Abstract
Aims: The G1249A variant of the multidrug resistance-associated protein 2 (ABCC2) gene may be associated with the development of antiepileptic drug (AED) resistance. Although numerous studies have investigated the association between the G1249A variant and the risk of drug resistance in epilepsy, the results of these studies have been inconclusive. To assess the role of G1249A polymorphism in drug resistance in epilepsy, a meta-analysis was performed. Materials and Methods: We systematically reviewed relevant studies retrieved by the PubMed and Embase. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated based on the date extracted from the studies to evaluate the strength of association. We also analyzed the heterogeneity and sensitivity of each report and the publication bias of the studies. Results: A total of 6 published studies, involving 2213 patients (1100 patients with drug-resistant epilepsy and 1113 controls with drug-responsive epilepsy) were reviewed in the present meta-analysis. The overall results indicated that the variant genotypes were associated with a significantly decreased risk of AED resistance (AA vs. GG: OR=0.372, 95% CI=0.182–0.762; recessive model: OR=0.399, 95% CI=0.200–0.795) (fixed-effects model). A stratified analysis by ethnicity showed similar findings for Caucasians in an additive model (A vs. G: OR=0.700, 95% CI=0.494–0.992). Conclusions: The meta-analysis suggests that the ABCC2 G1249A polymorphism is significantly associated with a decreased risk of AED resistance. However, further functional investigations are warranted to validate the association.
Introduction
Epilepsy is considered to be one of the most common and devastating neurological disorders and is characterized by a predisposition to having recurrent uncontrolled seizures (Annegers et al., 1995; Lakhan et al., 2009). Despite the availability of different antiepileptic drugs (AEDs) for therapy, about 30–40% of patients who begin a therapy do not attain seizure control, making drug resistance a major problem for managing epilepsy and creating a substantial public health burden (Kwan and Brodie, 2000; Elger and Schmidt, 2008). The precise reasons why some epilepsy patients fail to respond appropriately to AEDs remain unclear. However, some evidence suggests that ATP-binding cassette (ABC) efflux transporters in the blood–brain barrier (BBB) may contribute significantly to drug resistance in epilepsy by reducing the penetration of AEDs into the brain. Moreover, genetic variants of drug transporter genes, related to functional variations in efflux activity, may be responsible for the interindividual differences in drug resistance (Tishler et al., 1995; Loscher et al., 2009; Cascorbi, 2010).
The association between the overexpression of efflux transporters and the excess efflux of AEDs across the BBB, along with the resulting drug-resistant epilepsy, has been the focus of recent research. ABCC2 (also known asMRP2), located at the membrane of endothelial cells of the BBB, is a member of the ABC superfamily. Members of the ABC superfamily are important for the bioavailability of and response to medication in epilepsy. ABCC2 is also normally expressed in the BBB and may reduce the brain concentration of many AEDs, thereby leading to the AED failure (Sisodiya et al., 2003; Schmidt and Loscher, 2005; Kubota et al., 2006; Remy and Beck, 2006; Lazarowski et al., 2007).
The ABCC2 gene, localized on chromosome 10q23-24, exhibits various genetic polymorphisms (Ito et al., 2001; Itoda et al., 2002; Suzuki and Sugiyama, 2002); these polymorphisms may influence the function of MRPs, resulting in their functional changes (Rau et al., 2006; de Jong et al., 2007; Haenisch et al., 2007). The G1249A (Val417Ile, rs2273697) polymorphism, which is associated with the substitution of Val with an Ile at position 417, is one of the most common polymorphisms in the ABCC2 gene, and its role in epilepsy pharmacoresistance has been the focus of recent research (Ito et al., 2001; Obata et al., 2006; Kim et al., 2010).
It has recently been suggested that an association may exist between the ABCC2 G1249A polymorphism and risk of resistance to AEDs, but the results from various studies on this association remain inconclusive. Ufer et al. (2011) discovered a significant association between the ABCC2 G1249A polymorphism and drug-resistant epilepsy. However, other studies were unable to replicate this finding (Seo et al., 2008; Kim et al., 2009; Ufer et al., 2009; Kwan et al., 2011; Hilger et al., 2012). In view of the uncertainty surrounding this issue and considering the potential importance of this polymorphism for the response to anticonvulsant medication, we carried out this meta-analysis of all the relevant published literature using different genetic model analyses.
