Abstract
The retinoblastoma tumor suppressor protein (RB) is inactivated in a majority of cancers. RB restricts cell proliferation by inhibiting the E2F family of transcription factors. The current model for RB/E2F function describes its role in regulating transcription at gene promoters. Whether the RB or E2F proteins might play a role in gene expression beyond transcription initiation is not well known. This review describes evidence that points to a novel role for the RB/E2F network in the regulation of RNA processing, and we propose a model as a framework for future research. The elucidation of a novel role of RB in RNA processing will have a profound impact on our understanding of the role of this tumor suppressor family in cell and developmental biology.
Keywords: RB, Retinoblastoma tumor suppressor, E2F, RNA processing, Pre-mRNA splicing
Introduction
The retinoblastoma tumor suppressor protein (RB) is mutated or inactivated in a majority of cancers, and therefore its function has been the subject of much investigation. RB is highly conserved among metazoans and is a major component of the cell cycle, and it has a central role in development and differentiation. Thus, a detailed understanding of RB function is important to biology and medicine. The best described function of RB is to restrict cell proliferation by suppressing gene expression through direct inhibition of the E2F family of transcription factors [1]. The current model for RB/E2F network function describes its role in modulating gene expression at promoters. However, the question of whether the RB or E2F proteins play a role in gene expression beyond transcription initiation has never been systematically addressed. This review describes several lines of evidence that point to a novel role for the RB/E2F network in the regulation of RNA processing.
RB interacts with RNA-binding proteins
The RB protein has no known enzymatic function; its primary function is thought to be in coordinating the binding of different proteins to form diverse multiprotein complexes. Much work has been done to identify binding partners of RB in order to better understand its function in the regulation of cellular processes [2]. Among the many proteins identified are several RNA-binding proteins (Table 1). The proteins listed in Table 1 have been shown to affect a wide range of processes during RNA production, including transcription elongation, splicing, and transport. Future experiments will be required to determine how the retinoblastoma tumor suppressor might influence these processes through its interaction with RNA-binding proteins.
Table 1.
RNA-binding proteins that physically associate with RB.
| Symbol | Full name | Function with RB | References |
|---|---|---|---|
| PU.1/Spi1 | Spleen focus forming virus (SFFV) proviral integration oncogene spi1 |
RB and PU.1 are corepressors of transcription and erythroid differentiation |
[8,10] |
| p84/Hpr1/THOC1 | THO complex 1 | RB inhibits p84-induced apoptosis | [20,22] |
| RBQ-1/RBBP6/PACT/ P2P-R |
Retinoblastoma binding protein 6 | Unknown | [45–47] |
| Pur-alpha | Purine-rich element binding protein A | RB inhibits Pur-alpha ssDNA binding | [5] |
| Skip/SNW1 | SNW domain containing 1 | Skip inhibits RB to overcome transcriptional repression | [3] |
| Ssu72 | SSU72 RNA polymerase II CTD phosphatase homolog | Unknown | [48] |
| pp32/Anp32A | Acidic (leucine-rich) nuclear phosphoprotein 32 family, member A |
RB inhibits pp32-induced apoptosis and transactivation | [16,17] |
RB was found to interact with Skip, a binding partner of the oncoprotein Ski [3]. Skip binds to the Ski oncogene to synergistically overcome RB-mediated transcriptional repression and cell cycle arrest [3]. Skip is the functional orthologue of the yeast splicing factor Prp45 and thus physically links RB to RNA splicing control [4]. RB also physically binds to Pur-alpha, a protein with the ability to bind both single-stranded DNA and RNA. This RB interaction was shown to reduce the ability of Pur-alpha to bind single-stranded DNA in vitro [5]. It is not known whether RB affects the biological activity of Pur-alpha in vivo. However, the ability of Pur-alpha to bind and inhibit E2F suggests that it may modify the RB-E2F interaction to regulate the cell cycle [6]. Pur-alpha is known to function in transcription and mRNA transport and may work through the RB/E2F pathway to integrate RNA processing with cell cycle control [7].
