Abstract
Endogenous electric fields are instructive during embryogenesis by acting to direct cell migration, and postnatally, they can promote axonal growth after injury (McCaig 1991, Al-Majed 2000). However, the mechanisms for these changes are not well understood. Application of an appropriate electrical stimulus may increase the rate and success of nerve repair by directly promoting axonal growth. Previously, DC electrical stimulation at 50 mV/mm (1 mA, 8 h duration) was shown to promote neurite outgrowth and a more pronounced effect was observed if both peripheral glia (Schwann cells) and neurons were co-stimulated. If electrical stimulation is delivered to an injury site, both the neurons and all resident non-neuronal cells [e.g., Schwann cells, endothelial cells, fibroblasts] will be treated and this biophysical stimuli can influence axonal growth directly or indirectly via changes to the resident, non-neuronal cells. In this work, non-neuronal cells were electrically stimulated, and changes in morphology and neuro-supportive cells were evaluated. Schwann cell response (morphology and orientation) was examined after an 8 h stimulation over a range of DC fields (0–200 mV/mm, DC 1 mA), and changes in orientation were observed. Electrically prestimulating Schwann cells (50 mV/mm) promoted 30% more neurite outgrowth relative to co-stimulating both Schwann cells with neurons, suggesting that electrical stimulation modifies Schwann cell phenotype. Conditioned medium from the electrically prestimulated Schwann cells promoted a 20% increase in total neurite outgrowth and was sustained for 72 h poststimulation. An 11-fold increase in nerve growth factor but not brain-derived neurotrophic factor or glial-derived growth factor was found in the electrically prestimulated Schwann cell-conditioned medium. No significant changes in fibroblast or endothelial morphology and neuro-supportive behavior were observed poststimulation. Electrical stimulation is widely used in clinical settings; however, the rational application of this cue may directly impact and enhance neuro-supportive behavior, improving nerve repair.
Introduction
Hundreds of thousands of injuries to the peripheral nervous system (PNS) are reported annually in Europe and in the United States and are often caused by traumatic events (e.g., car accidents) or disease.1–3 Severe injuries may require surgical intervention with 50,000–200,000 performed annually.4,5 Injuries leaving small gaps in a nerve (<3 cm; small gap injury) are often able to spontaneously re-grow with or without surgical intervention; however, re-growth is limited in large-gap injuries >2–4 cm.6–8 Autografts are the current standard treatment for large-gap injuries, but only 50% of autograft-treated patients achieve full functional recovery and are at increased risk of co-morbidity.7–9 For large gap injuries (>4 cm), there are limited options and even autografts have low recovery rates, which may be partially attributed to a nonoptimal scaffold (e.g., the use of a sensory nerve graft for mixed or motor nerve repair).7,10 Due to limited functional recovery for large-gap injuries as well as a lack of available donor tissue, nerve guidance channels have been investigated since the 1800s.11 These guidance channels, however, remain inferior to natural autografts, highlighting the need for further research.11
To restore function, injured neurons should extend axons through the injury site to reach proper innervation targets. This repair is often impeded by scarring, apoptosis, and an unsupportive microenvironment at the injury site.9 Poor regeneration in large-gap injuries is accompanied by little or no Schwann cell (SC) re-population, supporting the hypothesis that Schwann cell participation and presence at the wound site is a rate-limiting factor in large-gap PNS repair.7,12–14 Schwann cells support re-growing axons through the release of soluble neurotrophic factors, removal of inhibitory myelin debris, expression of neuro-supportive surface ligands, and re-myelination of the re-grown axons.15–18 Due to the noted importance of Schwann cell participation in peripheral nerve repair, increases in neuro-supportive factors secreted by the Schwann cells may serve to enhance axonal growth through a large-gap injury.
Axonal re-growth is influenced by a multitude of exogenous factors (e.g., controlled release of neurotrophic factors, external mechanical or biophysical forces, and topographic features).9,19–21 In vivo, both neurons and non-neuronal cells are exposed to a naturally occurring endogenous current (biophysical cue) created by the epithelium during development and postnatally after injury.21,22 In the developing embryonic chick, electrical cues are known to be instructive to cell migration and tissue formation, and if disrupted, they can lead to abnormal limb bud formation.22 Furthermore, if sensitivity to this cue is retained postnatally, electrical stimulation may serve to enhance nerve repair.22–29
Application of exogenous electrical stimuli to peripheral nerves in adult rats in vivo have been shown to accelerate the rate of axonal regeneration, but not overall functionality, in both animal and human nerve injury models. In axotomized and repaired rodent nerve hind limb models, 1 h to 2 weeks of continuous electrical stimulation (20 Hz, 100 μS duration; 0.5–5 V amplitude) resulted in accelerated axonal regeneration.30–34 Electrical stimulation for longer than 1 h did not accelerate neuron regeneration, indicating an indifference to the duration of the biophysical cue.33 In these model systems, axonal regeneration is accompanied by increases in neurotrophins such as brain-derived neurotrophic factor (BDNF) and BDNF receptor (TrkA).30,33,34 It is not clear in these complex in vivo studies how electrical stimulation impacts non-neural support cells (Schwann cells, fibroblasts, and endothelial cells) that will also be resident in the injury site and may be influencing neuronal extension. While the effects of electrical stimulation to influence neuronal growth have been well characterized, changes to non-neuronal cells have not been explored.
When translated to treat human carpal tunnel syndrome (CTS) after surgical release of the transverse carpal ligament, bipolar electrical stimulation with similar parameters (20 Hz, 100 100 μS duration, and variable amplitude) was found to accelerate sensory and motor nerve regeneration with BDNF signaling, indicated as a major player in neuronal extension.35,36 However, the unstimulated control populations also recovered similar levels of functional recovery, but at a slower rate. While promising, this study utilized variable amplitudes to stimulate the median nerve, making it difficult to deduce the definitive local stimuli experienced by not only the neurons but also the resident support cells. Furthermore, these crush injuries are known to spontaneously regenerate without intervention due to the presence of intact guidance cues such as basal lamina and the presence of Schwann cells. It is unclear whether this method will translate to humans for large-gap or more complex nerve injuries. Large-gap injuries may have differences in wound size, influencing local signal attenuation within the tissue, intrinsic differences in cell responsiveness due to age, type and species, and/or nonoptimal presentation due to a limited mechanistic understanding of the downstream cellular changes after electrical stimulation.13,27,30–33,37
We recently reported a two-fold increase in neurite outgrowth after the exogenous application of a low-level DC electrical stimulation of 50 mV/mm (1 mA), with neurite extension similar in magnitude to unstimulated neurons grown in co-culture with growth-supportive Schwann cells.38 Furthermore, a 3.2-fold enhancement in outgrowth was found after the co-presentation of both electrical stimulation and Schwann cells relative to unstimulated neuron controls.38 There are no gross changes to Schwann cell morphology immediately after stimulation. At higher nonphysiological stimulations of 500 mV/mm (AC, 1 Hz), Huang et al. reported that electrically stimulated neonatal rat Schwann cells released a 1.6-fold greater amount of nerve growth factor (NGF) than unstimulated controls,39 and supplementation of NGF alone did not completely explain the increased outgrowth observed.38 Rather, it is likely that exogenous electrical stimulation alters the Schwann cell phenotype, potentially impacting growth factor(s) production (and/or release), changes to cell adhesion molecules presented to re-growing axons, and/or improved support cell migration and repopulation of the injury site. Moreover, it raises the question, are these changes Schwann-cell specific or does electrical stimulation have a global impact on other resident non-neuronal cell phenotypes?
To address this question, non-neural cells (Schwann cells, endothelial cells, and fibroblasts) were electrically stimulated (50 mV/mm, DC, 8 h, and 1 mA), and changes to both morphology and neuro-supportive behavior were quantified. A constant DC stimulus was applied to examine whether field and/or cathodal/anodal bias impacts support cell orientation. Electrically stimulated, non-neural cells supporting increased neurite outgrowth were evaluated by both the temporal collection of cell-conditioned medium for (1) quantitative analysis of neurotrophin release and (2) changes in neurite outgrowth after exposure to cell-conditioned medium. The characterization and mechanistic understanding of electrically induced, neuro-supportive changes in non-neuronal cells will aid the rational application of electrical stimulation to assist nerve repair.