Materials and Methods
Search strategy
We conducted a systematic literature search in the PubMed and Embase databases, attempting to identify all published human studies that had investigated the association between the risk of resistance to AEDs and ABCC2 G1249A polymorphisms (the last search update was conducted on March 31, 2013). We used the following Boolean search keywords “(ABCC2 OR Multidrug-resistance protein 2 OR MRP2 OR rs2273697) AND epilepsy.” The relevant articles were retrieved with no language restrictions. In addition, the reference lists of all pooled articles were carefully screened for additional eligible publications. Unpublished data and further information were also obtained from the authors. Only the case–control studies in which data were available on the role of ABCC2 G1249A polymorphism in the risk of resistance to AEDs were selected.
To be included in this meta-analysis, the studies had to (1) be related to AED treatment of patients with epilepsy, (2) have a human case–control design, and (3) have sufficient available data on genotype frequencies for both drug-responsive and drug-resistant patients. Studies were excluded from the analysis if they were (1) not about epilepsy research, (2) not human case–control design, (3) lacking usable data on genotype frequencies, and (4) a duplicate of previous publication.
Data extraction
Two reviewers independently extracted information from all eligible publications that met the inclusion criteria using an extraction template. Any disagreement in the extracted data was resolved by discussion. If a disagreement persisted despite the discussion, a third author was invited to assess those articles. The data extracted from each eligible publication included the following information: the first author's name, the year of publication, the country of origin of the article, the ethnic origin of the studied population (either Caucasian or Asian), the genotyping method, and the genotype and allele distributions for both drug-responsive and drug-resistant patients. We also converted genotype distributions that were reported in percentages to actual numbers and calculated allele frequencies from the corresponding genotype distributions.
Statistical analyses
All statistical analyses were performed using STATA software (version 11; Stata Corporation, College Station, TX). All probability values were two-sided, and p-values less than 0.05 were considered statistically significant. We assessed for violations of Hardy–Weinberg equilibrium (HWE) for each study, using a goodness-of-fit test (chi-square or Fisher's exact test). Based on the genotype frequencies in cases and controls, crude odds ratios (ORs) and their 95% confidence intervals (CIs) were employed to assess the strength of the association between the ABCC2 G1249A polymorphism and the risk of resistance to AEDs in a homozygote comparison (AA vs. GG), a heterozygote comparison (GA vs. GG), and dominant (AA+GA vs. GG), recessive (AA vs. GA+GG), and additive (A vs. G) genetic model comparisons. In addition, we carried out the stratified analyses by ethnicity for all genetic models. Ethnic groups were either Asian or Caucasian. The between-study heterogeneity was evaluated using the chi-squared-based Q-statistic test. When the p-value was greater than 0.05 (indicating that the between-study heterogeneity was not significant), the fixed-effects model that was selected (the Mantel–Haenszel method) (Mantel and Haenszel, 1959). For p-values<0.05 in the heterogeneity test, the pooled ORs were analyzed using the random-effects model (the DerSimonian and Laird method) (DerSimonian and Laird, 1986). We also used the I2 statistic to quantitatively estimate heterogeneity, with I2<25%, 25–75%, and >75% representing low, moderate and high degrees of inconsistency, respectively (Higgins and Thompson, 2002; Higgins et al., 2003). The significance of the combined OR was determined using the Z-test, and p-values<0.05 were considered statistically significant. In addition, we performed sensitivity analyses to evaluate the stability of the results in our meta-analyses through sequentially excluded individual studies. Finally, the Begg's test (Begg and Mazumdar, 1994) and Egger's test (Egger et al., 1997) were also used to statistically assess publication bias (a p-value<0.05 was considered statistically significant).
