Abstract
Prevalence of obesity has steadily increased over the past three decades both in the United States and worldwide. Recent studies have shown the role of dietary polyphenols in the prevention of obesity and obesity-related chronic diseases. Here we evaluated the impact of commonly consumed polyphenols, including green tea catechins and epigallocatechin gallates, resveratrol, and curcumin, on obesity and obesity-related-inflammation. Cellular studies demonstrated that these dietary polyphenols reduce viability of adipocytes and proliferation of preadipocytes, suppress adipocyte differentiation and triglyceride accumulation, stimulate lipolysis and fatty acid β-oxidation, and reduce inflammation. Concomitantly, the polyphenols modulate signaling pathways including the AMP-activated protein kinase, peroxisome proliferator activated receptor γ, CCAAT/enhancer binding protein α, PPAR gamma activator 1-alpha, sirtuin 1, sterol regulatory element binding protein-1c, uncoupling proteins 1 and 2, and nuclear factor kappa B that regulate adipogenesis, antioxidant and anti-inflammatory responses. Animal studies strongly suggest that commonly consumed polyphenols described in this review have a pronounced effect on obesity as shown by lower body weight, fat mass, and triglycerides through enhancing energy expenditure and fat utilization, and modulating glucose hemostasis. Limited human studies have been conducted in this area, and are inconsistent about the anti-obesity impact of dietary polyphenols, probably due to the various study designs and lengths, variation among subjects (age, gender, ethnicity), chemical forms of the dietary polyphenols used and confounding factors such as other weight reducing agents. Future randomized controlled trials are warranted to reconcile the discrepancies between preclinical efficacies and inconclusive clinic outcomes of these polyphenols.
Keywords: dietary polyphenols, antioxidants, obesity, molecular mechanism, cell, animal, human
1. Introduction
The prevalence of obesity has steadily increased over the past three decades both in the United States and worldwide [1]. Obesity has been associated with co-morbid metabolic and chronic diseases, such as type-2 diabetes, heart diseases, hypertension and several forms of cancer, and has added tremendous burden to the health care system [1]. Obesity is a multifactorial complex disease influenced by lifestyle, behavioral, environmental as well as genetic factors. Obesity emanates from energy imbalance due to excess caloric intake relative to energy expenditure; the latter primarily reflects sedentary lifestyle and lack of physical activity [2, 3] . Other obesigenic factors such as genetic susceptibility, family history and gene-environment interactions all contribute to the development of obesity [2, 3].
Obesity is defined by excess adipose mass and adipose tissue expansion, which occurs through adipocyte hypertrophy and hyperplasia [4]. Adipose tissue is an important energy storage organ and has only been progressively recognized over the past two decades as a key endocrine organ with active metabolism [5]. Indeed, adipose endocrine function is critical to overall energy balance and homeostasis with adipocyte-derived pro- and anti-inflammatory adipokines playing key roles. When the production and secretion of pro-inflammatory adipokines prevail, systemic inflammation, insulin resistance and obesity-related metabolic disorders arise [5]. In addition to adipocytes, stroma vascular cells – preadipocytes, adipose stem cells - and immune cells within adipose tissue such as macrophages and lymphocytes are rich sources of adipokines and contribute to obesity-associated inflammation [5]. Thus, obesity is a chronic low-grade inflammation.
