowed to vary. Institutional review board approval was obtained and all participants provided informed consent before initiation of study procedures.
Fatigue
Fatigue was measured by the 13-item Functional Assessment of Cancer Therapy for fatigue (FACT-F). This tool uses a 5-point Likert scale for each question with 0 indicating “not at all” to 4 indicating “very much”, resulting in a possible score range of 0 to 52. Items were scored so that a higher score indicated greater fatigue (Yellen et al., 1997).
Dietary Intake
Diet components were measured via nutritional analysis of 3-day food diaries. Participants were instructed to maintain diet records for two weekdays and one weekend day. Diet diaries were analyzed using Diet Analysis Plus software, version 7.0.1 (Thomson Corporation) for daily kilocalories (kcal), % kcal as fat, as well as carbohydrate and fibre content. Average daily fruit/vegetable servings consumed were also measured (by individual counts). The continuous variables for the individual diet components were analyzed separately. Three of the dietary components (dietary fibre, % kcal as fat, and daily kcal) were also dichotomized as “meeting” or “not meeting” recommendations based on the USDA Dietary Guidelines for 2005 (USDHHS and USDA, 2005). Recommended levels of intake for sedentary women within this age range are ≥ 25g / day of fibre, < 29% kcal as fat, and range of 1600-1700 kcal.
Weight Status and Body Composition
Standing height and weight measurements were completed with participants wearing light-weight clothing and no shoes. Weight and height were measured using mechanical physician beam scale with attached stadiometer (Health-O-Meter #400). Whole-body soft tissue composition was measured by dual-energy X-ray absorptiometry (DXA) using a Lunar Prodigy (software version 5.60.003, Lunar GE, Madison, WI). Fat mass as a percentage of total body mass is used here as “% body fat”. BMI was calculated by dividing body weight (kg) by height (m) squared (kg/m2).
Physical Activity
Physical activity was objectively measured via accelerometer (MT1 ActiGraph; based on both frequency and intensity of activity) for 7 consecutive days with ≥ four valid days required for assessment (Masse et al., 2005). An accelerometer is a motion sensor much like a pedometer that is worn at the waist and measures both frequency and intensity of movement allowing researchers to distinguish between low to moderate to vigorous physical activity. Weekly minutes of moderate plus vigorous physical activity (i.e., cut points of light: <1,952 counts/min; moderate: 1,952-5,724 counts/min; and vigorous: >5,724 counts/min) were dichotomized as meeting (≥150 weekly minutes) or not meeting (< 150 weekly minutes) national physical activity recommendations (Freedson et al., 1998, Haskell et al., 2007). The continuous variable of activity counts was also analyzed.
Data Analysis
Analyses were conducted using SPSS 18.0 for Windows (PASW Statistics, 2010, Chicago, IL). Means and standard deviations (SDs) for variables of interest were calculated. Bivariate correlational analyses were used to determine associations among primary variables of interest. Correlations for the primary dependent variable of interest (fatigue measure) and independent variable of interest (diet quality measures) with potential demographic (age), medical (treatment type, time since breast cancer diagnosis), body composition, and physical activity covariates which may require statistical control were also evaluated. Spearman’s correlations were chosen because of the skewed nature of several of the data distributions. Independent group t-tests were used to compare mean fatigue for the dichotomized diet components of fat, fibre, and kcal. P-values < 0.05 were considered significant.
Results
Participants
All results are mean ± SD, unless otherwise noted. Participants were women aged 53.9 ± 9.1 years old; two were Hispanic and all but three were Caucasian. All women had at least completed high school, while the mean grade completed was 14.6 ± 2.0, and most reported a household income of greater than $50,000 USD. All women were considered to be in remission; with mean time from diagnosis being 37 ± 35 months. Treatments the participants underwent before study participation included chemotherapy (81%), radiation (83%) and surgery (100%) with all being on adjuvant hormonal therapy at time of study enrolment (100%). At baseline, 81% of women reported being post-menopausal, 17% were pre-menopausal, and 2% (one participant) was unsure of menopausal status.