Results
Characteristics of eligible studies
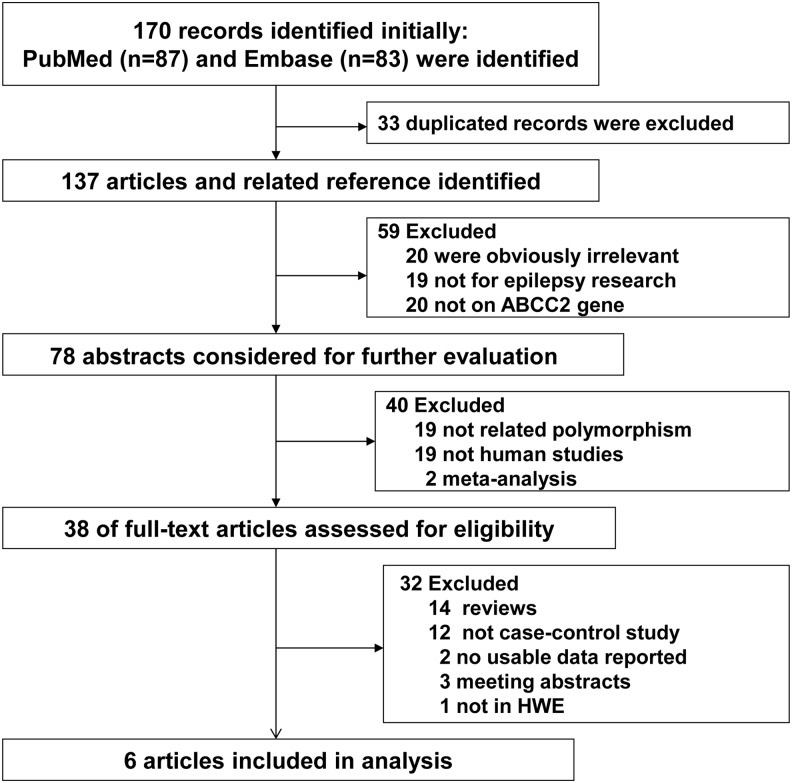
The selection process is shown in Figure 1. After excluding the duplicated records, a total of 137 articles were identified from the initial search in the PubMed and Embase databases. In this screening process, 20 studies were obviously irrelevant. Thirty-nine studies were excluded because they were not related to epilepsy research (19 studies) or were not associated with ABCC2 gene (20 articles). Nineteen articles that were not concerned with ABCC2 G1249A polymorphism were also excluded. Two of the articles were meta-analyses, and 19 of the publications were not human studies. Among the remaining 38 articles, 14 publications were reviews, 3 were meeting abstracts, 12 were not case–control studies, 2 did not present the usable data, and 1 was not in HWE; these studies were also excluded. Finally, a total of 6 published studies, comprising 1113 controls with drug-responsive epilepsy and 1100 patients with drug-resistant epilepsy, concerning G1249A polymorphism in the ABCC2 gene and the risk of resistance to AEDs were included in our meta-analysis (Seo et al., 2008; Kim et al., 2009; Ufer et al., 2009, 2011; Kwan et al., 2011; Qu et al., 2012). The characteristics of the included studies are listed in Table 1. Among the included publications, two studies included participants of Caucasian descent and four studies included participants of Asian descent. All studies indicated that the distribution of genotypes was consistent with HWE. The genotype frequencies of ABCC2 G1249A polymorphism were extracted from all eligible studies, and the main results are listed in Table 2.
FIG. 1.
The selection procedure for included and excluded studies.
Table 1.