This inflammation-associated obesity can be prevented or even reversed with weight reduction that can be achieved via energy restriction and increased physical activity [6]. Some studies demonstrated that physical activity directly reduced inflammation, others reported that the anti-inflammatory effects of physical activity are indirectly caused by reduced adiposity, which in turn reduces fat mass and fat-derived inflammatory adipokines [7–9]. Moreover, consumption of foods rich in bioactive anti-inflammatory compounds, such as omega-3 fatty acids and polyphenols, has been documented to decrease inflammation [4, 10]. Beneficial effects of some diets such as the Mediterranean diets are attributed for the most part to the significant amounts of bioactive components with recognized anti-inflammatory and anti-oxidant properties [4, 10]. Indeed, several cell, animal and human studies provide strong evidence that dietary bioactive compounds act as anti-oxidant and anti-inflammatory agents to increase thermogenesis and energy expenditure while decreasing inflammation and oxidative stress, further supporting progress towards weigh loss and/or decreased metabolic disorders [4, 5, 11]. In a systemic review by Esfahani et al. [5], the authors reported that daily consumption of mixed fruits and vegetable supplements significantly increases serum levels of antioxidant pro-vitamins and vitamins (β-carotene, vitamins C and E) and folate and reduces homocysteine and markers of oxidative stress [12]. These findings emphasize the beneficial effects of food components and further support the dietary recommendations for Americans that emphasize diets rich in fruits and vegetables for the prevention of chronic diseases including obesity [13].
Laboratory studies indicate that the anti-obesity effects of polyphenol-rich diets may be attributed to the ability of polyphenols to interact, directly or indirectly, with adipose tissues (preadipocytes, adipose stem cells, and immune cells). Therefore, in this review, we discuss possible mechanisms for the inhibitory effects of commonly consumed dietary antioxidants, namely epigallocatechin gallate (EGCG) and green tea extracts, resveratrol and curcumin, on obesity based on cell, animal, and human studies.
2. Epigallocatechin gallate and green tea extract
Green tea is made from the dried leaves of the Camellia Sinensis plant. Different from fermented black tea and partially fermented oolong tea, green tea is a non-fermented tea that is produced from direct drying of fresh green tea leaves by hot steam and air. During this process, polyphenol oxidase is inactivated and polyphenols are preserved [14]. Compared to black tea and oolong tea, green tea contains the highest amount of green tea catechins [15], the major polyphenols in green tea that constitutes about 35% of its total dry weight [14]. A 2-gram bag of green tea contains about 500 mg of green tea catechins. The most abundant green tea catechins are (−)-epigallocatechin gallate (EGCG), which accounts for about 68–69% of green tea catechins, followed by (−)-epigallocatechin (EGC, circa 15–18%) (−)-epicatechin gallate (ECG, circa 5–6%), and (−)-epicatechin (EC, circa 2–5%) [16].
The anti-obesity potential of green tea catechins, particularly EGCG, has been shown in cell culture, animal and human studies. Table 1 lists the in vitro activities of EGCG and green tea extracts (GTE) in inhibiting preadipocyte differentiation, decreasing adipocyte proliferation, inducing adipocyte apoptosis, suppressing lipogenesis, and promoting lipolysis and fatty acid beta (β)-oxidation [17–28].
Table 1.
Effect of green tea catechins on obesity in cell studies
| First author, yr [ref] |
Exerimental design and treatments | Results |
|---|---|---|
| Chan, 2011 [17] |