Materials and Methods
Isolation and culture of non-neural cells
Schwann cell isolation and purification
Primary Schwann cells were isolated from Sprague—Dawley postnatal day 2 (P2) neonatal rat sciatic nerves as previously described.38,40,41 Schwann cells migrating out of the explant tissue were purified using an anti-mitotic agent (10−5 M cytosine arabinoside [Sigma Aldrich]) and complement-mediated cell lysis.38,42 Purified Schwann cells were expanded in growth medium (Dulbecco's-modified Eagle's medium [DMEM; Mediatech, Inc.], 10% fetal bovine serum [Hyclone], 2 mM l-glutamine [Hyclone], and 50 U/mL penicillin/streptomycin [Mediatech, Inc.] supplemented with 6.6 mM Forskolin [Sigma Chemical] and 10 μg/mL Bovine Pituitary Extract [BD Biosciences]). The Schwann cells were determined to be 98%–99% pure, assessed by immunostaining with S100 (rabbit anti-S100 primary antibody (1:400 v:v in 2.5% goat serum [GS, CellGrow]; Dako North America, Inc.), a Schwann cell-specific marker. The cells are cryopreserved at 1×106 cells/mL in freezing medium (base medium supplemented with 10% dimethyl sulfoxide [Sigma Aldrich]) for use on demand. Schwann cells ranging from passage 4 to 10 were used for all experiments.
Fibroblasts
Primary fibroblasts were isolated from P2 neonatal rat skin from pups utilized for Schwann cell described earlier. The skin isolated from the torso was placed in Ham's F-12 in 150 mm Petri dishes (Mediatech, Inc.), was minced using sterile instruments to ∼2×2 mm pieces, dissociated in a trypsin-collagenase solution (0.1% trypsin [Mediatech, Inc.], and 1 mg/mL collagenase A in 1× Hanks Balanced Salt Solution [HBSS]) for 50 min at standard culture conditions (37°C, 5% CO2) with light mixing every 15–18 min. The tissue was pelleted and digested a second time in 0.1% trypsin for 10 min, rinsed in DMEM before mechanical trituration using a flame-polished pasture pipette. The dissociated cells were seeded in base medium and expanded before cryopreservation in freezing medium at 5×105 cells/mL. The cultures are purely fibroblasts, as any keratinocytes are not supported due to their relatively slow proliferation and absence of essential growth factors within the medium. Fibroblasts from passage 4 to 10 were utilized for experiments in this study and grown in base medium in standard culture conditions as described earlier.
Endothelial cells
A microvascular endothelial cell line (m.Bend.3) was purchased (American Type Culture Collection). All endothelial cells were used from passage 5–10 and grown in base medium in standard culture conditions. Primary endothelial cells are difficult to isolate and maintain. Endothelial cell lines are routinely used in both tissue engineering and vascular biology.
For all support cell types utilized in experiments, passage numbers were selected from 4–10 to allow expansion of both primary Schwann cells and fibroblasts as well as purchased endothelial cells while minimizing changes to cellular phenotype.
Neuron isolation
Neurons were isolated from P2 neonatal rat dorsal root ganglion (DRG) (Sprague—Dawley, P2; Taconic Farms, Inc.) as previously described.38,41,43 The collected DRG were digested in trypsin and collagenase solution (0.1% trypsin [Mediatech, Inc.] and 1 mg/mL collagenase A [Sigma Aldrich] solution diluted in 1×HBSS [Fisher Scientific]). The tissue was further digested in 0.1% trypsin and mechanically triturated in base medium. The dissociated cells were cryopreserved at a concentration of 1×106 cells/mL in freezing medium.43
Culture chamber design and construction
The custom electrical stimulation culture chambers utilized in this work are described in Koppes et al.38 based on previously described chambers used in McCaig and Rajnicek,21 Pedrotty et al.44 In brief, a nonconductive Sylgard 184 poly (dimethylsiloxane) base and curing agent (PDMS; Dow Corning) were mixed (10:1 wt./wt.) and allowed to cure in rectangular polystyrene plates (Thermo Fisher Scientific). Two separate chambers (7×30×1.5 mm) were excised from the PDMS to act as both the experimental and control chambers. Salt bridges (2% agarose in Steinberg's solution)45 flank each culture chamber on the plate to minimize cell exposure to any electrical byproducts or pH changes. An external agar salt bridge was applied to the anodal side of the chamber to add resistance in the system.46 Two biological-grade platinum electrodes (one anode, one cathode; 1 mm ID each; Sigma Aldrich) were inserted to apply voltage to the experimental chamber from a bench-top DC power supply (Marlin P. Jones & Assoc., Inc.). Biological-grade platinum wire electrodes were placed in each chamber, serving to measure the voltage drop across as detected by SCC-FT01 feed through components continuously using LabView (National Instruments). To minimize external noise during data collection, the system is grounded and lead shielding encases the instruments. The signal is continuously processed during stimulation via a moving average low-pass filter sampled at a frequency of 10 Hz, averaged once per second, and plotted using LabView software. The stimulation is checked and if voltage spikes or drops >2% of the set point, the sample is not used as a part of the data set.
Electrical stimulation of non-neural cells
Acid-etched glass coverslips (Belco Glass) were coated with 100 μg/mL of poly-l-Lysine (Sigma Aldrich) to allow cell attachment, and rinsed with sterile 1× Phosphate-Buffered Saline (PBS; Cambrex). Schwann cells, endothelial cells, and fibroblasts were seeded at 32,000 cells/cm2 in six-well tissue culture dishes and incubated for 12 h in growth medium at standard culture conditions. The sub-confluent population of cells permits visualization of individual cellular morphology and orientation. Before stimulation, the cell-seeded coverslips were placed in each chamber (experimental and control) containing warm growth medium, and incubated (37°C, 5% CO2) for 30 min.
The experimental chamber was stimulated with a constant current DC voltage (1 mA) to generate field strengths from 0 to 200 mV/mm for 8 h to treat Schwann cells. The control chamber remains unstimulated (0 mV/mm) for the duration of the experiment. The nonconductive polymer insulates the control unstimulated samples from any potential current leakage. Immediately, postelectrical stimulation, the power supply is disconnected from the stimulation chambers and samples are moved to fresh growth medium under standard culture conditions. Thus, current leakage is not a factor in our experimental stimulation device. To characterize any changes in cell morphology due to electrical stimulation, Schwann cells were visualized immediately after stimulation or 12 h poststimulation. The samples were fixed with a 4% paraformaldehyde, 4% sucrose (Sigma Aldrich) in 2×PHEM buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 2 mM MgSO4, pH 7.0 with KOH in DI H2O; Sigma Aldrich) for 30 min.
Based on an optimized response of neurons (maximum neurite outgrowth) Koppes et al.38 and Schwann cell alignment (see results), endothelial cells and primary fibroblast cultures were stimulated with 50 mV/mm for 8 h. Changes in morphology were investigated as described earlier.
Neurite outgrowth on electrically stimulated cells
To investigate neurosupportive phenotypic changes, Schwann cells, fibroblasts and endothelial cells were electrically stimulated with 50 mV/mm for 8 h and placed in fresh medium for an additional 12 h (37°C, 5% CO2). This treatment was based on maximal outgrowth for neurons and Schwann cell-neuronal co-cultures previously reported.38 Twelve hours poststimulation, primary dissociated DRG neurons were seeded at 13,000 cells/cm2 on electrically prestimulated cells or on the unstimulated paired controls. This density of neurons allowed visualization of individual neuronal morphology. The medium was changed 4 h postseeding to remove any nonadherent neurons and was replaced with fresh growth medium supplemented with 25 ng/mL NGF. The neurons were cultured for an additional 8 h (12 h in total poststimulation) before fixation, as described earlier in section Electrical stimulation of non-neural cells.
Neurite outgrowth in electrically stimulated Schwann cell conditioned medium
Immediately poststimulation, the medium was changed for both the stimulated and paired controls. Cell-conditioned medium was collected in full at 12 h intervals and replaced with fresh medium until 84 h poststimulation. The collected Schwann cell-conditioned medium was centrifuged at 100g, aliquoted, and stored at −20°C for no more than 1 month for cell culture experiments or ELISA analysis.