Descriptive statistics for fatigue, diet, body composition, and physical activity are provided in Table 1. On average, participants reported mild to moderate fatigue (mean score of 14.8 ± 10.7; median: 12.0) (Hwang et al., 2002). Participants exceeded recommended daily kilocalorie intake (1863 ± 453 compared with the recommended amount of 1600-1700kcal/day) and dietary fat as a percentage of kcal (35.8% ± 7.0% compared with the recommended amount of <29%/day). Mean intakes of daily dietary fibre and fruit and vegetable servings were well below recommended levels at 16.5g ± 6.5g and 2.7 ± 1.8 servings, respectively; with recommended daily intake levels being 25g of fibre and 9 servings of fruits and vegetables. Mean carbohydrate intake was 216.0g ± 59.0g. Mean % body fat was 44.1 ± 6.9% with a mean BMI of 30.5 ± 8.1 kg/m2 indicating that the sample was, on average, obese (BMI >30). Also related, 17 (40%) of participants had a BMI > 30. While self-report of physical activity by all women at time of enrolment indicated they were performing below recommended levels, objective measurement during baseline assessment using the accelerometer revealed that 29% met recommended levels and average counts per day equalled 208,015 ± 71,026.
Table 1.
Descriptive statistics for fatigue, diet, body composition, and physical activity (n=42)
| Variable | Mean ± SD or n (%) | Range |
|---|---|---|
| Fatigue | 14.8 ± 10.7 | 1.0-44.0 |
| Food energy (daily kcala) | 1863 ± 453 | 896-2793 |
| Fat (% kcal) | 35.8 ± 7.0 | 20-53 |
| Carbohydrate (g) | 216.0 ± 59.0 | 85.0-377.0 |
| Dietary fibre (g) | 16.5 ± 6.5 | 5.8-37.5 |
| Fruit / vegetable (daily servings) | 2.7± 1.8 | 0.0-8.7 |
| Body Fat (%) | 44.1 ± 6.9 | 28.1-54.3 |
| BMlb (kg/m2) | 30.5 ± 8.1 | 19.2-58.4 |
| Counts / day | 208015 ±71026 | 85400-410984 |
| Meets PAc recommendations | 12 (29%) | -- |
kcal – kilocalories;
BMI – body mass index;
PA – physical activity
Correlations among fatigue, diet, body composition, physical activity, demographic, and medical variables
Age, history of chemotherapy, history of radiation therapy, and time since diagnosis were not associated with fatigue or diet components (data not shown; all P > 0.05). Correlation matrix for the remaining variables provided in Table 2. Although the study aim focused on the associations between diet and fatigue, these factors would be expected to be interrelated with body composition and physical activity. Therefore, Table 2 is included to describe possible associations among the factors analyzed which may influence, confound, or assist in explaining our results. Neither body composition measures were associated with fatigue (% body fat: r = −0.03; BMI: r = 0.097) or with any diet measures. However, an association was suggested between BMI and dietary fibre (r = −.29, P = 0.065) and between BMI and kilocalories (r = 0.29, P = 0.060). The physical activity measurements in this sample were not correlated with fatigue (meets physical activity recommendations: r = −0.19; counts / day: r = −0.19). However, meeting physical activity recommendations was positively associated with dietary fibre (r = 0.38) and inversely related to % kcal as fat (r = 0.51).
Table 2.