3T3-L1 cells treated with EGCG (0–10 µM) for 18 days. |
↓ Cell viability ↓ Adipose conversion ↓ C/EBPα and PPARγ ↓ Fat accumulation in a dose-dependent manner |
| Furuyashiki, 2004 [18] |
3T3-L1 cells treated with EGCG (0–30 µM) for 5–8 days. |
↓ C/EBPα and PPARγ protein levels in a dose-dependent manner |
| Hung, 2005 [19] |
3T3-L1 cells treated with EC, EGC, ECG, or EGCG (0–400 µM) for 1–6 days or 2 hours pretreat. |
↓ Preadipocyte proliferation ↓ Phospho-ERK1/2, Cdk2, and cyclin D1 proteins, Cdk2 activity ↑ G0/G1 growth arrest, p21waf/cip, and p27kip1 |
| Hung, 2005 [19] |
3T3-L1 cells treated with EC, EGC, ECG, or EGCG (0–400 µM) for 24 or 48 hours. |
↑ Apoptosis and caspase-3 activity |
| Hwang, 2005 [20] |
3T3-L1 cells treated with EGCG (0 and 100 µM) for 48 hours. |
↑ AMPK activation ↑ Phosphorylated ACC |
| Kim, 2010 [21] |
3T3-L1 cells treated with EGCG (0–200 µM) for 2 days. |
↓ Fat accumulation in a dose-dependent manner ↓ Transcriptional activity of FOXO1 and SREBP-1c ↑ Phosphorylated FOXO1 protein levels |
| Ku, 2009 [22] |
3T3-L1 cells treated with EGCG (0–50 µM) for 1.5 hours. |
↓ Preadipocyte proliferation and mitogenesis ↓ Phosphorylation of IR-β, IRS1, IRS2, and MAPK proteins, RAF1, MEK1/2, and ERK1/2 |
| Ku, 2012 [23] |
3T3-L1 cells treated with EGCG (0–50µM) for 48 hours. |
↓ IGF-I-stimulated and IGF-I-stimulated mitogenesis |
| Lee, 2009 [24] |
3T3-L1 cells treated with EGCG (0, 1 or 10 µM) for 24 hours. |
↔ Cell viability At 10 µM EGCG: ↓ Intracellular TG accumulation ↑ Glycerol release ↑ HSL mRNA levels |
| Lee, 2009 [25] |
3T3-L1 cells treated with EGCG (0, 1, 5 or 10 µM) for 24 hours. |
↑ UCP-2 mRNA levels ↑ UCP-2 promoter activity |
| Lin, 2005 [26] |
3T3-L1 cells treated with EGCG (0, 50, 100, and 200 µM) for 0–24 hours. |
↓ Preadipocyte proliferation ↑ Apoptosis ↓ Fat accumulation in a dose-dependent manner |
| Liu, 2006 [27] |
3T3-L1 cells treated with EGCG (20 and 100 µM) for 0–2 hours. |
↓ Steady-state levels of Rstn ↓ Rstn mRNA and protein expression ↓ Phospho-ERK1/2 |
| Moon, 2007 [28] |
3T3-L1 cells treated with EGCG (0, 10, 50 and 100 µM) for 0–12 days. |
↓ Cell viability at ≥ 50 µM ↑ Increased generation of reactive oxygen species ↑ Phosphorylated AMPK levels and AMPK activation ↑ Phosphorylated ACC ↓ C/EBPα and PPARγ expression ↓ Fat accumulation in a time- and dose-dependent manner |
Abbreviations: ACC, acetyl-Coenzyme A carboxylase; AMPK, AMP-activated protein kinase; C/EBP, CCAAT/enhancer-binding protein; Cdk2, cyclin-dependent kinase 2; EC, epicatechin; ECG, epicatechin gallate; EGC, epigallocatechin; EGCG, epigallocatechin gallate; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; FOXO1, forkhead box protein O1; HSL, hormone sensitive lipase; IGF-I, insulin like growth factor-I; IR, insulin resistance; IRS, insulin receptor substrate; MAPK, mitogen-activated protein kinase; PPAR, peroxisome proliferators-activated receptor; Rstn, rat resistin; SREBP-1c, sterol regulatory element-binding protein-1c; UCP, uncoupling protein; ↑, increase; ↓, decrease; ↔, no change.
EGCG (10–100 µM) and with lower potencies, EC and EGC, induce dose- and time- dependent decrease in adipocyte viability [28, 29] and cell cycle arrest at the G0/G1 phase [19]. At lower concentrations (0–10 µM) EGCG induced G2/M growth arrest in a dose-dependent manner in mature 3T3-L1 adipocytes [17]. Concurrently, EGCG (0–400 µM) and less potently ECG, EGC and other catechins induce apoptosis in murine 3T3-L1 preadipocyte [29] and mature 3T3-L1 adipocytes [26] as shown by DNA fragmentation [29] and increased caspase-3 activity [29]. Furthermore, EGCG (0.5–10 µM) inhibits preadipocyte differentiation and at higher concentrations (50–200 µM) [17, 21, 24, 26, 28], cellular triglyceride accumulation in adipocytes in a dose- and time-dependent manner.