To determine the contribution of any Schwann cell-produced soluble factors impacting neurite outgrowth, dissociated DRG neurons were seeded at a density of 13,000 cells/cm2 on laminin-coated coverglass (50 μg/mL of laminin; BD Biosciences) cultured in growth medium supplemented with 25 ng/mL NGF. Neurons were allowed to adhere for 4 h before a gentle rinse with warm, 1×PBS to remove nonadherent cells. Schwann cell-conditioned medium with no supplemental NGF was collected from either the experimental, electrically stimulated cells (8 h; 50 mV/mm) or control, unstimulated cells (0 mV/mm). Conditioned medium collected immediately poststimulation (0 h), 0–12 h, or 60–72 h intervals poststimulation was used to treat the neurons to determine whether any temporal differences in neurotrophic soluble factors secreted by the Schwann cells existed. Neurons were cultured in the Schwann cell-conditioned medium in the absence of any supplemental NGF for 8 h before fixation.
ELISA assay of growth factor secretion
Schwann cells were plated and stimulated as described earlier (Electrical stimulation of non-neural cells). 50 mV/mm was chosen, as it maximized neurite outgrowth and poststimulation promoted the greatest re-alignment of Schwann cells.38 Medium was collected from Schwann cells immediately postelectrical stimulation (0 h) and in 12 h intervals until 84 h poststimulation (3.5 days). The Schwann cell-conditioned medium was stored at −20°C for no more than 30 days and used on demand.
Common Schwann cell-produced neurotrophins NGF, BDNF, and Glial-Derived Growth Factor (GDNF) were measured to determine changes in release by the Schwann cells in response to the electrical stimulus. ELISA kits were utilized as per manufacturer's instructions (Rat GDNF ELISA Kit, Rat BDNF ELISA Kit, Rat NGF ELISA Kit; Insight Genomics). The plates were read at 450 nm and analyzed using an InfiniteM200 (Tecan). The total amount of NGF, BDNF, and GDNF per mL secreted by Schwann cells was calculated based on a plate standard curve.
Immunofluorescent staining
Fixed samples were rinsed thrice with warm PBS and permeabilized for 5 min with 0.1% Triton X-100 (Sigma Chemical) in PBS. All samples were then blocked to prevent background staining with 2.5% GS in PBS (Invitrogen) for 1 h at room temperature.
Neurons
To visualize neurons, the samples were incubated for 1 h at room temperature with mouse anti-β-III-tubulin primary antibody (1:500 in 2.5% GS) and later rinsed thrice for 5 min each in HBSS. Samples were incubated for 1 h at room temperature with goat-anti-mouse Alexa Fluor 488 IgG2b secondary antibody (Invitrogen) at room temperature (1:1000 v:v in 2.5% GS).
Non-neural cells and co-cultures
After blocking, the neuron and support cell (Schwann cells, fibroblasts, and endothelial cells) co-cultures were incubated with mouse-anti-β-III-tubulin primary antibody for 1 h to label neurons. After primary antibody incubation, the co-culture samples were rinsed thrice in HBSS for 5 min. Goat-anti-mouse IgG2b Alexa Fluor 488 (Invitrogen; 1:1000 v:v in 2.5% GS) secondary antibody and rhodamine phalloidin (1:1000 in 2.5% GS) were then applied for 1 h at room temperature to visualize all cells.
All labeled samples were rinsed thrice for 5 min in PBS before mounting on a glass slide (Fisher) with Prolong Gold Anti-fade containing 4′,6-diamidino-2-phenylindol (Invitrogen) to label all cell nuclei; mounted slides were stored at −20°C before imaging.
Microscopy and image analysis
Samples were imaged using an Olympus IX81 inverted microscope (Olympus) with a 10× dry objective as previously described, and images were acquired with Metamorph image acquisition software38 (Molecular Devices). For the non-neural cell samples, five images were taken at evenly spaced, predetermined locations across both the experimental and control chamber, avoiding edges. For the neurons and neuron-support cell co-cultures, 20 individual neurons (not contacting adjacent neurons) were imaged using a 20× dry objective.38
Support cell orientation for sub-confluent cultures was approximated by measuring the orientation of nuclei using NIH ImageJ 1.41 (National Institutes of Health) as previously described.41,47 Images of nuclei were inverted, thresholded, and best-fit ellipses were assigned to each nucleus. The major and minor axis for each ellipse was determined, and the resultant nuclear orientation was calculated. Nuclear orientation angles were binned every 10°, and the frequency distribution was plotted between 0° and 180°, with 90°, representing the direction of the electric field.48 The frequency distributions were generated per experiment. Cell number was quantified by counting nuclei per image using ImageJ. Neuronal morphology was quantified using Neurolucida software (MBF Bioscience) as previously described in detail.43 This software allows quantification of neuronal morphometrics, including total neurite outgrowth, number of primary neurites, number of branch points, length of longest neurite, and directionality of neurite outgrowth.
Statistics
Schwann cell morphology data were calculated from three separate experiments (n=3) for each treatment condition (0, 10, 50, 100, 150, and 200 mV/mm), with five images per sample, and three samples per experiment. Fibroblast and endothelial cell experiments were also collected from three separate experiments (n=3) at a field strength of 50 mV/mm. Neuron-support cell co-culture electrical stimulation data were collected from three separate experiments (n=3) with 10 neurons per condition per experiment randomly selected from all the individual neurons imaged. ELISAs were repeated in triplicate for each growth factor (NGF, BDNF, and GDNF), with duplicates of each time point (m=2, n=3).
Statistical significance was determined using Excel to perform a two-tailed ANOVA. p-values<0.05 were considered statistically significant. A minimum of 10 neurons per experimental treatment was calculated using a Student's t-test in order for each experiment to have a 99% power to detect (α=0.05) a maximum difference of 251 μm within total outgrowth of control (unstimulated) neuron populations, assuming an average variability of 143 μm based on preliminary experiments as previously described.38
Results
Electrical stimulation of non-neural cells induces morphological changes in Schwann cells but not endothelial cells or fibroblasts
Application of electric stimulation delivered to an injury site will influence all resident cells, including neurons and non-neuronal cells (e.g., Schwann cells, endothelial cells, and fibroblasts). To investigate cellular changes to the non-neuronal cells, Schwann cells were treated for 8 h with a DC stimulation ranging from 0–200 mV/mm and exhibited a varied response to treatment. Schwann cells exposed to 10 mV/mm appeared morphologically similar to the unstimulated control cells (0 mV/mm; Fig. 1A, B); however, at higher stimuli of 25–75 mV/mm, the Schwann cells exhibited morphological differences that were only manifesting during the 12 h poststimulation (Fig. 1C–E), but not during stimulation or immediately after treatment.38 The stimulated Schwann cells exhibited an elongated, bipolar morphology (Fig. 1C–E). At 50 mV/mm, some of the Schwann cells reorganized, forming clusters of cells, aligning parallel to the previously applied electric field (Fig. 1D). These clusters varied in length and alignment, but were only observed after stimulation with 50 mV/mm, again only manifesting within the 12 h poststimulation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). At higher stimuli ranging from 100–150 mV/mm, no significant changes in morphology were observed relative to unstimulated controls (Fig. 1D–G); however, abnormal cell morphology was observed along with a 37% decrease in cell number after a stimulus of 200 mV/mm (Fig. 1H, Supplementary Fig. S2). 200 mV/mm represents the upper limits of previously reported physiologic field strengths measured during development and wound healing.21–29,37 Based on this evidence, the magnitude should be maintained at <200 mV/mm, and/or duration of stimulus should be <8 h for viable Schwann cells (Supplementary Fig. S2).
FIG. 1.
