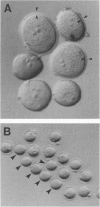
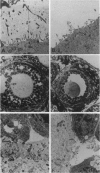
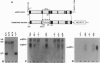Abstract
Mammalian eggs are surrounded by a thick extracellular coat, the zona pellucida, that plays important roles during early development. The mouse egg zona pellucida is constructed of three glycoproteins, called mZP1, mZP2, and mZP3. The gene encoding mZP3 is expressed only by growing oocytes during a 2- to 3-week period of oogenesis. Here, the mZP3 gene was disrupted by targeted mutagenesis using homologous recombination in mouse embryonic stem cells. Viable female mice homozygous for the mutated mZP3 allele (mZP3-/-) were obtained. These mice are indistinguishable in appearance from wild-type (mZP3+/+) and heterozygous (mZP3+/-) littermates. However, although ovaries of juvenile and adult mZP3-/- females possess growing and fully grown oocytes, the oocytes completely lack a zona pellucida. Consistent with this observation, eggs recovered from oviducts of superovulated, adult mZP3-/- females also lack a zona pellucida. Thus far, mZP3-/- females mated with wild-type males have failed to become pregnant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Wassarman P. M. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev Biol. 1983 Feb;95(2):317–324. doi: 10.1016/0012-1606(83)90032-5. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Synthesis of zona pellucida proteins by denuded and follicle-enclosed mouse oocytes during culture in vitro. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1029–1033. doi: 10.1073/pnas.77.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson R. A., McLaren A. Transfer to the mouse oviduct of eggs with and without the zona pellucida. J Reprod Fertil. 1970 Jun;22(1):129–137. doi: 10.1530/jrf.0.0220129. [DOI] [PubMed] [Google Scholar]
- Epifano O., Liang L. F., Familari M., Moos M. C., Jr, Dean J. Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development. 1995 Jul;121(7):1947–1956. doi: 10.1242/dev.121.7.1947. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Salzmann G. S., Roller R. J., Wassarman P. M. Biosynthesis of the major zona pellucida glycoprotein secreted by oocytes during mammalian oogenesis. Cell. 1982 Dec;31(3 Pt 2):749–759. doi: 10.1016/0092-8674(82)90329-4. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Wassarman P. M. Mouse egg extracellular coat is a matrix of interconnected filaments possessing a structural repeat. J Mol Biol. 1985 Jan 20;181(2):253–264. doi: 10.1016/0022-2836(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Kinloch R. A., Sakai Y., Wassarman P. M. Mapping the mouse ZP3 combining site for sperm by exon swapping and site-directed mutagenesis. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):263–267. doi: 10.1073/pnas.92.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Wassarman P. M. Profile of a mammalian sperm receptor gene. New Biol. 1989 Dec;1(3):232–238. [PubMed] [Google Scholar]
- Lau M. M., Stewart C. E., Liu Z., Bhatt H., Rotwein P., Stewart C. L. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994 Dec 15;8(24):2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- Liu C., Litscher E. S., Wassarman P. M. Transgenic mice with reduced numbers of functional sperm receptors on their eggs reproduce normally. Mol Biol Cell. 1995 May;6(5):577–585. doi: 10.1091/mbc.6.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Modliński J. A. The role of the zona pellucida in the development of mouse eggs in vivo. J Embryol Exp Morphol. 1970 Jun;23(3):539–547. [PubMed] [Google Scholar]
- Myles D. G. Molecular mechanisms of sperm-egg membrane binding and fusion in mammals. Dev Biol. 1993 Jul;158(1):35–45. doi: 10.1006/dbio.1993.1166. [DOI] [PubMed] [Google Scholar]
- Roller R. J., Kinloch R. A., Hiraoka B. Y., Li S. S., Wassarman P. M. Gene expression during mammalian oogenesis and early embryogenesis: quantification of three messenger RNAs abundant in fully grown mouse oocytes. Development. 1989 Jun;106(2):251–261. doi: 10.1242/dev.106.2.251. [DOI] [PubMed] [Google Scholar]
- Rosiere T. K., Wassarman P. M. Identification of a region of mouse zona pellucida glycoprotein mZP3 that possesses sperm receptor activity. Dev Biol. 1992 Dec;154(2):309–317. doi: 10.1016/0012-1606(92)90070-w. [DOI] [PubMed] [Google Scholar]
- Salzmann G. S., Greve J. M., Roller R. J., Wassarman P. M. Biosynthesis of the sperm receptor during oogenesis in the mouse. EMBO J. 1983;2(9):1451–1456. doi: 10.1002/j.1460-2075.1983.tb01607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen R. A., Wassarman P. M. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976 Jun;50(2):531–536. doi: 10.1016/0012-1606(76)90172-x. [DOI] [PubMed] [Google Scholar]
- Tong Z. B., Nelson L. M., Dean J. Inhibition of zona pellucida gene expression by antisense oligonucleotides injected into mouse oocytes. J Biol Chem. 1995 Jan 13;270(2):849–853. doi: 10.1074/jbc.270.2.849. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Upadhyay S., Zamboni L. Ectopic germ cells: natural model for the study of germ cell sexual differentiation. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6584–6588. doi: 10.1073/pnas.79.21.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M., Litscher E. S. Sperm--egg recognition mechanisms in mammals. Curr Top Dev Biol. 1995;30:1–19. doi: 10.1016/s0070-2153(08)60562-1. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Mortillo S. Structure of the mouse egg extracellular coat, the zona pellucida. Int Rev Cytol. 1991;130:85–110. doi: 10.1016/s0074-7696(08)61502-8. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Profile of a mammalian sperm receptor. Development. 1990 Jan;108(1):1–17. doi: 10.1242/dev.108.Supplement.1. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Towards molecular mechanisms for gamete adhesion and fusion during mammalian fertilization. Curr Opin Cell Biol. 1995 Oct;7(5):658–664. doi: 10.1016/0955-0674(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]