EGCG-mediated suppression of adipocyte differentiation may be attributed to its impact on genes playing crucial roles in adipocyte differentiation (Figure 1). Peroxisome proliferator activator receptor γ (PPARγ) and CCAAT/enhancer binding protein α (C/EBPα) are two key regulators of adipocyte differentiation that orchestrate the expression of adipogenic and lipogenic genes; these genes include acetyl-coenzyme A carboxylase (ACC) that converts acetyl-CoA to malonyl-CoA, a building block for fatty acid synthesis and an inhibitor for fatty acid oxidation [30], and the transcriptional factor sterol regulatory element-binding protein 1c (SREBP-1c) that enhances lipogenesis and adipogenesis [21]. EGCG’s effect on adipocyte differentiation is accompanied by down-regulation of the expression of PPARγ and C/EBPα at the mRNA and protein levels [17] and activation of AMP-activated protein kinase (AMPK), a suppressor of PPARγ and C/EBPα expression [17, 20].
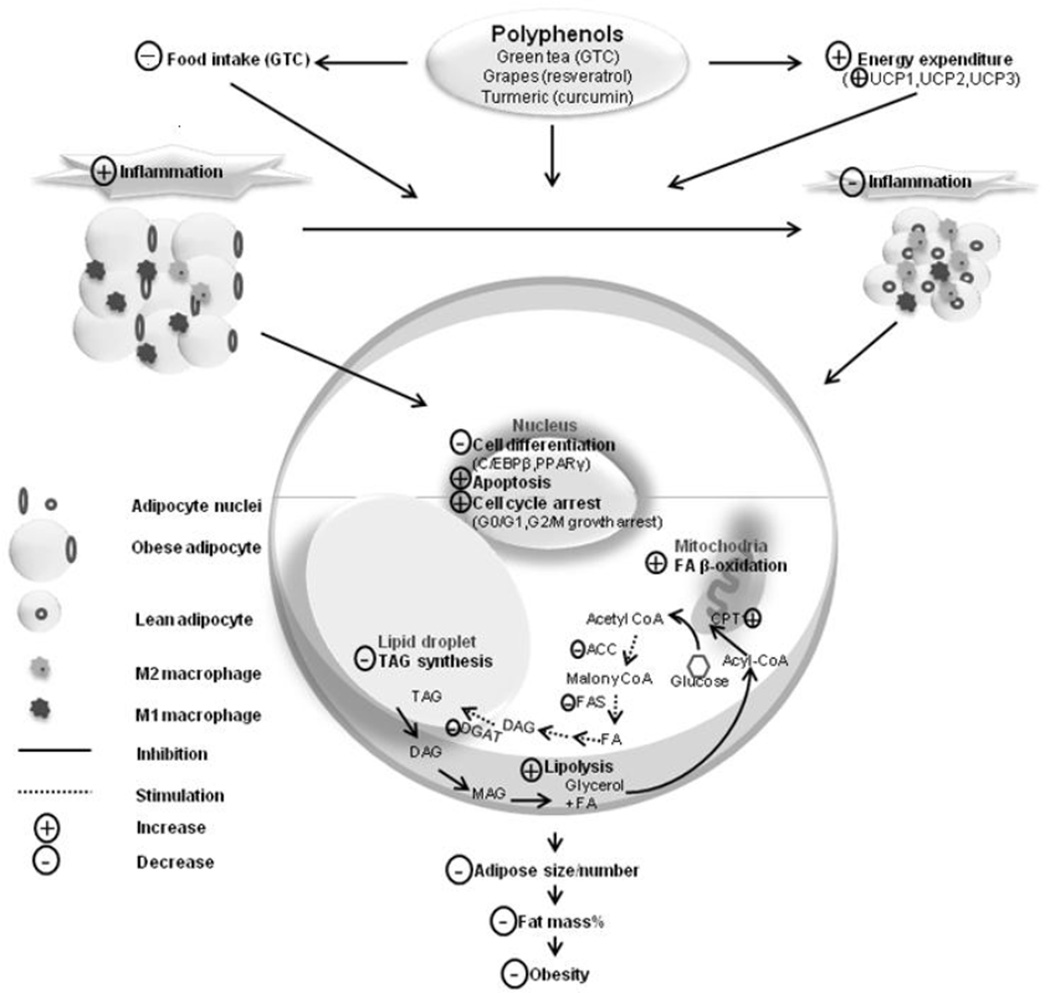
Figure 1.