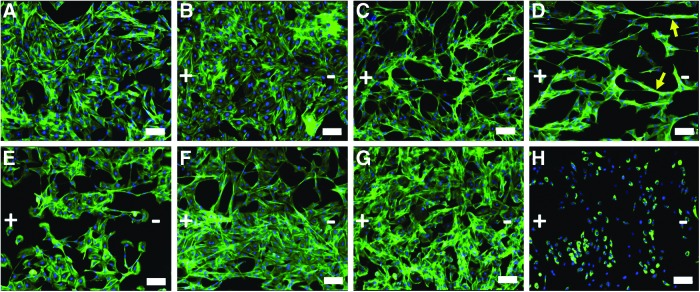
Schwann cell response to DC electrical stimulation. Schwann cells were stimulated for 8 h in DC fields of (A) 0 mV/mm (control), (B) 10 mV/mm, (C) 25 mV/mm (D) 50 mV/mm, (E) 75 mV/mm, (F) 100 mV/mm, (G) 150 mV/mm, and (H) 200 mV/mm (direction of EF is shown in the above image). Significant changes in morphology were observed at 25 and 50 mV/mm with the highest degree of Schwann cell alignment and clustering at 50 mV/mm. In contrast, (H), cells stimulated at 200 mV/mm appeared abnormal compared with the unstimulated controls (A). Samples were fixed 12 h culture period poststimulation. Schwann cell cultures were stained with phalloidin to visualize actin (green) cytoskeleton and 4′,6-diamidino-2-phenylindol (DAPI) to visualize the nuclei (blue). Despite the morphological differences in Schwann cells at 50 mV/mm, similar cell densities were found compared with other electrically stimulated cell populations. At 200 mV/mm, Schwann cell density is reduced and cells depict an atypical morphology (Supplementary Fig. S2). Arrows denote clusters in (D). Images were taken at 10×, bar=200 μm. Color images available online at www.liebertpub.com/tea
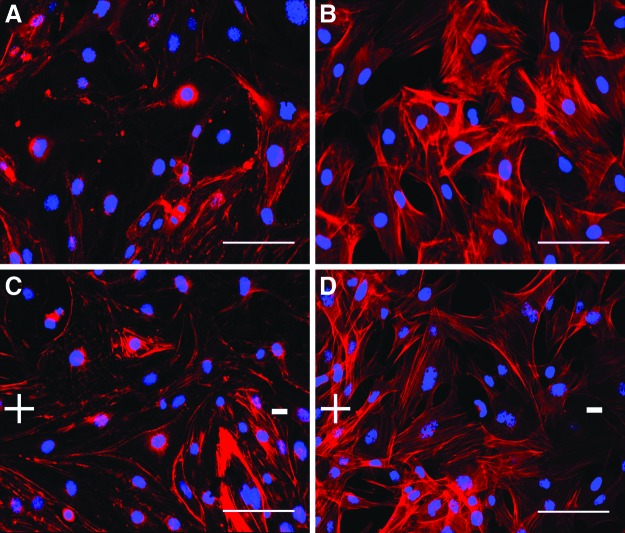
Due to the observed clustering and reorganization of Schwann cells relative to the applied field as well as previously reported increase in neurite outgrowth at 50 mV/mm, both primary fibroblasts and endothelial cells were examined at this optimized field strength (50 mV/mm; Fig. 2). Unlike the Schwann cells (Fig. 1D), both the primary endothelial cells and fibroblasts did not exhibit any apparent morphological changes or re-organization either immediately after stimulation or 12 h poststimulation (Fig. 2). No changes in cell number or viability were observed.
FIG. 2.
Representative images of endothelial cells and fibroblasts 12 h post DC stimulation (50 mV/mm). Both the electrically stimulated endothelial cells (A, C) and fibroblasts (B, D) appeared morphologically similar to unstimulated paired controls. Samples were fixed and stained with phalloidin for actin (red) cytoskeleton and DAPI for nuclei (blue). Images were taken at 20×, bar=100 μm, direction of electric field is shown. Color images available online at www.liebertpub.com/tea
Schwann cells, but not endothelial cells and fibroblasts exhibit a directional bias after electrical stimulation
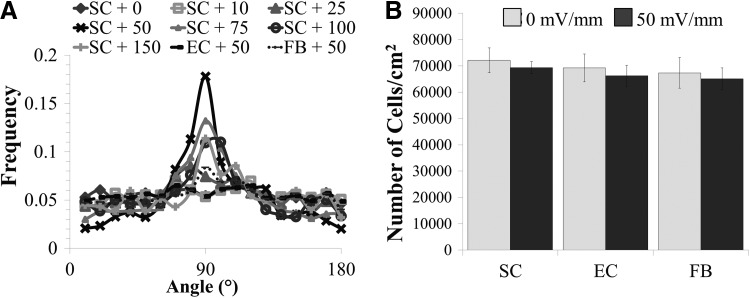
To quantify directional bias after electrical stimulation, cell orientation was plotted as a frequency distribution and is in agreement with the qualitative findings.38,49 Results indicate that at low fields of 0–10 mV/mm or high fields (100–150 mV/mm), Schwann cells do not exhibit any significant bias in any direction (Fig. 3A). After stimulation in a moderate field strength of 25–75 mV/mm, Schwann cells exhibited bias parallel to the previously applied electric field with 50 mV/mm eliciting the most dominant response (Fig. 3A). At 50 mV/mm, 47% of Schwann cells were aligned parallel to the previously applied electrical stimulus (+/−10° of 90°). Interestingly, this bias is only observed in the 12 h poststimulation, and not during stimulation or immediately after stimulation (Supplementary Fig. S138). Neither endothelial cells nor fibroblasts exhibited any significant changes in alignment during or after stimulation (Fig. 3A). Regardless of the cell type examined (Schwann cells, endothelial cells, and fibroblasts), all electrically stimulated cultures exhibited no significant differences in cell density from their paired controls (Fig. 3B, p>0.05).
FIG. 3.
Non-neural alignment and number poststimulation. (A) The frequency distribution of Schwann cell orientation measured using ImageJ after 12 h poststimulation exhibits maximal bias at 50 mV/mm parallel to the previously generated electric field, while endothelial cells and fibroblasts do not exhibit a directional bias. 90° represents the direction of the previously applied electric field. (B) At 50 mV/mm, no significant changes were observed in cell density of stimulated Schwann cells (SC), endothelial cells (EC), and fibroblasts (FB) in comparison to unstimulated paired controls. Thresholded and binarized cell nuclei were counted before analysis of major angle for each 10× image captured. n=3, standard deviation shown.
Electrically prestimulated Schwann cells support greater neurite outgrowth
Poststimulation, Schwann cells exhibit differences in morphology and alignment, as shown in Figures 2 and 3. In a previous work, we demonstrated that co-stimulation of neurons and Schwann cells resulted in a significant increase in outgrowth, presumably due to the stimulus-induced changes to the neurons and indirectly via changes to the Schwann cells.38 It is unclear whether this enhancement is Schwann cell specific or indicative of all non-neural cells localized within a PNS injury site. To isolate any non-neuronal electrically induced neuro-supportive changes, non-neuronal cells (Schwann cells, endothelial cells, and fibroblasts) were exogenously stimulated (DC 50 mV/mm for 8 h) and placed in standard culture (0 mV/mm) overnight before the addition of unstimulated sensory neurons.
After 12 h of growth on prestimulated non-neural support cells, neurite outgrowth was visualized. Qualitatively, Schwann cells supported the most robust neurite outgrowth, regardless of electrical stimulation (Fig. 4A, B, Supplementary Fig. S3) in comparison to either endothelial cells (Fig. 4C, D) or fibroblasts (Fig. 4E, F). The electrically prestimulated Schwann cells supported longer neurites compared with the already highly supportive, unstimulated Schwann cell controls (Fig. 4A, B). In contrast to the Schwann cells, electrically stimulated endothelial cells or fibroblasts did not positively or negatively alter neuronal growth compared with unstimulated paired controls (Fig. 4E, F, Supplementary Fig. S3).
FIG. 4.