Characteristics of Eligible Studies Considered in the Meta-Analysis
| Drug-resistant | Drug-responsive | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | First author | Year | Ethnicity | Genotyping methods | GG | GA | AA | G | A | GG | GA | AA | G | A | HWE |
| 1 | Seo (Japan) | 2008 | Asian | PCR | 102 | 29 | 2 | 233 | 33 | 104 | 35 | 7 | 243 | 49 | 0.087 |
| 2 | Kim (Korea) | 2009 | Asian | RT-PCR | 162 | 35 | 1 | 359 | 37 | 165 | 27 | 1 | 357 | 29 | 0.926 |
| 3 | Ufer (Germany) | 2009 | Caucasian | PCR-RFLP | 66 | 48 | 4 | 180 | 56 | 56 | 37 | 10 | 149 | 57 | 0.298 |
| 4 | Ufer (Germany) | 2011 | Caucasian | PCR | 119 | 48 | 9 | 286 | 66 | 14 | 16 | 2 | 44 | 20 | 0.355 |
| 5 | Kwan (China) | 2011 | Asian | Mass array | 193 | 65 | 0 | 451 | 65 | 255 | 60 | 4 | 570 | 68 | 0.825 |
| 6 | Qu (China) | 2012 | Asian | PCR-RFLP | 181 | 35 | 1 | 397 | 37 | 262 | 56 | 2 | 580 | 60 | 0.593 |
PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism; HWE, Hardy–Weinberg equilibrium; RT-PCR, reverse transcription PCR.
Table 2.
Total and Stratified Analysis of ABCC2 G1249A Polymorphism on the Risk of Drug Resistance in Epilepsy
| AA vs. GG | GA vs. GG | A vs. G | Recessive model | Dominant model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | pa | I2 (%) | OR (95% CI) | pa | I2 (%) | OR (95% CI) | pa | I2 (%) | OR (95% CI) | pa | I2 (%) | OR (95% CI) | pa | I2 (%) |
| Total | 0.372 (0.182–0.762) | 0.916 | 0.0 | 0.977 (0.704–1.355)b | 0.046 | 55.7 | 0.914 (0.761–1.098) | 0.104 | 45.3 | 0.399 (0.200–0.795) | 0.827 | 0.0 | 0.976 (0.795–1.199) | 0.056 | 53.6 |
| Ethnicity | |||||||||||||||
| Asian | 0.357 (0.123–1.041) | 0.731 | 0.0 | 1.133 (0.893–1.437) | 0.310 | 16.2 | 1.010 (0.814–1.252) | 0.239 | 28.9 | 0.356 (0.123–1.035) | 0.726 | 0.0 | 1.075 (0.852–1.358) | 0.260 | 25.3 |
| Caucasian | 0.388 (0.148–1.013) | 0.666 | 0.0 | 0.646 (0.212–1.966)b | 0.021 | 81.1 | 0.700 (0.494–0.992) | 0.206 | 37.5 | 0.441 (0.178–1.092) | 0.368 | 0.0 | 0.699 (0.453–1.078) | 0.052 | 73.5 |
The results that are in bold type show statistical significance.
p-Value of Q-test for heterogeneity test.
Random-effects model was used when p-value for heterogeneity test<0.05; otherwise, fixed-effects model was used.
CI, confidence interval; OR, odds ratio.
Meta-analysis results
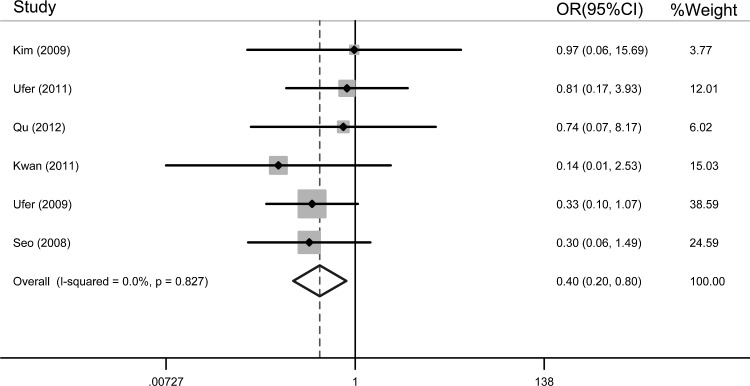
Table 2 shows the association between ABCC2 G1249A polymorphism and the risk of drug resistance in epilepsy. Overall, the results indicated that ABCC2 AA genotype was associated with a significantly decreased risk of AED resistance (AA vs. GG: OR=0.372, 95% CI=0.182–0.762; recessive model: OR=0.399, 95% CI=0.200–0.795) (fixed-effects model). Homozygous carriers of variant allele of the ABCC2 1249G>A polymorphism contributed greatly to the response to AEDs. Figure 2 shows the overall meta-analysis of the ABCC2 G1249A polymorphism and the risk of drug resistance in epilepsy in the recessive model.