RB regulates differentiation of a variety of tissues during development. For example, RB controls erythrocyte differentiation in part through a physical interaction with PU.1 [8]. The PU.1 oncoprotein belongs to the Ets family of transcription factors and is necessary for proper differentiation during hematopoiesis [9]. PU.1 requires RB to suppress transcription of the target gene GATA-1 [10]. PU.1 can also bind to RNA and interact with splicing factors to influence alternative splicing of RNA transcripts [11,12]. Interestingly, it was found that splicing regulation by PU.1 was dependent upon its promoter binding and transactivation activities [13]. Similar promoter-dependent splicing control was also demonstrated by E2F [14]. Integration of transcription with RNA splicing control is a common theme in RNA biology [15], and we predict that RB controls differentiation by regulating alternative splicing through its interaction with RNA-binding proteins such as PU.1 (Fig. 2).
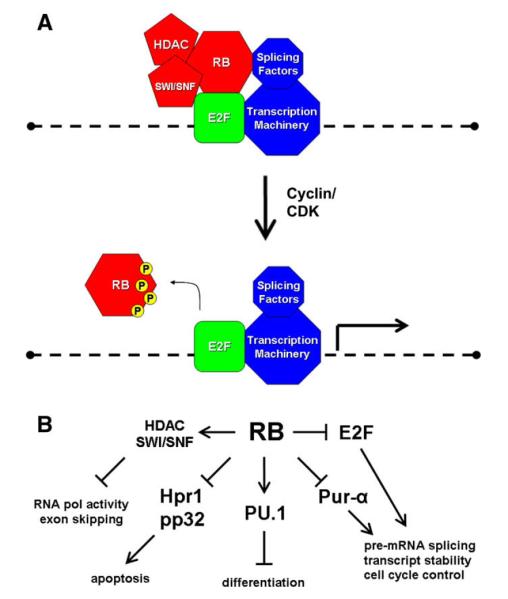
Fig. 2.
Models of RB/E2F and RNA processing control. (A) RB is bound to gene promoters through E2F. RB may inhibit splicing factors at promoters to suppress splicing of nacent RNA transcripts. Alternatively, RB may directly recruit splicing repressors. As the cell cycle proceeds toward S phase RB is phosphorylated and released from the promoter, which relieves splicing suppression and allows E2F to stimulate transcription. (B) RB interacts with several RNA processing factors that may serve to regulate diverse cellular processes. The role of RB in RNA processing control is a relatively unexplored area of research that may yield many insights into RB function.
The RB/E2F pathway regulates apoptosis, and RB inhibition of apoptosis is an important mechanism of tumor suppression whereby cells deficient for RB function can be eliminated by apoptosis. One manner through which RB can inhibit apoptosis is through its binding to RNA processing factors. Although the mechanism for inhibition in this context is not known, it may occur through modification of RNA processing pathways. For example, the pp32 protein is a positive regulator of apoptosis and physically associates with hyperphosphorylated RB [16]. RB is a suppressor of pp32 transactivation and is found in a pp32 complex with splicing machinery [17]. Importantly, RB inhibits pp32-induced apoptosis [16].
RB can also inhibit apoptosis induced by overexpression of the RNA-binding protein Hpr1. Hpr1, also called p84N5 or Thoc1, is part of the evolutionarily conserved THO/TREX complex that has been implicated in many stages of RNA processing, including transcription elongation, splicing, and nuclear export [18,19]. The gene was first identified in mammals by a yeast two-hybrid screen for protein interactions with the amino-terminus of RB. RB suppression of Hpr1-induced apoptosis depends upon a direct physical association with the RB amino-terminal domain [20]. The requirement for E2F in this context is not clear. However, a comparison of gene expression data from Drosophila cell culture studies suggests that Hpr1 may cooperate with E2F to regulate many of the same genes [18,21].
Hpr1 colocalizes with RB in nuclear speckles that are associated with RNA processing centers in the nuclear matrix of mammalian cells [22]. RB is known to bind tightly to the nuclear matrix and is resistant to nuclear extraction protocols [23]. RB may associate with the nuclear matrix directly through association with nuclear lamins A and C or by association with RNA processing factors such as Hpr1 [22,23].