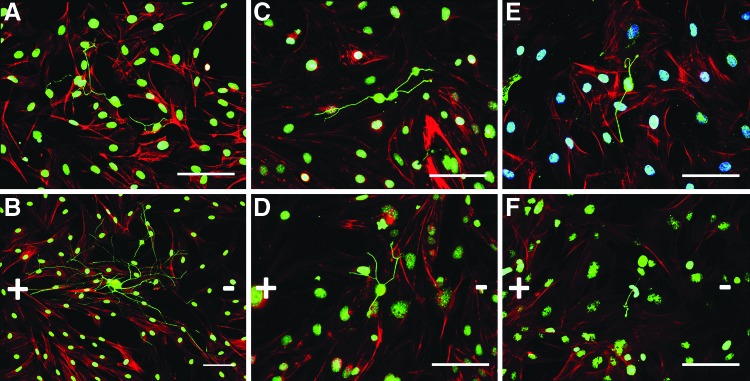
Electrically prestimulated Schwann cells support the greatest neurite outgrowth. Neurite outgrowth was investigated in electrically prestimulated support cells (Schwann cells, endothelial cells, and fibroblasts). The non-neural cells were stimulated in a 50 mV/mm DC field for 8 h and placed in standard culture (12 h @ 0 mV/mm) before co-culture with neurons. Schwann cells (A, B) supported the greatest outgrowth, irrespective of electrical stimulation. Electrically stimulated Schwann cells (B) appear to support greater neurite outgrowth in comparison to unstimulated Schwann cell controls (A). In contrast, no visible differences were evident for neurons on prestimulated endothelial cells (D) or fibroblasts (F) relative to paired controls (C, E). Direction of the electrical field (+/−) is depicted above. Green=anti-β-III-tubulin (neurons), Red=Phalloidin actin cytoskeleton (all cells), Blue=DAPI (nuclei); bar=100 μm, n=3. Color images available online at www.liebertpub.com/tea
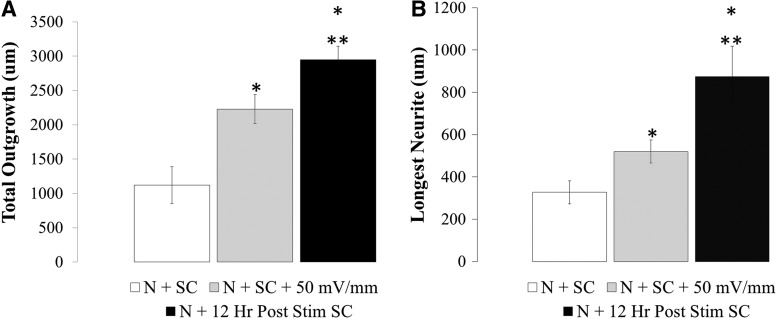
To quantify neurite extension from neurons cultured with electrically prestimulated Schwann cells (Fig. 4), neuron morphometrics were examined with Neurolucida software. Results indicate that the prestimulated Schwann cells (N+12 h Post Stim SC, Black) supported a significant 3.2-fold increase in total neurite outgrowth and 2.8-fold longest neurite relative to paired unstimulated Schwann cells (N+SC, White) (Fig. 5A, p<0.05). Interestingly there is a benefit to prestimulating Schwann cells before co-culture with neurons compared with our previously reported concurrent stimulation of neurons with Schwann cells, resulting in a 1.3-fold increase in neurite outgrowth and a 1.7-fold increase in longest neurite being relative to co-stimulation of neurons and Schwann cells (N+SC+50 mV/mm, Grey)38 (Fig. 5). This increased outgrowth occurs without changes in branching or number of primary neurites (not shown). Unlike Schwann cells, no significant benefit was observed for neurons grown on the electrically stimulated endothelial cells or fibroblasts relative to unstimulated, paired controls (Supplementary Fig. S3.).
FIG. 5.
Total neurite outgrowth (A) and longest primary neurite (B) are significantly enhanced on electrically prestimulated Schwann cells relative to their co-stimulated and unstimulated Schwann cell counterparts. The prestimulated Schwann cells were exposed to 50 mV/mm for 8 h (DC, 0.01 mA) followed by 12 h at 0 mV/mm in co-culture with neurons. Co-stimulated neuron-Schwann cells were treated with an 8 h electrical followed by 4 h at 0 mV/mm. In all cases, the neurons were allowed to remain in co-culture with Schwann cells for 12 h to allow a direct comparison between the conditions. *p<0.05 compared with N+SC, **p<0.05 compared with all conditions, n=3 for all conditions. Error bars represent standard deviation.
Electrically stimulated Schwann cell-conditioned medium elicits sustained increases in neurite outgrowth
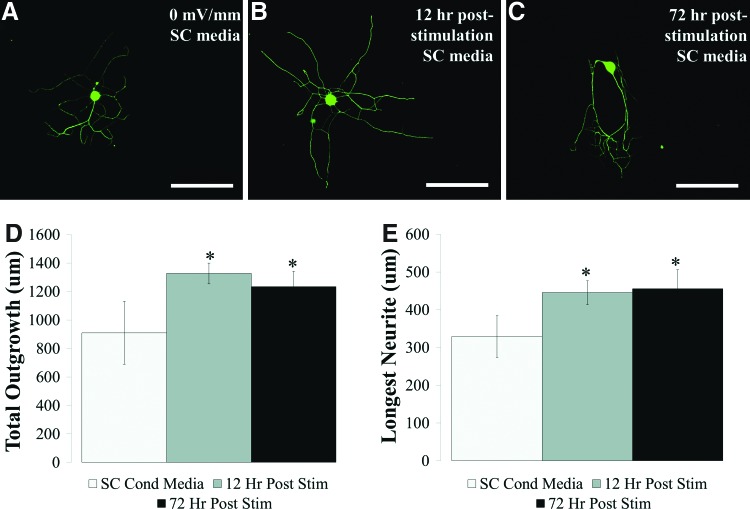
Neurite outgrowth is influenced by electrical stimulation38 and a further increase in outgrowth was observed after contact with either prestimulated or co-stimulated Schwann cells. Schwann cell-mediated neurosupportive behavior may be attributed to soluble factor release and/or surface ligand presentation. To explore changes in soluble factor release as one possible mechanism, conditioned medium collected from electrically stimulated and unstimulated Schwann cells was collected every 12 h poststimulation. Schwann cells are known to produce neurotrophic factors to support neurite outgrowth; however, electrically induced changes to the Schwann cell phenotype may enhance neurotrophin release.16 Neurons cultured in Schwann cell-conditioned medium collected from prestimulated Schwann cells at either 12 (0–12 h poststimulation) or 72 h (60–72 h poststimulation) without any supplemental NGF appeared visually larger than neurons grown in control, paired unstimulated Schwann cell-conditioned medium (Fig. 6A–C). Figure 6 suggests that the conditioned medium contains neurotrophic factors and appears for at least 3 days poststimulation. Using Neurolucida to quantify neuronal morphometrics, there is a 1.5-fold increase in outgrowth and a 1.4-fold increase in the longest neurite compared with the unstimulated paired controls 12 h poststimulation (Fig. 6D, E). Similar increases are sustained 72 h poststimulation with both a 1.4-fold increase in total outgrowth and longest neurite relative to medium collected from the unstimulated paired control.
FIG. 6.
Neurite outgrowth is enhanced in the electrically stimulated Schwann cell conditioned medium. Representative immunofluorescent images of neurons grown for 12 h in Schwann cell-conditioned medium collected from (A) unstimulated (control, 0 mV/mm) (B) 0–12 h poststimulation and (C) 60–72 h poststimulation. Medium was changed immediately after stimulation, replaced with fresh medium, and collected at 12 h intervals to maintain comparable samples. Both total neurite outgrowth (D) and the longest primary neurite (E) increase in the conditioned medium collected at either the 0–12 or 60–72 h poststimulation relative to control. *p<0.05 to control Schwann cell conditioned medium (0 mV/mm), n=3 for all conditions. Error bars represent standard deviation. Color images available online at www.liebertpub.com/tea
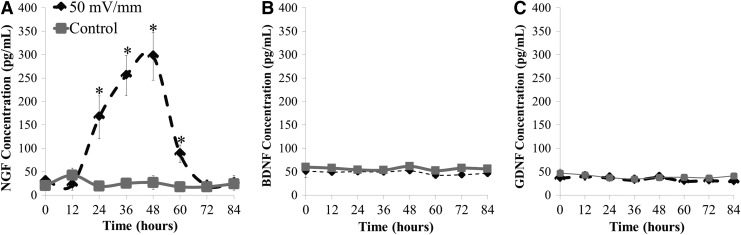
To identify differences in Schwann cell-produced factors poststimulation, the cell-conditioned medium was analyzed via ELISA against common neurotrophins (NGF, BNDF, and GDNF). Both the control and electrically stimulated Schwann cells secrete these three common neurotrophins. A single 8 h electrical stimulus (50 mV/mm) was sufficient to elicit a sustained increase in NGF. This increase manifests 12 h poststimulation and remains significantly elevated compared with paired, unstimulated control Schwann cells for more than 60 h poststimulation before returning to basal levels by 72 h. (Fig. 7A; p<0.05). Similar changes in NGF but not morphology were observed for cells stimulated for only 4 h (data not shown). Figure 7 presents data from 0–84 h poststimulation, as intervals after 84 h did not exhibit changes from the unstimulated paired controls. We report that the electrically prestimulated Schwann cells release approximately 11.1-fold more NGF than in the control, unstimulated Schwann cells (296.7 pg/mL vs. 26.7 pg/mL; p<0.05). By 84 h poststimulation, NGF returns to baseline levels. No differences were observed in either BDNF or GDNF release as a result of the applied electrical stimulus (Fig. 7B, C).