Unadjusted correlations among fatigue, diet, body composition, and physical activity in breast cancer survivors (n=42)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Fatigue | 1.00 | −0.11 | 0.31 * | −0.31 * | −0.38 * | −0.29 | −0.03 | 0.10 | −0.19 | −0.19 |
| 2. Food energy (kcala) | 1.00 | 0.37* | 0.75** | 0.26 | 0.14 | 0.26 | 0.29 | 0.12 | −0.02 | |
| 3.Fat(%kcal) | 1.00 | −0.16 | -0.32* | -0.33* | 0.07 | 0.07 | −0.22 | -0.51* | ||
| 4. Carbohydrates (g) | 1.00 | 0.55** | 0.44** | −0.01 | 0.05 | 0.19 | 0.17 | |||
| 5. Dietary fibre (g) | 1.00 | 0.56** | −0.26 | −0.29 | 0.19 | 0.38* | ||||
| 6. Fruit/vegetable intake | 1.00 | −0.23 | −0.25 | 0.13 | 0.23 | |||||
| 7. % Body fat | 1.00 | 0.88** | −0.07 | −0.05 | ||||||
| 8. BMIb | 1.00 | −0.02 | 0.01 | |||||||
| 9. Accelerometer counts/day | 1.00 | 0.68** | ||||||||
| 10. Meets PAc recommendations | 1.00 |
kcal - kilocalories;
BMI - body mass index;
PA - physical activity
p < 0.05
p < 0.01
Carbohydrate and daily fibre intake were both inversely related to fatigue (r = −0.31 and r = −0.38, respectively). Percent of kcal from dietary fat was positively associated with fatigue (r = 0.31). Mean daily servings of fruits and vegetables followed this trend but was not statistically significant (r = −0.29, P = 0.058). Total daily kcal was not associated with fatigue (r = −0.11).
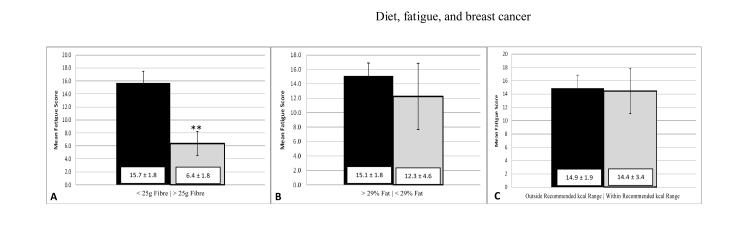
We further analyzed fatigue by dichotomizing three of the diet variables: dietary fibre, % kcal as fat, and kcal into “meeting or not meeting current dietary guidelines”. Presenting mean fatigue in Figure 1 facilitates comparison of the three diet variables with regard to magnitude of fatigue difference and similarity to the “minimal clinically important difference” of 3 units) (Cella et al., 2002) for mean fatigue. As noted in Figure 1, the largest and only significant difference in mean fatigue for the three diet variables was noted for > 25g per day of dietary fibre resulted in an average report of 60% less fatigue than those not meeting dietary guidelines (P = 0.004). Also noted in Figure 1, the association with fatigue was smaller and non-significant for meeting diet recommendations for % of kcal as fat and almost non-existent for daily energy intake (kcal) (P > 0.05).
Figure 1. Mean fatigue (± SEM) for breast cancer survivors who meet or do not meet the specific diet recommendation (n=42).
(A) Breast cancer survivors who met dietary fibre recommendations (>25g fibre / day; N = 4; **P < 0.005) reported significantly less fatigue. (B) There were no differences in reported fatigue between groups breast cancer survivors who met dietary fat recommendations (<29% kcal as fat; N = 4; P = 0.60) or (C) kilocalorie recommendations (1600-1700 kcal; N = 7; P = 0.91).
Discussion
The aim of this study was to examine the associations between measures of diet quality and fatigue in breast cancer survivors, recognizing the potential interactive effects of weight status and physical activity. The most salient findings were that breast cancer survivors who reported less fatigue had a higher dietary quality as assessed by fibre and dietary fat intake. Specifically, participants meeting current USDA recommendations for fibre intake (i.e., > 25 grams/day) reported significantly lower fatigue when compared with those not meeting recommendations with this difference in fatigue scores (i.e., 9 units) exceeding the minimal clinically important difference of 3 units (Cella et al., 2002). Similarly, neither adiposity nor physical activity was directly associated with fatigue; however, participants meeting physical activity recommendations were more apt to report eating less fat and more fibre (noted in Table 2).