Based upon the understanding that RB associates with RNA processing factors through an interaction with its amino-terminal domain, we wondered whether this function might also be conserved in Drosophila. We examined larval salivary gland cells that express the amino-terminal domain of the Drosophila RB, Rbf, fused to a red fluorescent protein (RbfN-RFP) in a genetic background that expresses a GFP-tagged core spliceosomal protein (SmD3-GFP)[24,25]. We observed that RbfN-RFP strongly colocalized with SmD3-GFP in salivary gland nuclei (Fig. 1). This observation is consistent with previous experiments which show that human RB colocalizes with RNA processing centers in the nuclei of mammalian cells [22]. Recent experiments from our lab also show that Drosophila RbfN physically interacts with the RNA-binding protein Squid [49] which is known to be involved in several stages of RNA processing including alternative splicing and mRNA transport [26,27]. We infer that the RB protein may have a conserved role in RNA processing that will reveal new insights into the function of this versatile tumor suppressor.
Fig. 1.
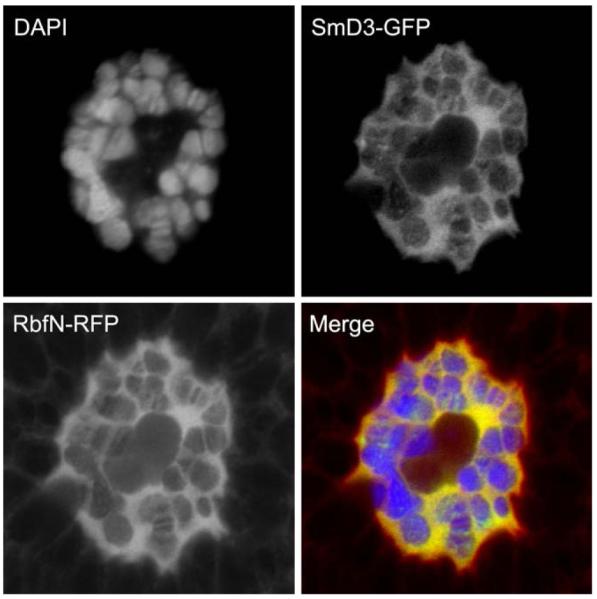
The Drosophila retinoblastoma tumor suppressor colocalizes with the spliceosomal component SmD3 in salivary gland nuclei. The amino-terminal domain of Drosophila Rbf fused to RFP (RbfN-RFP) colocalizes with a GFP-tagged SmD3 in larval salivary gland cells. The DNA stain DAPI was used to show the bands of the characteristic polytene chromosomes. Confocal imaging shows extensive colocalization of SmD3-GFP and Rbf1N-RFP in the nucleoplasm and along the chromsomes. Salivary glands were isolated from mature larvae and fixed with 4% formaldehyde. Confocal images were obtained using a Zeiss LSM510 Meta.
The RB/E2F pathway regulates RNA processing
Interesting observations have emerged which support a model involving the RB/E2F pathway in RNA processing either indirectly through transcriptional regulation of RNA-binding proteins or by direct physical interaction with RNA processing factors. Mammalian studies have shown that E2F can regulate the production of splicing factors [28,29]. One study identified SC35, a member of the SR family of splicing factors, as a direct transcriptional target of E2F1. Significantly, SC35 is required for E2F-induced pre-mRNA alternative splicing of pro-apoptotic factors [30].
Experiments using invertebrate organisms have also revealed potential links between the RB/E2F pathway and RNA processing. Microarray analysis shows that Drosophila RB/E2F regulates the transcription of splicing factors Hel25E and Hrb87F [21]. Another study identified the RNA-binding protein Api5 as a regulator of dE2F1-induced apoptosis [31]. Furthermore, a genome-wide RNAi screen in Caenorhabditis elegans recently showed that the RB homologue lin-35 genetically interacts with many core components of the splicesosome to regulate vulval development [32]. Taken together, these observations suggest that the RB/E2F pathway genetically interacts with the RNA processing machinery in a conserved manner to regulate developmental processes.
E2F can influence gene expression through regulation of the half-life of RNA molecules, which may occur by controlling expression of several genes involved in mRNA stability [29,33]. Indeed, E2F expression was shown to stabilize axin2 mRNA in mammalian cells [34]. E2F also regulates microRNA expression which in turn has an impact on mRNA stability [35,36]. In line with these observations, several studies in C. elegans have shown a functional role for the RB pathway in RNA interference [37–40].