FIG. 2.
Overall meta-analysis of the ABCC2 G1249A polymorphism and the risk of drug resistance in epilepsy in the recessive model (odds ratio [OR]=0.399, 95% confidence interval [CI]=0.200–0.795) (the fixed-effects model).
In the analysis stratified by ethnicity, being Caucasian was associated with a significantly reduced risk of drug resistance in additive model (A vs. G: OR=0.700, 95% CI=0.494–0.992) (fixed-effects model). However, this association was not found among Asians in the additive model (A vs. G: OR=1.010, 95% CI=0.814–1.252). In addition, we failed to find any association between the G1249A polymorphism in the ABCC2 gene and response to anticonvulsant drugs in the other genetic models. Table 2 depicts the results of the other genetic comparisons models.
Test of heterogeneity
There was significant heterogeneity among the studies in the heterozygote comparison (GA vs. GG: pheterogeneity=0.046, I2=55.7%), but not in the homozygote comparison (AA vs. GG: pheterogeneity=0.916, I2=0.0%), the recessive model comparison (AA vs. GA+GG: pheterogeneity=0.848, I2=0.0%), the additive model comparison (A vs. G: pheterogeneity=0.104, I2=45.3%), and the dominant model comparison (AA+GA vs. GG: pheterogeneity=0.056, I2=53.6%). In the analysis by the ethnicity, heterogeneity was not observed for the Asian group in the heterozygote comparison (GA vs. GG: pheterogeneity=0.310, I2=16.2%). Heterogeneity was observed for the Caucasian group, however (GA vs. GG: pheterogeneity=0.021, I2=81.1%).
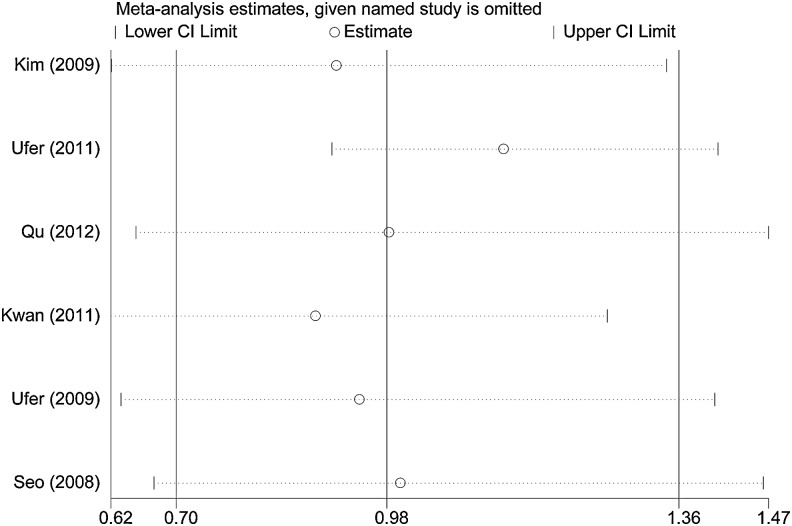
Sensitivity analysis
We performed sensitivity analyses to assess the influence of each individual study on the pooled OR by sequentially removing each eligible study (Fig. 3). The results indicated that the independent study conducted by Ufer et al. (2011) made the greatest contribution to the heterogeneity. This heterogeneity was effectively removed by excluding this study from the analysis. In addition, we did not find that any single study influenced the quality of the pooled ORs, suggesting that the results of this meta-analysis were stable and reliable.
FIG. 3.
Results of the cumulative meta-analysis examining the association between the ABCC2 G1249A polymorphism and the risk of drug resistance in epilepsy in the heterozygote comparison model.