Diagram illustrates the potential actions of dietary polyphenols, particularly green tea catechins (GTC), resveratrol and curcumin on obesity. GTC decrease food intake. These polyphenols increased energy expenditure through up-regulating uncoupled proteins (UCP1-3). They also decrease inflammatory response in adipose tissue, induce apoptosis and cell cycle arrest, inhibit adipogenesis and lipognenesis, stimulate lipolysis and fatty acid p-oxidation. These actions would lead to decreased adipocyte size and number, lowered fat mass% and decreased obesity risk.
Abbreviation: ACC, acetyl-CoA carboxylase; C/EBPp, CCAAT/enhancer binding protein beta; CPT-1, carnitine palmitoyltransferase-1; DAG, diglyceride; DGAT, diglyceride acyltransferase; FA, fatty acid; FAS, fatty acid synthase; MAG, monoglyceride; PPARy, Peroxisome proliferator activator receptor gamma; TAG, triglyceride.
Complementing EGCG’s effect on adipocyte differentiation is EGCG’s ability to up-regulate lipolysis and thermogenesis [24]. EGCG was found to increase glycerol release and the expression of hormone sensitive lipase and carnitine palmitoyltransferase-1 (CPT-1) that is involved in fatty acid β-oxidation in 3T3-L1 cells [17]. The mRNA levels of uncoupling protein (UCP)-2, a key protein in fat-supported thermogenesis, was increased by EGCG (0–10 µM) in a dose-dependent manner [25].
In addition to the effects of EGCG described above, reduced expression of resistin [27], an adipocyte-derived inflammatory adipokine that is associated with insulin resistance and increased risk of cardiovascular disease (CVD) [31], may also contribute to the EGCG-mediated anti-inflammatory response [31] and insulin sensitization [24, 25, 27]. In 3T3-L1 adipocytes, incubation with 100 µM EGCG for 3 hours decreased both mRNA and protein levels of resistin by 50% [27].
Preclinical studies in animals [32–54] further confirmed the beneficial effects of EGCG or GTE on obesity-related parameters including decreased BW [34, 35, 37, 39, 42–45, 47–54], adipose mass [32–35, 37, 39–42, 44, 45, 47, 50–54], total lipids, cholesterol, triglyceride (TG), and triacylglycerol (TAG) in liver and plasma [33, 34, 37, 40, 41, 45, 47, 48, 50, 53], and improved glucose homeostasis (increased glucose tolerance and decreased serum glucose, insulin resistance, and homeostasis model assessment-insulin resistance (HOMA-IR)) [32, 34, 36, 37, 47, 49, 52–54] (Table 2). Two common obesity models, a high-fat (HF) or cholesterol-rich diet-induced obesity model and leptin-deficient ob/ob mice model, were used in these studies. Various mechanisms have been proposed including suppressing dietary fat absorption [34, 37, 49], enhancing fat oxidation in adipose tissue and skeletal muscle [36, 39, 43, 52], increasing glucose utilization [32, 34, 37, 52], and decreasing de novo lipogenesis [33, 41, 43] (Table 2).
Table 2.