Another intriguing study showed a role for E2F in the suppression of RNA splicing during the cell cycle. E2F regulates transcription of the PFK-2 gene, which encodes an enzyme that is critical for energy production during glycolysis. Transcription and splicing of the PFK-2 transcript is suppressed in quiescent cells. Upon serum stimulation, PFK-2 transcription increases and its pre-mRNA is more efficiently spliced, leading to a dramatic increase in mature transcript as the cell cycle progresses. However, mutation of an intronic E2F binding site results in a loss of cell cycle sensitive splicing control. Thus, the suppression of pre-mRNA splicing of PFK-2 in G0/G1 is dependent upon the binding of E2F [14]. Significantly, this data implies that an RB/E2F complex may act to suppress RNA splicing during the cell cycle (Fig. 2). The ability of RB/E2F to influence mRNA processing may provide an additional layer of control over gene expression and cell cycle regulation.
Coupling of transcription and RNA processing
Transcription and RNA processing have commonly been considered as separate events. Consequently, transcription factors are generally examined for their ability to upregulate or down-regulate transcription of target genes without giving much consideration to transcript processing. However, mounting evidence supports a model that integrates transcription initiation with downstream processing events, including pre-mRNA splicing. For example, placing a different promoter sequence upstream of a gene can affect the alternative splicing of its transcripts [41]. Transcription factors may affect alternative splicing by several means. First, they may physically recruit splicing factors to the promoter region. Second, a transcription factor may favor the exclusion or inclusion of alternatively spliced exons by modulating the elongation rate of transcription. Finally, transcription factors may recruit chromatin modifying enzymes to alter chromatin structure, affecting RNA polymerase activity that can lead to exon skipping [15].
Coordination of promoter activity with RNA processing may be a common mechanism of gene regulation. The transcription factor PU.1 can regulate splicing in a promoter-dependent manner [13]. PCG-1 can also regulate mRNA processing only when it is bound upstream to the promoter [42]. E2F has likewise been implicated in promoter regulation of pre-mRNA splicing [14]. In light of this knowledge we propose the following model based upon the genetic and biochemical data of RB/E2F interaction with RNA processing factors (Figure 2A). The RB protein binds to E2F, masking its transactivation domain, and recruits histone deacetylases (HDAC) and chromatin remodeling enzymes to attenuate transcription [43,44]. At the same time, RB interacts with RNA processing factors to suppress pre-mRNA splicing. As the cell cycle proceeds towards S phase, RB is hyperphosphorylated by Cyclin/CDK complexes and is released from E2F, relieving splicing suppression and allowing E2F to activate transcription. E2F upregulates RNA processing factors which stimulates alternative splicing of transcripts to promote either cell proliferation or apoptosis, depending on the cellular context. Misregulation of alternative splicing upon RB inactivation may be one mechanism that contributes to cancer progression.
Conclusion
The RB tumor suppressor protein interacts with RNA processing factors that help it to regulate a diverse array of processes (Figure 2B). Future experiments will reveal how the retinoblastoma tumor suppressor might influence RNA splicing, transport, and stability. The elucidation of a novel role of RB in RNA processing will have a profound impact on our understanding of the role of this tumor suppressor family in cell and developmental biology, and it will place this versatile protein at almost every level of gene regulation. Most importantly, this new knowledge will help us better understand how mutation of RB leads to cancer and may provide new therapeutic targets.
Acknowledgments
This work was supported by the National Institutes of Health grant GM069462. We thank Maureen Peterson for helpful comments during the preparation of this manuscript.