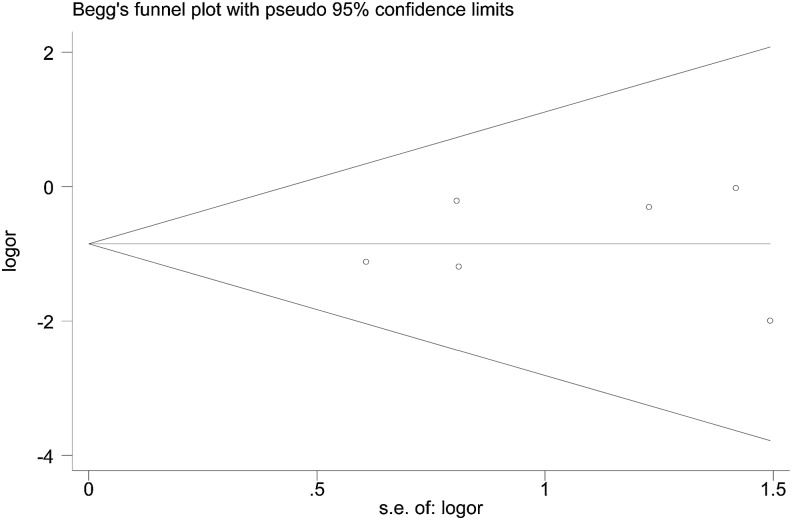
Publication bias
The shape of the funnel plot seemed symmetrical, suggesting that there was no obvious publication bias. In addition, the results of Egger's test showed no significant publication bias in this meta-analysis (t=0.34, p=0.748 for AA vs. GA+GG, Figure 4).
FIG. 4.
Funnel plot to determine publication bias of the meta-analysis of the association between the G1249A variant and the risk of drug resistance in epilepsy in the recessive model.
Discussion
Epilepsy, which is characterized by its significant morbidity and mortality, affects ∼0.5–2.0% of the general population (Annegers et al., 1995; Lakhan et al., 2009). About one in three of patients with epilepsy fails to respond to medication, as a result of the resistance to AEDs, constituting a significant public health challenge (Kwan and Brodie, 2000; Sills et al., 2005; Elger and Schmidt, 2008). Recent studies have confirmed that multidrug resistance-associated protein (ABCC2), an important member of the ABC efflux transporters, when overexpressed in the BBB in patients with epilepsy, could limit the penetration of AEDs into the brain and reduce the intracellular accumulation of the drug, leading to pharmacoresistance in epileptics (Dombrowski et al., 2001; Sisodiya et al., 2003; Schmidt and Loscher, 2005; Kubota et al., 2006; Remy and Beck, 2006; Lazarowski et al., 2007; Szakacs et al., 2008).
Single-nucleotide polymorphisms (SNPs) are extremely common sources of human genetic variation, and pharmacogenetic studies have revealed that such miniscule variation in genome sequence could influenced the drug disposition and pharmacokinetics, thereby affecting individual response to treatment (Sun et al., 2010). Recent studies have identified numerous polymorphisms of the ABCC2 gene. The G1249A variant, a G to A base change that causes a substitution of isoleucine for valine at position 417 in the ABCC2 gene, has been suggested to influence ABCC2 function (Ito et al., 2001; Itoda et al., 2002; Suzuki and Sugiyama, 2002). Kim et al. (2010) confirmed that this polymorphism reduced the ABCC2 transportation of carbamazepine, one of the most commonly used first-line drugs in epilepsy. In addition, research has demonstrated that the functional effects of the combination of other SNPs in the haplotypes could lead to an alteration of MRP protein expression. Thus, the gene–gene interactions might play an important role in the functional alteration of the MRP protein (Laechelt et al., 2011; Grover et al., 2012).
Overall, this research is a systematic review to evaluate the association between G1249A polymorphism in the ABCC2 gene and the risk of developing drug resistance in epilepsy. Our meta-analysis of eligible case–control studies, comprising a total of 2213 patients (1100 patients with drug-resistant epilepsy and 1113 patients with drug-responsive epilepsy), indicated that ABCC2 G1429A polymorphism was associated with a significant decreased risk of drug resistance in epilepsy in a homozygote comparison (AA vs. GG) and a recessive genetic model comparison (AA vs. GA+GG). A stratified analysis by ethnicity reviewed a similar association for Caucasians but not for Asians in additive comparison model, suggesting that this polymorphism plays different roles in populations with different genetic backgrounds and from different living environments. However, we failed to draw a clear conclusion using other genetic effects models.