Importantly, focusing on diet components in this analysis suggests novel hypotheses related to understanding the aetiology of fatigue in breast cancer survivors and potential intervention targets. To our knowledge, this is the first study to demonstrate that fatigue in breast cancer survivors is related to both higher dietary fibre and lower fat intakes. It is well-established that a poor diet can result in morbidity and mortality in healthy populations (USDHHS and USDA, 2005). The dietary guidelines were established and are regularly updated to reflect current understanding of key role of diet in health (USDHHS and USDA, 2005). One study simply found that the affect of dietary intake on fatigue in breast cancer survivors is difficult to assess (Porock et al., 2005). This may be partially due to, the relationship between diet and fatigue being more important when intakes are deficient or patients are underweight (Porock et al., 2005) as opposed to when patients are meeting dietary needs and are normal weight or obese. However, as previously discussed, dietary intake does have some bearing on fatigue in breast cancer survivors, indirectly through influencing BMI and adiposity (Demark-Wahnefried et al., 2001). The associations between dietary fibre and fatigue as well as dietary fat and fatigue are intriguing. We postulate that the association may be due to these dietary components’ respective influence on inflammation. Dietary fibre has an inverse relationship with circulating inflammatory markers interleukin 6 (IL-6) and tumour necrosis factor α receptor 2 (TNF-α-R2) in postmenopausal women (Ma et al., 2008) as well as C-reactive protein (CRP) in breast cancer survivors (Villasenor et al., 2011). The relationship between dietary fat and inflammation is more complicated as not all dietary fats have the same effect on inflammation. Saturated fats are positively associated with inflammation, while poly-unsaturated fatty acids (PUFAs), such as those ω-3 and ω-6, are inversely related to inflammation. The present data do not allow us to distinguish type of dietary fat. This may explain, in part, the smaller magnitude of fatigue difference noted in Figure 1. However, future work will continue to examine the role of diet on inflammation. Along with others, our previous work has demonstrated that a chronic pro-inflammatory state does contribute to fatigue (Valentine et al., 2011).
The inverse association between carbohydrate intake and fatigue is worthy of discussion. First, carbohydrates are a major source of dietary energy because 49-50% of the average American diet consists of this macronutrient, which is equivalent to ~250g of CHO which is similar to our sample (National Academy of Sciences, 2005). Indeed, in our sample the relation between carbohydrate intake and total daily energy intake was strong (r = 0.79, see Table 2). Second, carbohydrates and fats are often inversely related in the diet with protein intake tending to remain fairly constant in the American diet (National Academy of Sciences, 2005). Often, when fat is reduced in the diet it is replaced with carbohydrates. Therefore, it is conceivable that the association between carbohydrate intake and fatigue reflects either a) reduced fat intake rather than an increase in carbohydrates or b) an increase in energy intake overall. Further research is needed to determine the associations of fatigue with dietary carbohydrate intake independent of fat. Furthermore, carbohydrate sources have variable nutritional value. For example, brown rice provides more fibre than the more refined white rice resulting in slower digestion time and a slower rise in postprandial blood glucose, which potentially aids in more predictable and stable insulin and glucose kinetics (National Academy of Sciences, 2005). Future work is needed to examine whether the type of carbohydrate rather than the total amount plays a more important role in fatigue in breast cancer survivors.
Unlike the diet, the effect of physical activity on fatigue has been well studied. In a meta-analysis conducted to clarify the role of physical activity in cancer survivors, Speck and colleagues suggest that improved fitness level mediates the effect of a physical activity intervention on fatigue (Speck et al., 2010). The association between physical activity and fatigue was not statistically significant in our sample, possibly due to the small sample size. Also, the lack of variability in physical activity resulting from the constraints of the parent grant on recruitment of individuals who were not physically active may have also contributed to this finding. Nevertheless, this lack of variability also facilitated our ability to test associations between diet and fatigue independent of physical activity.