References
- [1].van den Heuvel S, Dyson NJ. Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell Biol. 2008;9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- [2].Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv. Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- [3].Prathapam T, Kuhne C, Banks L. Skip interacts with the retinoblastoma tumor suppressor and inhibits its transcriptional repression activity. Nucleic Acids Res. 2002;30:5261–5268. doi: 10.1093/nar/gkf658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Figueroa JD, Hayman MJ. The human ski-interacting protein functionally substitutes for the yeast PRP45 gene. Biochem. Biophys. Res. Commun. 2004;319:1105–1109. doi: 10.1016/j.bbrc.2004.05.096. [DOI] [PubMed] [Google Scholar]
- [5].Johnson EM, Chen PL, Krachmarov CP, Barr SM, Kanovsky M, Ma ZW, Lee WH. Association of human pur alpha with the retinoblastoma protein, rb, regulates binding to the single-stranded DNA pur alpha recognition element. J. Biol. Chem. 1995;270:24352–24360. doi: 10.1074/jbc.270.41.24352. [DOI] [PubMed] [Google Scholar]
- [6].Darbinian N, Gallia GL, Kundu M, Shcherbik N, Tretiakova A, Giordano A, Khalili K. Association of pur alpha and E2F-1 suppresses transcriptional activity of E2F-1. Oncogene. 1999;18:6398–6402. doi: 10.1038/sj.onc.1203011. [DOI] [PubMed] [Google Scholar]
- [7].White MK, Johnson EM, Khalili K. Multiple roles for puralpha in cellular and viral regulation. Cell. Cycle. 2009;8:1–7. doi: 10.4161/cc.8.3.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hagemeier C, Bannister AJ, Cook A, Kouzarides T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc. Natl. Acad. Sci. USA. 1993;90:1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kastner P, Chan S. PU.1: a crucial and versatile player in hematopoiesis and leukemia. Int. J. Biochem. Cell Biol. 2008;40:22–27. doi: 10.1016/j.biocel.2007.01.026. [DOI] [PubMed] [Google Scholar]
- [10].Rekhtman N, Choe KS, Matushansky I, Murray S, Stopka T, Skoultchi AI. PU.1 and pRB interact and cooperate to repress GATA-1 and block erythroid differentiation. Mol. Cell Biol. 2003;23:7460–7474. doi: 10.1128/MCB.23.21.7460-7474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hallier M, Tavitian A, Moreau-Gachelin F. The transcription factor spi-1/PU.1 binds RNA and interferes with the RNA-binding protein p54nrb. J. Biol. Chem. 1996;271:11177–11181. doi: 10.1074/jbc.271.19.11177. [DOI] [PubMed] [Google Scholar]
- [12].Hallier M, Lerga A, Barnache S, Tavitian A, Moreau-Gachelin F. The transcription factor spi-1/PU.1 interacts with the potential splicing factor TLS. J. Biol. Chem. 1998;273:4838–4842. doi: 10.1074/jbc.273.9.4838. [DOI] [PubMed] [Google Scholar]
- [13].Guillouf C, Gallais I, Moreau-Gachelin F. Spi-1/PU.1 oncoprotein affects splicing decisions in a promoter binding-dependent manner. J. Biol. Chem. 2006;281:19145–19155. doi: 10.1074/jbc.M512049200. [DOI] [PubMed] [Google Scholar]
- [14].Darville MI, Rousseau GG. E2F-dependent mitogenic stimulation of the splicing of transcripts from an S phase-regulated gene. Nucleic Acids Res. 1997;25:2759–2765. doi: 10.1093/nar/25.14.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kornblihtt AR. Promoter usage and alternative splicing. Curr. Opin. Cell Biol. 2005;17:262–268. doi: 10.1016/j.ceb.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [16].Adegbola O, Pasternack GR. Phosphorylated retinoblastoma protein complexes with pp32 and inhibits pp32-mediated apoptosis. J. Biol. Chem. 2005;280:15497–15502. doi: 10.1074/jbc.M411382200. [DOI] [PubMed] [Google Scholar]
- [17].Adegbola O, Pasternack GR. A pp32-retinoblastoma protein complex modulates androgen receptor-mediated transcription and associates with components of the splicing machinery. Biochem. Biophys. Res. Commun. 2005;334:702–708. doi: 10.1016/j.bbrc.2005.06.153. [DOI] [PubMed] [Google Scholar]
- [18].Rehwinkel J, Herold A, Gari K, Kocher T, Rode M, Ciccarelli FL, Wilm M, Izaurralde E. Genome-wide analysis of mRNAs regulated by the THO complex in drosophila melanogaster. Nat. Struct. Mol. Biol. 2004;11:558–566. doi: 10.1038/nsmb759. [DOI] [PubMed] [Google Scholar]