In addition, the Egger's test and funnel plot suggested that there was no publication bias for the association between this polymorphism and drug resistance risk in epilepsy in this meta-analysis.
There may be limitations to the results of this meta-analysis. First, the number of published studies in our meta-analysis was insufficient, and the studies with small samples may not have had enough statistical power for evaluating the association. Second, the complex gene–gene or gene–environment interactions, as well as the different polymorphism of the same gene, may contribute to response to AEDs among epileptics. Third, heterogeneity between the included studies, which might affect the results of the meta-analysis, was detected in the heterozygote comparison model. Despite these limitations, our meta-analysis maintains some validity. First, a systematic review of the relationship between ABCC2 G1249A polymorphism and response to AEDs has more statistical powerful compared with any single study. Moreover, the studies that enrolled in our meta-analysis statistical strictly met the selection criteria set. Furthermore, no publication bias was shown, indicating that there was no bias among the pooled results.
In summary, our meta-analysis showed that the ABCC2 G1249A polymorphism is significantly associated with decreased risk of resistance to AEDs through a homozygote comparison and a recessive genetic comparison model. This association was also present in the Caucasian subpopulation. However, additional well-designed functional studies, particularly studies investigating gene–gene and gene–environment interactions, need to be conducted, to further understand the association between ABCC2 polymorphism and the risk of drug resistance in epilepsy.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (grant 81172694); the Grant for the 135 Key Medical Project of Jiangsu Province (No. XK201117); the practice innovation training program projects for the Jiangsu College students; and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author Disclosure Statement
The authors declare they have no conflicts of interest.
References
- Annegers JF, Hauser WA, Lee JR, et al. (1995) Incidence of acute symptomatic seizures in Rochester, Minnesota, 1935–1984. Epilepsia 36:327–333 [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101 [PubMed] [Google Scholar]
- Cascorbi I. (2010) ABC transporters in drug-refractory epilepsy: limited clinical significance of pharmacogenetics? Clin Pharmacol Ther 87:15–18 [DOI] [PubMed] [Google Scholar]
- de Jong FA, Scott-Horton TJ, Kroetz DL, et al. (2007) Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther 81:42–49 [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188 [DOI] [PubMed] [Google Scholar]
- Dombrowski SM, Desai SY, Marroni M, et al. (2001) Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 42:1501–1506 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, et al. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger CE, Schmidt D. (2008) Modern management of epilepsy: a practical approach. Epilepsy Behav 12:501–539 [DOI] [PubMed] [Google Scholar]
- Grover S, Gourie-Devi M, Bala K, et al. (2012) Genetic association analysis of transporters identifies ABCC2 loci for seizure control in women with epilepsy on first-line antiepileptic drugs. Pharmacogenet Genomics 22:447–465 [DOI] [PubMed] [Google Scholar]
- Haenisch S, Zimmermann U, Dazert E, et al. (2007) Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J 7:56–65 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, et al. (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger E, Reinthaler EM, Stogmann E, et al. (2012) Lack of association between ABCC2 gene variants and treatment response in epilepsy. Pharmacogenomics 13:185–190 [DOI] [PubMed] [Google Scholar]
- Ito S, Ieiri I, Tanabe M, et al. (2001) Polymorphism of the ABC transporter genes, MDR1, MRP1 and MRP2/cMOAT, in healthy Japanese subjects. Pharmacogenetics 11:175–184 [DOI] [PubMed] [Google Scholar]
- Itoda M, Saito Y, Soyama A, et al. (2002) Polymorphisms in the ABCC2 (cMOAT/MRP2) gene found in 72 established cell lines derived from Japanese individuals: an association between single nucleotide polymorphisms in the 5′-untranslated region and exon 28. Drug Metab Dispos 30:363–364 [DOI] [PubMed] [Google Scholar]