Weight status (high BMI) and adiposity are positively correlated with fatigue in an older independently living population (Valentine et al., 2011). More importantly, previous studies with breast cancer survivors have found similar associations between adiposity and fatigue (Wratten et al., 2004, Winters-Stone et al., 2008, Lim et al., 2005). However, in the present study, these associations were not found. This may have been related to the participants on average, being obese (i.e., lacked sufficient BMI variability) as well as the present sample size being small. Although these limitations may have contributed to the lack of association between fatigue and habitual physical activity and weight status, our results suggest that diet components are associated with fatigue in breast cancer survivor populations that are less physically active and more obese. Moreover, external validity of our results is supported by the fact that the percentage of the sample that was obese is similar to the 38.2% reported for American women aged 40-59 years (Flegal et al., 2010). With regard to physical activity, accelerometer assessment of physical activity in national samples has not been frequently reported limiting comparison. However, the 29% prevalence of meeting physical activity recommendations is higher than the prevalence of meeting recommendations when only bouts of ≥ 10 minutes are counted (i.e., 2.3%) (Troiano et al., 2008) but lower than self-report prevalence of 43% (Macera et al., 2005).
This highlights an on-going conundrum within the energy balance and public health fields, impacting not only breast cancer survivors but nearly all sectors of the population. An adult who consistently exercises often has a higher diet quality, does not smoke, and is not obese. This “health behaviour clustering” is consistent with the associations we report in Table 2 among physical activity, dietary fat and fibre. Because there are known relations among health outcomes (our dependent variable being fatigue), weight status (adiposity), habitual dietary intake and physical activity, disentangling the factors in order to elucidate the primary target intervention to enhance health status, is difficult, if not impossible. Alternatively, it is accepted in public health research and practice that future studies and interventions should simultaneously address these multiple health behaviours to maximize the positive changes in physiological and psychological outcomes (Aziz, 2002). Indeed, the community of obesity researchers and practitioners embrace the importance of both sides of the energy balance equation to successfully manage weight (Center, 2005).
As a healthy lifestyle is known to reduce cancer risk as well as reoccurrence, it is often found that breast cancer diagnosis serves as a teachable moment resulting in improvements in nutritional intake and physical activity (Alfano et al., 2009). Presently, the relatively poor diet quality of the participants, particularly the low intakes of fruits, vegetables and dietary fibre, provides an excellent opportunity for an intervention that would offer benefits in both cancer prevention and improvements in quality of life. Wanchai and colleagues suggest improving quality of life variables, specifically fatigue, through non-pharmacologic therapies is appropriate as medications alone do not appear to be as effective as once thought (Wanchai et al., 2011).
Although this pilot study provides intriguing data on which to build our research agenda aimed at enhancing quality of life in breast cancer survivors, our study, similar to other research, is not without limitations. Our small sample size did not permit multivariate analysis examining the independent associations of the diet components with fatigue. Also, our data distributions suggested a lack of variability in several outcomes (e.g., no participant reported eating at least 9 servings of fruits and vegetables per day). This lack of variability may have interfered with our ability to fully examine the associations among fatigue, diet quality, physical activity and weight status. Similarly, we had a very small number of women meeting dietary intake recommendations (see Figure 1). Again, although not desirable from a statistical analysis perspective, these reported dietary intake values, similar to BMI values, are typical of older women in the United States (Roberts et al., 2005). We believe our findings are important, particularly in regard to dietary fibre, because the difference in reported fatigue presented in Figure 1 is notable. This difference exceeds the value needed for a “minimal clinically important difference” (i.e., 3 units) (Cella et al., 2002). If these associations are consistent, intervention studies examining the effect of diet quality change, in the absence of weight loss, on fatigue may be warranted. Finally, future studies should also consider potential covariates such as depression or sleep quality, both of which are associated with fatigue in breast cancer survivors (Bower, 2008, Bower et al., 2000, Cella et al., 2001, Massie, 2004).
Additional research is needed to better understand the associations among fatigue, body composition, and the related lifestyle behaviours of diet and physical activity. While diet may play a role in fatigue that is experienced by breast cancer survivors, the extent to which improvement in fatigue is possible through lifestyle modifications of diet behaviour is relatively unexplored. Future work is planned to examine the relation between diet quality, specifically dietary fibre, and systemic inflammation as a possible explanation for the elevated fatigue levels experienced by breast cancer survivors. Moreover, the effects of diet quality, independent of habitual physical activity/exercise or weight status, are of interest.