- [19].Li Y, Lin AW, Zhang X, Wang Y, Wang X, Goodrich DW. Cancer cells and normal cells differ in their requirements for Thoc1. Cancer Res. 2007;67:6657–6664. doi: 10.1158/0008-5472.CAN-06-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Doostzadeh-Cizeron J, Evans R, Yin S, Goodrich DW. Apoptosis induced by the nuclear death domain protein p84N5 is inhibited by association with rb protein. Mol. Biol. Cell. 1999;10:3251–3261. doi: 10.1091/mbc.10.10.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dimova DK, Stevaux O, Frolov MV, Dyson NJ. Cell cycle-dependent and cell cycle-independent control of transcription by the drosophila E2F/RB pathway. Genes Dev. 2003;17:2308–2320. doi: 10.1101/gad.1116703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Durfee T, Mancini MA, Jones D, Elledge SJ, Lee WH. The amino-terminal region of the retinoblastoma gene product binds a novel nuclear matrix protein that co-localizes to centers for RNA processing. J. Cell Biol. 1994;127:609–622. doi: 10.1083/jcb.127.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mancini MA, Shan B, Nickerson JA, Penman S, Lee WH. The retinoblastoma gene product is a cell cycle-dependent, nuclear matrix-associated protein. Proc. Natl. Acad. Sci. USA. 1994;91:418–422. doi: 10.1073/pnas.91.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ahlander J, Chen XB, Bosco G. The N-terminal domain of the drosophila retinoblastoma protein Rbf1 interacts with ORC and associates with chromatin in an E2F independent manner. PLoS One. 2008;3:e2831. doi: 10.1371/journal.pone.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Quinones-Coello AT, Petrella LN, Ayers K, Melillo A, Mazzalupo S, Hudson AM, Wang S, Castiblanco C, Buszczak M, Hoskins RA, Cooley L. Exploring strategies for protein trapping in drosophila. Genetics. 2007;175:1089–1104. doi: 10.1534/genetics.106.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nilson LA, Schupbach T. EGF receptor signaling in drosophila oogenesis. Curr. Top. Dev. Biol. 1999;44:203–243. doi: 10.1016/s0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- [27].Park JW, Parisky K, Celotto AM, Reenan RA, Graveley BR. Identification of alternative splicing regulators by RNA interference in drosophila. Proc. Natl. Acad. Sci. USA. 2004;101:15974–15979. doi: 10.1073/pnas.0407004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Young AP, Nagarajan R, Longmore GD. Mechanisms of transcriptional regulation by rb-E2F segregate by biological pathway. Oncogene. 2003;22:7209–7217. doi: 10.1038/sj.onc.1206804. [DOI] [PubMed] [Google Scholar]
- [29].Li Z, Kreutzer M, Mikkat S, Mise N, Glocker MO, Putzer BM. Proteomic analysis of the E2F1 response in p53-negative cancer cells: new aspects in the regulation of cell survival and death. Proteomics. 2006;6:5735–5745. doi: 10.1002/pmic.200600290. [DOI] [PubMed] [Google Scholar]
- [30].Merdzhanova G, Edmond V, De Seranno S, Van den Broeck A, Corcos L, Brambilla C, Brambilla E, Gazzeri S, Eymin B. E2F1 controls alternative splicing pattern of genes involved in apoptosis through upregulation of the splicing factor SC35. Cell Death Differ. 2008 doi: 10.1038/cdd.2008.135. [DOI] [PubMed] [Google Scholar]
- [31].Morris EJ, Michaud WA, Ji JY, Moon NS, Rocco JW, Dyson NJ. Functional identification of Api5 as a suppressor of E2F-dependent apoptosis in vivo. PLoS Genet. 2006;2:e196. doi: 10.1371/journal.pgen.0020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ceron J, Rual JF, Chandra A, Dupuy D, Vidal M, van den Heuvel S. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 2007;7:30. doi: 10.1186/1471-213X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ma Y, Croxton R, Moorer RL, Jr., Cress WD. Identification of novel E2F1-regulated genes by microarray. Arch. Biochem. Biophys. 2002;399:212–224. doi: 10.1006/abbi.2002.2761. [DOI] [PubMed] [Google Scholar]