FIG. 7.
A significant increase in Schwann cell-produced nerve growth factor was sustained for more than 60 h after a single 8 h stimulation (50 mV/mm) (A). In contrast, no changes in either brain-derived neurotrophic factor (B) or glial-derived neurotrophic factor (C) were observed. Schwann cell conditioned medium was collected from electrically stimulated cells (50 mV/mm; 8 h; 1 mA) (T=0), and every 12 h for approximately 1 week poststimulation. *p<0.05 to control Schwann cell-conditioned medium (0 mV/mm); m=3, n=3 for all conditions. Error bars represent standard deviation. NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor.
Discussion
Current treatment options for peripheral nerve repair are limited and large-gap injuries typically do not regain full function, motivating the development of new therapies to promote nerve re-growth. In vivo and in vitro studies provide evidence of robust growth after electrical stimulation; however changes to resident non-neuronal cells after electrical stimulation are not well characterized.30,31,33,36,50 Our goal sought to evaluate the sensitivity of neural and non-neuronal cells to electrical cues in vitro that could be translated as one feature in a novel guidance channel for improved repair of large-gap injuries. We report that 12 h after exogenous electrical stimulation, Schwann cells, but not endothelial cells or fibroblasts, exhibit moderate alignment to the applied field, but no bias is observed during or immediately poststimulation. Significant increases in neurite outgrowth and length are reported for neurons cultured on exogenously prestimulated Schwann cells in comparison to neurons and Schwann cells that are concurrently stimulated. These increases are, in part, attributed to Schwann cell-secreted factors, including an 11-fold increase in NGF release poststimulation that remains elevated for approximately 3 days. An a priori knowledge of electrically mediated neurosupportive/inhibitory changes would be important to guide the rational implementation of electrical stimulation and motivate temporal delivery of the exogenous stimulation to treat nerve injury.
The stimuli evaluated in this study (0–200 mV/mm) lies within the physiological range of endogenous fields measured (21–140 mV/mm) during embryogenesis and/or after injury.21–24,28,51 Schwann cells exhibit varied degrees of responsiveness to the applied stimulus. Twelve hours poststimulation, changes to Schwann cell morphology were readily observed (25–75 mV/mm)38 with Schwann cells exhibiting a moderate bias, with 47% aligning parallel to the applied field (Figs. 1, 3), but no differences were evident during or immediately after stimulation. 50 mV/mm elicited the greatest alignment parallel to the previously applied field (Fig. 3A) with cells clustering and re-organizing to varying degrees from an evenly seeded sub-confluent monolayer (Fig. 2D, Supplementary Fig. S2). Shorter stimulation durations of 1 and 4 h followed by an unstimulated overnight incubation were insufficient to elicit any change in alignment (not shown). In contrast, presentation of lower (10 mV/mm) and higher (100–150 mV/mm) field strengths had no effect on alignment or morphology. The biased Schwann cell alignment may be due to the polarization of positively- and negatively charged surface ligands and proteins in the cell membrane, inducing change in cell shape after the applied stimulus.52,53 DC stimulation was used to see whether anodal/cathodal differences existed and none were observed. Studies are currently underway to understand the underlying mechanism in guiding the electrically mediated changes of cell alignment and/or re-organization/clustering. Sensitivity to exogenous stimulation is observed in a variety of cell types, specifically astrocytes (glia from the central nervous system) align perpendicular to high, nonphysiological fields of 500 mV/mm, within 24 h.25,54 Control of cellular alignment through electrical stimulation in the absence of topographical cues or chemical gradients would be an important tool to manipulate cells in vivo.
While the electrically stimulated Schwann cells exhibited a modest directional bias, aligning parallel to the previously applied field (Fig. 3), neurons exhibited no significant directional preference. In these experiments, the Schwann cells are sub-confluent and only moderately aligned (47%). The extending neurites respond to local cell orientation as well as the presence or absence of Schwann cells, which is likely too modest to globally guide all neurites. The ability to manipulate glial orientation with a biophysical cue is exciting, but further work is needed to more robustly control glial alignment. Application of a repeated exogenous electrical stimulation may serve to efficiently direct alignment without the need to insert a biomaterial containing an oriented topography or soluble factor delivery to generate gradients to direct axonal re-growth.
Increases in neurite extension were observed in electrically prestimulated Schwann cells (50 mV/mm, 8 h, 1 mA, and 12 h recovery) with 1.3- and 3.2-fold greater total outgrowth compared with concurrently stimulated neurons and Schwann cells or unstimulated co-culture of neurons and Schwann cells, respectively (Fig. 5A38). Schwann cells are known to have a neurosupportive phenotype that appears from this study to be further enhanced by electrical stimulation.16,38,55 Enhancement of neurosupportive changes triggered by stimulation became more potent with time in comparison to concurrently stimulated cells. Based on the conditioned medium collected from prestimulated Schwann cells, there is either an up-regulation or increased release of neurotrophic factors to, in part, support the robust outgrowth measured that is sustained for more than 3 days poststimulation. Huang et al. stimulated Schwann cells with a high, nonphysiologic AC stimulus (500 mV/mm, 1 Hz) and reported a 1.6-fold, calcium-dependent increase in NGF release relative to unstimulated, control Schwann cell.39 Increases in both brain-derived growth factor (BDNF) and BDNF receptor tyrosine receptor kinase B (trkB) mRNA were reported to increase in neurons after the high, but brief electrical stimulation of repaired rat and mouse femoral nerve injuries (100 μs, 0.5–5 V, 20 Hz, 1 h).30,33,34,50 BDNF increases were observed as early as 1 h post stimulation, with a significant three-fold increase seen 8 h poststimulation. Growth factor release increases transiently over time, reaching 6-fold (BDNF) and 4.5-fold (trkB) levels by 2 days poststimulation before returning to the unstimulated sham levels.30 Axonal transection and subsequent repair to the motor nerve are known to up-regulate BDNF and trkB mRNA, but the electrical stimulation results in a benefit observed over multiple days.30,56–61 These studies only focused on the axonal response to the electrical stimulus, and it remains unknown whether an altered phenotype, such as those reported from Schwann cells here, are also playing a role in the acceleration of axonal extension in vivo.