Acknowledgments
Funding: This project was supported by the National Cancer Institute Grant #3R01CA136859-03S1, Southern Illinois University School of Medicine Excellence in Academic Medicine Award (E200634), Brooks Medical Research Fund, and Memorial Medical Center Foundation and Regional Cancer Center.
References
- Alfano CM, Day JM, Katz ML, Herndon JE, 2nd, Bittoni MA, Oliveri JM, Donohue K, Paskett ED. Exercise and dietary change after diagnosis and cancer-related symptoms in long-term survivors of breast cancer: CALGB 79804. Psychooncology. 2009;18:128–133. doi: 10.1002/pon.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz NM. Cancer survivorship research: challenge and opportunity. J Nutr. 2002;132:3494S–3503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- Banning M. Employment and breast cancer: a meta-ethnography. European journal of cancer care. 2011;20:708–719. doi: 10.1111/j.1365-2354.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19:3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- Center NHI. In: Aim for a Healthy Weight. USDHHS, editor. NIH; Bethesda, MD: 2005. [Google Scholar]
- De Jong N. Fatigue in female breast cancer patients: might its origins be more generic than we think? European journal of cancer care. 2011;20:701–702. doi: 10.1111/j.1365-2354.2011.01296.x. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- Donohoe CL, Ryan AM, Reynolds JV. Cancer cachexia: mechanisms and clinical implications. Gastroenterol Res Pract. 20112011:601434. doi: 10.1155/2011/601434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AM. The role of nutrition in the prevention of breast cancer. AACN Clin Issues. 2004;15:119–135. doi: 10.1097/00044067-200401000-00011. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. Jama. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Hanf V, Gonder U. Nutrition and primary prevention of breast cancer: foods, nutrients and breast cancer risk. Eur J Obstet Gynecol Reprod Biol. 2005;123:139–149. doi: 10.1016/j.ejogrb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Hwang SS, Chang VT, Cogswell J, Kasimis BS. Clinical relevance of fatigue levels in cancer patients at a Veterans Administration Medical Center. Cancer. 2002;94:2481–2489. doi: 10.1002/cncr.10507. [DOI] [PubMed] [Google Scholar]
- Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, Dipietro L, Mayne ST, Yu H. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jevtic M, Velicki R, Popovic M, Cemerlic-Adjic N, Babovic SS, Velicki L. Dietary influence on breast cancer. J BUON. 2010;15:455–461. [PubMed] [Google Scholar]
- Lee ES, Lee MK, Kim SH, Ro JS, Kang HS, Kim SW, Lee KS, Yun YH. Health-related quality of life in survivors with breast cancer 1 year after diagnosis compared with the general population: a prospective cohort study. Ann Surg. 2011;253:101–108. doi: 10.1097/sla.0b013e3181f662ce. [DOI] [PubMed] [Google Scholar]
- Lim W, Hong S, Nelesen R, Dimsdale JE. The association of obesity, cytokine levels, and depressive symptoms with diverse measures of fatigue in healthy subjects. Arch Intern Med. 2005;165:910–915. doi: 10.1001/archinte.165.8.910. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hebert JR, Li W, Bertone-Johnson ER, Olendzki B, Pagoto SL, Tinker L, Rosal MC, Ockene IS, Ockene JK, Griffith JA, Liu S. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Nutrition. 2008;24:941–949. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macera CA, Ham SA, Yore MM, Jones DA, Ainsworth BE, Kimsey CD, Kohl HW., 3rd Prevalence of physical activity in the United States: Behavioral Risk Factor Surveillance System, 2001. Prev Chronic Dis. 2005;2:A17. [PMC free article] [PubMed] [Google Scholar]
- Masse LC, Fuemmeler BF, Anderson CB, Matthews CE, Trost SG, Catellier DJ, Treuth M. Accelerometer data reduction: a comparison of four reduction algorithms on select outcome variables. Med Sci Sports Exerc. 2005;37:S544–554. doi: 10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5–13. doi: 10.1007/s10549-007-9831-1. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences I. O. M., Food and Nutrition Board . DRI Report - Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids (Macronutrients) National Academies Press; 2005. [Google Scholar]