- Kim DW, Lee SK, Chu K, et al. (2009) Lack of association between ABCB1, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res 84:86–90 [DOI] [PubMed] [Google Scholar]
- Kim WJ, Lee JH, Yi J, et al. (2010) A nonsynonymous variation in MRP2/ABCC2 is associated with neurological adverse drug reactions of carbamazepine in patients with epilepsy. Pharmacogenet Genomics 20:249–256 [DOI] [PubMed] [Google Scholar]
- Kubota H, Ishihara H, Langmann T, et al. (2006) Distribution and functional activity of P-glycoprotein and multidrug resistance-associated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res 68:213–228 [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319 [DOI] [PubMed] [Google Scholar]
- Kwan P, Wong V, Ng PW, et al. (2011) Gene-wide tagging study of the association between ABCC2, ABCC5 and ABCG2 genetic polymorphisms and multidrug resistance in epilepsy. Pharmacogenomics 12:319–325 [DOI] [PubMed] [Google Scholar]
- Laechelt S, Turrini E, Ruehmkorf A, et al. (2011) Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J 11:25–34 [DOI] [PubMed] [Google Scholar]
- Lakhan R, Misra UK, Kalita J, et al. (2009) No association of ABCB1 polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy Behav 14:78–82 [DOI] [PubMed] [Google Scholar]
- Lazarowski A, Czornyj L, Lubienieki F, et al. (2007) ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia 48(Suppl 5):140–149 [DOI] [PubMed] [Google Scholar]
- Loscher W, Klotz U, Zimprich F, et al. (2009) The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia 50:1–23 [DOI] [PubMed] [Google Scholar]
- Mantel N, Haenszel W. (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748 [PubMed] [Google Scholar]
- Obata H, Yahata T, Quan J, et al. (2006) Association between single nucleotide polymorphisms of drug resistance-associated genes and response to chemotherapy in advanced ovarian cancer. Anticancer Res 26:2227–2232 [PubMed] [Google Scholar]
- Qu J, Zhou BT, Yin JY, et al. (2012) ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci Ther 18:647–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau T, Erney B, Gores R, et al. (2006) High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther 80:468–476 [DOI] [PubMed] [Google Scholar]
- Remy S, Beck H. (2006) Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain 129:18–35 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Loscher W. (2005) Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia 46:858–877 [DOI] [PubMed] [Google Scholar]
- Seo T, Ishitsu T, Oniki K, et al. (2008) ABCC2 haplotype is not associated with drug-resistant epilepsy. J Pharm Pharmacol 60:631–635 [DOI] [PubMed] [Google Scholar]
- Sills GJ, Mohanraj R, Butler E, et al (2005) Lack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and response to antiepileptic drug treatment. Epilepsia 46:643–647 [DOI] [PubMed] [Google Scholar]
- Sisodiya SM, Martinian L, Scheffer GL, et al. (2003) Major vault protein, a marker of drug resistance, is upregulated in refractory epilepsy. Epilepsia 44:1388–1396 [DOI] [PubMed] [Google Scholar]
- Sun N, Sun X, Chen B, et al. (2010) MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 65:437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. (2002) Single nucleotide polymorphisms in multidrug resistance associated protein 2 (MRP2/ABCC2): its impact on drug disposition. Adv Drug Deliv Rev 54:1311–1331 [DOI] [PubMed] [Google Scholar]
- Szakacs G, Varadi A, Ozvegy-Laczka C, et al. (2008) The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox). Drug Discov Today 13:379–393 [DOI] [PubMed] [Google Scholar]
- Tishler DM, Weinberg KI, Hinton DR, et al. (1995) MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia 36:1–6 [DOI] [PubMed] [Google Scholar]
- Ufer M, Mosyagin I, Muhle H, et al. (2009) Non-response to antiepileptic pharmacotherapy is associated with the ABCC2-24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics 19:353–362 [DOI] [PubMed] [Google Scholar]
- Ufer M, von Stulpnagel C, Muhle H, et al. (2011) Impact of ABCC2 genotype on antiepileptic drug response in Caucasian patients with childhood epilepsy. Pharmacogenet Genomics 21:624–630 [DOI] [PubMed] [Google Scholar]