In our study, we observe increases in neurite outgrowth on prestimulated Schwann cells as well as in Schwann cell-conditioned medium isolated 0–72 h poststimulation (Fig. 6). Our results and previous work together support the hypothesis that electrical stimulation promotes soluble neurotrophic factor release. Here, an 11-fold increase in NGF was observed at 48 h and significant increases were sustained over 3 days poststimulation, but no significant changes to BDNF and GDNF were found as measured by ELISA. Both control and electrically stimulated Schwann cells release BDNF and GDNF cytokines as expected (Fig. 7). Since all experiments are paired with controls, electrical stimulation is responsible for the increased NGF release. The BDNF increases reported in Al-Majed et al. in vivo was an injury of a motor nerve, and there are noted differences between motor and sensory Schwann cells.30,31,62 Furthermore, injury itself is noted to increase growth factor release, including BDNF.30,31,61,63–65 Schwann cells release all three neurotrophins in vitro and in vivo; however, postinjury NGF is up-regulated first with BDNF reaching a maximum at 4 weeks postinjury, suggesting that the two growth factors act through different pathways and are induced differently.66–70 Our observed differences in neurotrophin induction may be due to the relatively short in vitro experiment or a less complex response of exogenously stimulated, nonmyelinating Schwann cells stimulated in the absence of sensory neurons, or phenotypic differences due to Schwann cell maturity and/or sensory/motor origin.62 It is unlikely that NGF is the only factor altered via electrical stimulation, as medium containing the additional NGF does not promote the full benefit that we observe with the conditioned medium. Other soluble factors may be at play, and a broader panel of growth factors will be explored in the future. Differences are expected from that of intact nerves where there is considerable cross-talk between neurons and myelinating glia.71 Both our results and those by Huang et al. and Al-Majed et al.30,39 report that neurite outgrowth is mediated, in part, by soluble factor release; however, cell contact also likely plays a role in the increased outgrowth. While changes to surface ligands need to be considered and may play a role, they are beyond the scope of this study.72–77
Since the electrical stimulation would be nonspecifically applied to the entire injury site, we extended this study to briefly evaluate changes to other resident cells (fibroblast and endothelial cells). A wide variety of cell types such as karatocytes, epithelial cells, avian glia, and hippocampal neurons are sensitive to exogenous electrical stimulation and exhibit varied responses such as galvanotaxis toward the cathode or neuronal turning toward the anode or cathode.52,78–86 In this work, primary fibroblasts and an endothelial cell line exhibited no visible changes in morphology during, immediately after, or poststimulation. Unlike electrically stimulated Schwann cells, these prestimulated non-neural support cells did not enhance or inhibit neurite extension (Fig. 2, Supplementary Fig. S3). While it is possible that the cells may be responsive (alignment or neurosupportive phenotype) given a different magnitude or duration of stimulus, our long-term goal is related to nerve repair; therefore we were only interested in testing parameters known to be neurosupportive of neurite outgrowth.38
In this article, electrical stimulation of Schwann cells in vitro enhanced an already neuro-supportive phenotype. Results indicate that a single electrical stimulation of Schwann cells manifests in extended soluble factor release which supports increased outgrowth over 3 days poststimulation, and this increase can be reproduced with a second stimulation at 4 days (data not shown). Therefore, repeated electrical stimulation within an appropriate range may aid in the treatment of nerve injuries by causing local cells, primarily mediated by the Schwann cells, to release factors that promote neurite survival and extension. This model system allows parameter optimization and provides a framework to carefully investigate how this biophysical cue serves to promote re-growth after injury. Investigations are ongoing to identify how NGF-release is mediated as well as to explore how altered surface ligands may be up/down-regulated by exogenous electrical stimulation, resulting in increased neurite outgrowth. A mechanistic understanding of cellular response to electrical stimulation is vital to translate this developmental cue to the clinic to aid in the repair of nerve injuries.
Supplementary Material
Acknowledgments
This work was completed in the Center for Biotechnology and Interdisciplinary Studies. This work was funded by NIH # 1R01EB013281 (DMT) and NYSCIRB # C022067 (DMT). The authors thank Professors Jonathan Newell and Sheppard Salon (RPI) for their discussions related to this project as well as members of the Thompson lab.
Disclosure Statement
The authors of this work have no conflicts to disclose.
References
- 1.Selecki B.R., Ring I.T., Simpson D.A., Vanderfield G.K., and Sewell M.F.Trauma to the central and peripheral nervous systems: Part I: an overview of mortality, morbidity and costs; N.S.W. 1977. Aust N Z J Surg 52,93, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Noble J., Munro C.A., Prasad V.S., and Midha R.Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45,116, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Robinson L.R.Traumatic injury to peripheral nerves. Muscle Nerve 23,863, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Evans G.R.Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec 263,396, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Schlosshauer B., Dreesmann L., Schaller H.E., and Sinis N.Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 59,740, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Shergill G., Bonney G., Munshi P., and Birch R.The radial and posterior interosseous nerves. Results fo 260 repairs. J Bone Joint Surg Br 83,646, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Kuffler D.P.Accurate reinnervation of motor end plates after disruption of sheath cells and muscle fibers. J Comp Neurol 250,228, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Campbell W.W.Evaluation and management of peripheral nerve injury. Clin Neurophysiol 119,1951, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt C.E., and Leach J.B.Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng 5,293, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Reyes O., Sosa I., and Kuffler D.P.Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J 24,215, 2005 [PubMed] [Google Scholar]
- 11.Lee S.K., and Wolfe S.W.Peripheral nerve injury and repair. J Am Acad Orthop Surg 8,243, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Gordon T., and Fu S.Y.Long-term response to nerve injury. Adv Neurol 72,185, 1997 [PubMed] [Google Scholar]
- 13.Gordon T., Brushart T.M., Amirjani N., and Chan K.M.The potential of electrical stimulation to promote functional recovery after peripheral nerve injury—comparisons between rats and humans. Acta Neurochir Suppl 100,3, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Gordon T., Sulaiman O., and Boyd J.G.Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst 8,236, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Guenard V., Kleitman N., Morrissey T.K., Bunge R.P., and Aebischer P.Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J Neurosci 12,3310, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunge R.P.The role of the Schwann cell in trophic support and regeneration. J Neurol 242,S19, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Hadlock T., Sundback C., Hunter D., Cheney M., and Vacanti J.P.A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng 6,119, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Ramon-Cueto A., Plant G.W., Avila J., and Bunge M.B.Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci 18,3803, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark P., Britland S., and Connolly P.Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J Cell Sci 105 (Pt 1),203, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Lu P., Jones L.L., and Tuszynski M.H.Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol 203,8, 2007 [DOI] [PubMed] [Google Scholar]
- 21.McCaig C.D., and Rajnicek A.M.Electrical fields, nerve growth and nerve regeneration. Exp Physiol 76,473, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Hotary K.B., and Robinson K.R.Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol 166,789, 1994 [DOI] [PubMed] [Google Scholar]
- 23.McGinnis M.E., and Vanable J.W., Jr., Voltage gradients in newt limb stumps. Prog Clin Biol Res 210,231, 1986 [PubMed] [Google Scholar]
- 24.Barker J.L.Multiple excitability functions in cultured mouse spinal neurones. Electroencephalogr Clin Neurophysiol Suppl 36,19, 1982 [PubMed] [Google Scholar]
- 25.Borgens R.B., Shi R., Mohr T.J., and Jaeger C.B.Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Exp Neurol 128,41, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Borgens R.B., Toombs J.P., Breur G., Widmer W.R., Waters D., Harbath A.M., et al. . An imposed oscillating electrical field improves the recovery of function in neurologically complete paraplegic dogs. J Neurotrauma 16,639, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Shapiro S., Borgens R., Pascuzzi R., Roos K., Groff M., Purvines S., et al. . Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine 2,3, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hotary K.B., and Robinson K.R.Endogenous electrical currents and the resultant voltage gradients in the chick embryo. Dev Biol 140,149, 1990 [DOI] [PubMed] [Google Scholar]
- 29.McCaig C.D., Rajnicek A.M., Song B., and Zhao M.Controlling cell behavior electrically: current views and future potential. Physiol Rev 85,943, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Al-Majed A.A., Brushart T.M., and Gordon T.Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci 12,4381, 2000 [PubMed] [Google Scholar]