- [34].Hughes TA, Brady HJ. E2F1 up-regulates the expression of the tumour suppressor axin2 both by activation of transcription and by mRNA stabilisation. Biochem. Biophys. Res. Commun. 2005;329:1267–1274. doi: 10.1016/j.bbrc.2005.02.102. [DOI] [PubMed] [Google Scholar]
- [35].Woods K, Thomson JM, Hammond SM. Direct regulation of an oncogenic micro-RNA cluster by E2F transcription factors. J. Biol. Chem. 2007;282:2130–2134. doi: 10.1074/jbc.C600252200. [DOI] [PubMed] [Google Scholar]
- [36].Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, Iliopoulos D, Pilozzi E, Liu CG, Negrini M, Cavazzini L, Volinia S, Alder H, Ruco LP, Baldassarre G, Croce CM, Vecchione A. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008;13:272–286. doi: 10.1016/j.ccr.2008.02.013. [DOI] [PubMed] [Google Scholar]
- [37].Wang D, Kennedy S, Conte D, Jr, Kim JK, Gabel HW, Kamath RS, Mello CC, Ruvkun G. Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature. 2005;436:593–597. doi: 10.1038/nature04010. [DOI] [PubMed] [Google Scholar]
- [38].Lehner B, Calixto A, Crombie C, Tischler J, Fortunato A, Chalfie M, Fraser AG. Loss of LIN-35, the Caenorhabditis elegans ortholog of the tumor suppressor p105Rb, results in enhanced RNA interference. Genome Biol. 2006;7:R4. doi: 10.1186/gb-2006-7-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ouellet J, Roy R. The lin-35/Rb and RNAi pathways cooperate to regulate a key cell cycle transition in C. elegans. BMC Dev. Biol. 2007;7:38. doi: 10.1186/1471-213X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Grishok A, Hoersch S, Sharp PA. RNA interference and retinoblastoma-related genes are required for repression of endogenous siRNA targets in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2008;105:20386–20391. doi: 10.1073/pnas.0810589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cramer P, Pesce CG, Baralle FE, Kornblihtt AR. Functional association between promoter structure and transcript alternative splicing. Proc. Natl. Acad. Sci. USA. 1997;94:11456–11460. doi: 10.1073/pnas.94.21.11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Monsalve M, Wu Z, Adelmant G, Puigserver P, Fan M, Spiegelman BM. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell. 2000;6:307–316. doi: 10.1016/s1097-2765(00)00031-9. [DOI] [PubMed] [Google Scholar]
- [43].Hagemeier C, Cook A, Kouzarides T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 1993;21:4998–5004. doi: 10.1093/nar/21.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang HS, Gavin M, Dahiya A, Postigo AA, Ma D, Luo RX, Harbour JW, Dean DC. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-rb-hSWI/SNF and rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]
- [45].Sakai Y, Saijo M, Coelho K, Kishino T, Niikawa N, Taya Y. CDNA sequence and chromosomal localization of a novel human protein, RBQ-1 (RBBP6), that binds to the retinoblastoma gene product. Genomics. 1995;30:98–101. doi: 10.1006/geno.1995.0017. [DOI] [PubMed] [Google Scholar]
- [46].Simons A, Melamed-Bessudo C, Wolkowicz R, Sperling J, Sperling R, Eisenbach L, Rotter V. PACT: Cloning and characterization of a cellular p53 binding protein that interacts with rb. Oncogene. 1997;14:145–155. doi: 10.1038/sj.onc.1200825. [DOI] [PubMed] [Google Scholar]
- [47].Witte MM, Scott RE. The proliferation potential protein-related (P2P-R) gene with domains encoding heterogeneous nuclear ribonucleoprotein association and Rb1 binding shows repressed expression during terminal differentiation. Proc. Natl. Acad. Sci. USA. 1997;94:1212–1217. doi: 10.1073/pnas.94.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].St-Pierre B, Liu X, Kha LC, Zhu X, Ryan O, Jiang Z, Zacksenhaus E. Conserved and specific functions of mammalian ssu72. Nucleic Acids Res. 2005;33:464–477. doi: 10.1093/nar/gki171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ahlander J, Bosco G. Sqd interacts with the Drosophila retinoblastoma tumor suppressor Rbf. Biochem. Biophys. Res. Commun. 2009;383:363–367. doi: 10.1016/j.bbrc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]