- Nccn NCCN. Cancer-Related Fatigue Version 1.2011. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2011 [Google Scholar]
- Porock D, Beshears B, Hinton P, Anderson C. Nutritional, functional, and emotional characteristics related to fatigue in patients during and after biochemotherapy. Oncol Nurs Forum. 2005;32:661–667. doi: 10.1188/05.ONF.661-667. [DOI] [PubMed] [Google Scholar]
- Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv. 2010;4:405–414. doi: 10.1007/s11764-010-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HE, Carter EA, Aloia M, Phillips B. Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: results from the third national health and nutrition examination survey. J Clin Sleep Med. 2006;2:163–169. [PubMed] [Google Scholar]
- Roberts SB, Hajduk CL, Howarth NC, Russell R, Mccrory MA. Dietary variety predicts low body mass index and inadequate macronutrient and micronutrient intakes in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2005;60:613–621. doi: 10.1093/gerona/60.5.613. [DOI] [PubMed] [Google Scholar]
- Rock CL, Demark-Wahnefried W. Nutrition and survival after the diagnosis of breast cancer: a review of the evidence. J Clin Oncol. 2002;20:3302–3316. doi: 10.1200/JCO.2002.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LQ, Hopkins-Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, Hoelzer K, Naritoku C, Edson B, Jones L, Dunnington G, Verhulst S. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009a;18:1410–1418. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- Rogers LQ, Hopkins-Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, Verhulst S, Hoelzer K, Naritoku C, Jones L, Dunnington G, Lanzotti V, Wynstra J, Shah L, Edson B, Graff A, Lowy M. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009b;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- Tramm R, Mccarthy AL, Yates P. Dietary modification for women after breast cancer treatment: a narrative review. Eur J Cancer Care (Engl) 2011;20:294–304. doi: 10.1111/j.1365-2354.2011.01238.x. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, Mcdowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Tyczynski JE, Bray F, Parkin DM. Breast Cancer in Europe. European Network of Cancer Registries (ENCR) Cancer Fact Sheets. 2002;2:1–4. [Google Scholar]
- Usdhhs, Usda . Dietary guidelines for Americans. U.S. Department of Health & Human Services, U.S. Department of Agriculture; Washington, DC: 2005. 2005. [Google Scholar]
- Valentine RJ, Mcauley E, Vieira VJ, Baynard T, Hu L, Evans EM, Woods JA. Sex differences in the relationship between obesity, C-reactive protein, physical activity, depression, sleep quality and fatigue in older adults. Brain Behav Immun. 2009;23:643–648. doi: 10.1016/j.bbi.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Valentine RJ, Woods JA, Mcauley E, Dantzer R, Evans EM. The associations of adiposity, physical activity and inflammation with fatigue in older adults. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Villasenor A, Ambs A, Ballard-Barbash R, Baumgartner KB, Mctiernan A, Ulrich CM, Neuhouser ML. Dietary fiber is associated with circulating concentrations of C-reactive protein in breast cancer survivors: the HEAL study. Breast Cancer Res Treat. 2011;129:485–494. doi: 10.1007/s10549-011-1474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanchai A, Armer JM, Stewart BR. Nonpharmacologic supportive strategies to promote quality of life in patients experiencing cancer-related fatigue: a systematic review. Clin J Oncol Nurs. 2011;15:203–214. doi: 10.1188/11.CJON.203-214. [DOI] [PubMed] [Google Scholar]
- Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35:815–821. doi: 10.1188/08.ONF.815-821. [DOI] [PubMed] [Google Scholar]
- Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O'brien PC, Denham JW. Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys. 2004;59:160–167. doi: 10.1016/j.ijrobp.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]