Effect of green tea catechins on obesity in animal studies
| First author, yr [ref] |
Experimental design and treatments | Results |
|---|---|---|
| Ashida, 2004 [32] |
Male Wistar rats (3-week-old). Groups including control group (laboratory chow) or GT group (bottled commercial product) for 3 weeks. |
↓ Adipose tissue weight ↓ Total-, free-, HDL-C and LDL-C and FFA ↓ Glucose uptake activity and translocation of GLUT4 on the plasma membrane in adipose tissue and skeletal muscle ↓ PPARγ and cleavage of SREBP-1 in adipose tissue ↔ BW, TG, glucose, and leptin |
| Axling, 2012 [33] |
Female C57BL/6J mice (8-week-old) in a HF diet-induced obesity model. Groups including HF group, HF+LP group, HF+GT (4%, w/w in diet) group, or HF+LP+GT(4%) group for 22 weeks. |
↓ Body fat content ↓ Plasma glucose, insulin, fructosamine, cholesterol, TAG, adiponectin ↓ mRNA expression of inflammatory markers (MCP-1, TNF-α) ↓ mRNA expression of lipogenesis (i.e, ACC, SREBP1c, PPARγ, CD36, LXR) |
| Bose, 2008 [34] |
C57BL/6J male mice (6-week-old) in a HF diet-induced obesity model. Experiments 1 and 2: Groups including LF group, HF group, HF+EGCG group (3.2 g/kg diet) for 16 weeks. Experiment 3: After LF and HF for 9 weeks, groups including LF group, HF group, HF+EGCG (3.2 g/kg) for 4 weeks. |
↓ BW, percent body fat, and visceral fat mass ↑ Fecal lipid content ↓ Liver weight, size and TG ↓ Plasma ALT, cholesterol, and MCP-1 ↓ Blood glucose and insulin resistance, HOMA-IR |
| Bruno, 2008 [35] |
Male ob/ob mice (5-week-old) and their C57BL/6J lean littermates in a HF dietinduced obese model. Groups including ob/ob group, ob/ob+1%GTE (w/w) group, ob/ob+2%GTE group, lean group, lean+1%GTE group, or lean+2%GTE group for 6 weeks. |
↓ BW, adipose tissue weight ↓ Hepatic steatosis histologically, total lipid, TG, hepatic α-tocopherol ↓ Serum ALT and AST ↔ Serum adiponectin, hepatic cholesterol, serum ALP |
| Chen, 2009 [36] |
Male Sprague Dawley rats (4-week-old) in a HF diet-induced obesity model. Groups including GT group or EGCG group (1 mg/kg/d) for 27 weeks. |
Compared to the control group, the GT group: ↓ FM and ↑ lean mass ↔ Liver TAG and plasma cholesterol, TG, FFA, LDL, HDL, total protein, glucose ↑ Glucose tolerance ↑ FA synthesis (SREBP-1c, FAS, MCD, ACC) ↑ FA oxidation (PPAR-α, CPT-1, ACO) ↓ Adipogenic expression (C/EBP-β, PPAR-γ) differentiation and FA update ↓ LPL, HSL, and UCP-2 Compared to the control group, the EGCG group ↑ Adipogenic expression (C/EBP-β, PPAR-γ, Pref-1, UCP-2) |
| Chen, 2011 [37] |
Male C57BL/6J mice (5-week-old) in a HF /Western-style diet-induced obesity and metabolic syndrome model. Groups including LF group, HF group, HF group, and HF+GT group (3.2g EGCG/kg diet) for 17 weeks. |
↓ BW, liver TG and damage ↓ Blood glucose, IR, and HOMA-IR ↓ Plasma cholesterol and ALT ↓ Inflammation markers (MCP-1, CRP, IL-6, GM-CSF) ↑ fecal lipids |
| Chung, 2012 [38] |
Male ob/ob mice (5-week-old) and their C57BL/6J lean littermates in a HF diet-induced obese model. Groups including ob/ob group, ob/ob+0.5%GTE (w/w) group, ob/ob+1%GTE group, lean group, lean+0.5%GTE group, or lean+1%GTE group for 6 weeks. |