- 31.Al-Majed A.A., Neumann C.M., Brushart T.M., and Gordon T.Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20,2602, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahlborn P., Schachner M., and Irintchev A.One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol 208,137, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Geremia N.M., Gordon T., Brushart T.M., Al-Majed A.A., and Verge V.M.Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol 205,347, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Brushart T.M., Jari R., Verge V., Rohde C., and Gordon T.Electrical stimulation restores the specificity of sensory axon regeneration. Exp Neurol 194,221, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Gordon T., Amirjani N., Edwards D.C., and Chan K.M.Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol 223,192, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Gordon T., Brushart T.M., and Chan K.M.Augmenting nerve regeneration with electrical stimulation. Neurol Res 30,1012, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Borgens R.B.Electrically mediated regeneration and guidance of adult mammalian spinal axons into polymeric channels. Neuroscience 91,251, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Koppes A.N., Seggio A.M., and Thompson D.M.Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J Neural Eng 8,046023, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Huang J., Ye Z., Hu X., Lu L., and Luo Z.Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia 58,622, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Assouline J.G., and Pantazis N.J.Localization of the nerve growth factor receptor on fetal human Schwann cells in culture. Exp Cell Res 182,499, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Seggio A., Narayanaswamy A., Roysam B., and Thompson D.M.Local orientation of Schwann cells directs neurite outgrowth. J Neural Eng 7,046001, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Morrissey T.K., Kleitman N., and Bunge R.P.Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci 11,2433, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seggio A.M., Ellison K.S., Hynd M.R., Shain W., and Thompson D.M.Cryopreservation of transfected primary dorsal root ganglia neurons. J Neurosci Methods 173,67, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Pedrotty D.M., Koh J., Davis B.H., Taylor D.A., Wolf P., and Niklason L.E.Engineering skeletal myoblasts: roles of three-dimensional culture and electrical stimulation. Am J Physiol Heart Circ Physiol 288,H1620, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Tanaka E.M., and Kirschner M.W.Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol 115,345, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCaig C.D.Nerve branching is induced and oriented by a small applied electric field. J Cell Sci 95 (Pt 4),605, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Thompson D.M., and Buettner H.M.Oriented Schwann cell monolayers for directed neurite outgrowth. Ann Biomed Eng 32,1120, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Thompson D.M.An in vitro model for characterization of neuronal-Schwann cell interactions during peripheral nerve regeneration. Ph.D. thesis, Rutgers, The State University of New Jersey, Piscataway, NJ, 2001 [Google Scholar]
- 49.Thompson D.M., and Buettner H.M.Neurite outgrowth is directed by schwann cell alignment in the absence of other guidance cues. Ann Biomed Eng 34,161, 2006 [DOI] [PubMed] [Google Scholar]
- 50.English A.W., Schwartz G., Meador W., Sabatier M.J., and Mulligan A.Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev Neurobiol 67,158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi R., and Borgens R.B.Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn 202,101, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Zhao M., Dick A., Forrester J.V., and McCaig C.D.Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin. Mol Biol Cell 10,1259, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song B., Zhao M., Forrester J.V., and McCaig C.D.Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci U S A 99,13577, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander J.K., Fuss B., and Colello R.J.Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol 2,93, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu X.M., Guenard V., Kleitman N., and Bunge M.B.Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol 351,145, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Kanazawa O., Kondo T., Kobayashi I., Hirokawa Y., and Ohta Y.Tracheal, circulatory, and respiratory responses to femoral nerve stimulation. Jpn J Physiol 46,319, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Ohbayashi K., Inoue H.K., Awaya A., Kobayashi S., Kohga H., Nakamura M., et al. . Peripheral nerve regeneration in a silicone tube: effect of collagen sponge prosthesis, laminin, and pyrimidine compound administration. Neurol Med-Chir 36,428, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Piehl F., Frisen J., Risling M., Hokfelt T., and Cullheim S.Increased trkB mRNA expression by axotomized motoneurones. Neuroreport 5,697, 1994 [DOI] [PubMed] [Google Scholar]
- 59.Koliatsos V.E., Price D.L., Gouras G.K., Cayouette M.H., Burton L.E., and Winslow J.W.Highly selective effects of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 on intact and injured basal forebrain magnocellular neurons. J Comp Neurol 343,247, 1994 [DOI] [PubMed] [Google Scholar]
- 60.Clatterbuck R.E., Price D.L., and Koliatsos V.E.Further characterization of the effects of brain-derived neurotrophic factor and ciliary neurotrophic factor on axotomized neonatal and adult mammalian motor neurons. J Comp Neurol 342,45, 1994 [DOI] [PubMed] [Google Scholar]
- 61.Funakoshi H., Frisen J., Barbany G., Timmusk T., Zachrisson O., Verge V.M., et al. . Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol 123,455, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoke A., Redett R., Hameed H., Jari R., Zhou C., Li Z.B., et al. . Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J Neurosci 26,9646, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obata K., Yamanaka H., Dai Y., Tachibana T., Fukuoka T., Tokunaga A., et al. . Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci 23,4117, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arendt T., Bruckner M.K., Krell T., Pagliusi S., Kruska L., and Heumann R.Degeneration of rat cholinergic basal forebrain neurons and reactive changes in nerve growth factor expression after chronic neurotoxic injury—II. Reactive expression of the nerve growth factor gene in astrocytes. Neuroscience 65,647, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Kishino A., Ishige Y., Tatsuno T., Nakayama C., and Noguchi H.BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol 144,273, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Meyer M., Matsuoka I., Wetmore C., Olson L., and Thoenen H.Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol 119,45, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menei P., Montero-Menei C., Whittemore S.R., Bunge R.P., and Bunge M.B.Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci 10,607, 1998 [DOI] [PubMed] [Google Scholar]
- 68.Munson J.B., and McMahon S.B.Effects of GDNF on axotomized sensory and motor neurons in adult rats. Eur J Neurosci 9,1126, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Rossi J., and Airaksinen M.S.GDNF family signalling in exocrine tissues: distinct roles for GDNF and neurturin in parasympathetic neuron development. Adv Exp Med Biol 506,19, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Airaksinen M.S., and Saarma M.The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3,383, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Chen S., Rio C., Ji R.R., Dikkes P., Coggeshall R.E., Woolf C.J., et al. . Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat Neurosci 6,1186, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Nieke J., and Schachner M.Expression of the neural cell adhesion molecules L1 and N-CAM and their common carbohydrate epitope L2/HNK-1 during development and after transection of the mouse sciatic nerve. Differentiation 30,141, 1985 [DOI] [PubMed] [Google Scholar]
- 73.Seilheimer B., and Schachner M.Regulation of neural cell adhesion molecule expression on cultured mouse Schwann cells by nerve growth factor. EMBO J 6,1611, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seilheimer B., and Schachner M.Studies of adhesion molecules mediating interactions between cells of peripheral nervous system indicate a major role for L1 in mediating sensory neuron growth on Schwann cells in culture. J Cell Biol 107,341, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martini R., Xin Y., and Schachner M.Restricted localization of L1 and N-CAM at sites of contact between Schwann cells and neurites in culture. Glia 10,70, 1994 [DOI] [PubMed] [Google Scholar]
- 76.Martini R., and Schachner M.Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J Cell Biol 106,1735, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martini R., and Schachner M.Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol 103,2439, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lois N., Reid B., Song B., Zhao M., Forrester J., and McCaig C.Electric currents and lens regeneration in the rat. Exp Eye Res 90,316, 2010 [DOI] [PubMed] [Google Scholar]
- 79.Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., et al. . Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442,457, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Zhao M., Agius-Fernandez A., Forrester J.V., and McCaig C.D.Directed migration of corneal epithelial sheets in physiological electric fields. Invest Ophthalmol Vis Sci 37,2548, 1996 [PubMed] [Google Scholar]
- 81.Rajnicek A.M., Foubister L.E., and McCaig C.D.Prioritising guidance cues: directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch. Dev Biol 312,448, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Yao L., Shanley L., McCaig C., and Zhao M.Small applied electric fields guide migration of hippocampal neurons. J Cell Physiol 216,527, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Yao L., McCaig C.D., and Zhao M.Electrical signals polarize neuronal organelles, direct neuron migration, and orient cell division. Hippocampus 19,855, 2009 [DOI] [PubMed] [Google Scholar]
- 84.McKasson M.J., Huang L., and Robinson K.R.Chick embryonic Schwann cells migrate anodally in small electrical fields. Exp Neurol 211,585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwartz R.G., Enwemeka C.S., Kloth L.C., Unger P.G., and Feedar J.A.Electrotherapy for wound healing. Rehab Manage 4,38, 1991 [PubMed] [Google Scholar]
- 86.Feedar J.A., Kloth L.C., and Gentzkow G.D.Chronic dermal ulcer healing enhanced with monophasic pulsed electrical stimulation. Phys Ther 71,639, 1991 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.