Abstract
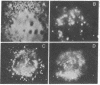
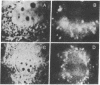
We have studied in detail the binding of fluorescent derivatives of insulin and epidermal growth factor to 3T3 fibroblasts. We have used two types of fluorescent analogues of insulin and epidermal growth factor: highly fluorescent derivatives which have seven to eight rhodamine molecules or fluorescent derivatives which have a single rhodamine molecule per one molecule of insulin or epidermal growth factor. Both types of analogue retained substantial binding affinity as determined by radioreceptor assays and biological activity. The cells labeled with the fluorescent analogues were visualized with a sensitive video intensification microscopic system that enabled us to directly observe the location of the fluorescent hormone on the surface and within the living fibroblasts. We found that both insulin and epidermal growth factor initially bound diffusely to the cell surface and, at 4°, remained dispersed. Within a few minutes at 23° or 37° the hormone-receptor complexes aggregated into patches that could be readily removed by trypsin but not by excess native hormone. The hormone-receptor complexes, which were initially mobile in the plane of the membrane, become immobilized later as the consequence of the receptor aggregation or internalization. Within ∼30 min at 37°, much of the labeled hormone was found within the cell in endocytic vesicles that moved about in the cytoplasm in a saltatory manner. The aggregation and immobilization of the hormone-receptor complexes could be due to either hormone-hormone interactions on the cell membrane or a hormone-induced conformational change in the hormone-receptor complex. Aggregation and internalization of hormone-receptor complexes could be associated with certain aspects of hormone action, hormone degradation, down regulation of receptors, or negative cooperativity of hormone binding.
Keywords: hormone receptors, fluorescent hormones, lateral diffusion, patching, endocytosis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Armelin H. A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2702–2706. doi: 10.1073/pnas.70.9.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. ISOLATION AND BIOLOGICAL EFFECTS OF AN EPIDERMAL GROWTH-STIMULATING PROTEIN. Natl Cancer Inst Monogr. 1964 Apr;13:13–37. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Human epidermal growth factor and the proliferation of human fibroblasts. J Cell Physiol. 1976 Jun;88(2):227–237. doi: 10.1002/jcp.1040880212. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Lembach K. J., Morrison M. M., Cohen S. Characterization of the binding of 125-I-labeled epidermal growth factor to human fibroblasts. J Biol Chem. 1975 Jun 10;250(11):4297–4304. [PubMed] [Google Scholar]
- DeMeyts P., Bainco A. R., Roth J. Site-site interactions among insulin receptors. Characterization of the negative cooperativity. J Biol Chem. 1976 Apr 10;251(7):1877–1888. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Roth J., Neville D. M., Jr, de Meyts P., Buell D. N. Insulin-dependent regulation of insulin receptor concentrations: a direct demonstration in cell culture. Proc Natl Acad Sci U S A. 1974 Jan;71(1):84–88. doi: 10.1073/pnas.71.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J., Wong K. Y., Jones A. L. Cellular uptake and nuclear binding of insulin in human cultured lymphocytes: evidence for potential intracellular sites of insulin action. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1368–1372. doi: 10.1073/pnas.74.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The natural occurrence of insulin receptors in groups on adipocyte plasma membranes as demonstrated with monomeric ferritin-insulin. J Supramol Struct. 1977;6(1):45–59. doi: 10.1002/jss.400060104. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Roth J., Macchia V. Binding of hormone to tissue: the first step in polypeptide hormone action. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1802–1809. doi: 10.1073/pnas.56.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt R. M., Pastan I. Decreased binding of epidermal growth factor to BALB/c 3T3 mutant cells defective in glycoprotein synthesis. Nature. 1978 Mar 2;272(5648):68–70. doi: 10.1038/272068a0. [DOI] [PubMed] [Google Scholar]
- Richter P. H., Eigen M. Diffusion controlled reaction rates in spheroidal geometry. Application to repressor--operator association and membrane bound enzymes. Biophys Chem. 1974 Oct;2(3):255–263. doi: 10.1016/0301-4622(74)80050-5. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Schlessinger J., Jacobs S., Chang K. J., Cuatrecasas P. Fluorescent labeling of hormone receptors in viable cells: preparation and properties of highly fluorescent derivatives of epidermal growth factor and insulin. Proc Natl Acad Sci U S A. 1978 May;75(5):2135–2139. doi: 10.1073/pnas.75.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos P., Roth J., Lovelace E., Pastan I. Insulin receptors in normal and transformed fibroblasts: relationship to growth and transformation. Cell. 1976 Jul;8(3):417–423. doi: 10.1016/0092-8674(76)90154-9. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]