↓ Hepatic steatosis and inflammation histologically ↓ Protein nitration (↓ NO metabolites) ↓ Oxidative stress and pro-inflammation (↓ iNOS and MPO mNRA) ↓ Lipid peroxidation (↓ LPO, 4-HNE, NADPH oxidase activity) |
| Cunha, 2013 [39] |
Male Swiss mice (8-week-old). Groups including control group (chow diet), GTE (chow diet+GTE at 400 mg/kg BW by oral gavage), HF group, or HF+GTE group for 2 months. |
↓ BW, adipose tissue ↑ HDL, adiponectin ↑ Lipolytic pathway (↑ HSL, ABHD5, perilipin in mesenteric adipose tissue) ↓ Proinflammatory signaling (↓ TNF-α, TLR4, MYD88, TRAF6) |
| Friedrich, 2012 [40] |
Male C57BL/6N mice (10-12-week-old) in a HF diet-induced obesity model. Experiment1: Groups including control group, 0.5% (w/w) EGCG group, or 1% EGCG group for 4 days. Experiment 2: Groups including semi-synthetic 13C-enriched HF diet, standard rodent chow, 0.25% EGCG, or 0.5% (w/w) EGCG for 7 days. |
↓ Food digestibility, BW and fat gain, liver weight, and epdidymal fat pad weight ↑ Palmitate oxidation, energy excretion, fat and nitrogen excretion ↓ Plasam NEFA, plasma and liver TG, liver glycogen content ↓ Lipogenic genes (ACC, FAS and SCD1) and lipid synthesis in liver and skeletal muscle ↓ Intestinal substrate transporters (CD36, FATP4, SGLT1) |
| Kim, 2009 [41] |
Male C57BL/6J–Lepob/ob mice (4-week-old). Groups including HF group or HF+GTE (0.05 g/100 g diet) group for 12 weeks. |
↓ Perirenal and total white adipose tissue weight ↑ Plasma HDL-C, HTR, and phospholipids ↓ TG synthesis enzymes (PAP) and FA synthesis enzymes (G6PD and ME) ↔ FA oxidation enzymes (β-oxidation and CPT-1) |
| Klaus, 2005 [42] |
Male New Zealand black mice (1–4 week-old) in a HF diet-induced obesity model. Experiment 1 (short-term feeding effect): Groups including HF group, HF+0.5% (w/w in diet) EGCG, or HF+1.0% EGCG for 4 weeks. Experiment 2 (acute effect): Groups including control group (water) or EGCG group (500 mg/kg in water) for 3 days. |
Experiment 1: ↓ BW, body fat content, epididymal fat pads ↑ Fecal energy content ↓ Leptin and SCD1 gene expression in white fat ↑ UCP2 gene expression in liver ↓ Gene expression of SCD1, ME, and GK in liver ↔ SCD1 and UCP1 gene expression in brown fat Experiment 2: ↔ Body temperature, activity, and energy expenditure ↓ RQ during night |
| Lu, 2012 [43] |
Female SD rats (3-month-old) in a high-fat diet-induced obesity model. Groups including LF group, HF group or HF+0.5% (w/v) GTP for 8 months (4 month with GTP). |
↓ BW ↓ Protein expression of IL-1β and IL-6 in serum ↑ Orexigenic genes (Agrp, Ghrl, Nr3c1) ↓ Anorectic genes (Apoa4, Cntfr, Ghr, IL-1β, Ins1, Lepr, Sort1) ↑ Energy expenditure (Adcyap1r1, Adrb 1) ↑ Antioxidant activity genes (SOD1 and COMT in liver |
| Monteiro, 2008 [44] |
Male Wistar rats (12-month-old). Groups including control group or GT group (drinking beverage 52.8±6.4 mL/d) for 6 months. |
↓ BW and adipocyte size ↑ Aromatase expression in adipose tissue ↑ Apoptotic cell number in visceral adipose tissue ↓ Plasma testosterone |
| Park, 2011 [45] |
Male obese (ob/ob) mice (5-week-old) and their lean littermates. Groups including lean control group, obese control group, obese+0.5% GTE, or obese+1% GTE for 6 weeks. |
↓ BW, FM, liver lipids and ALT ↓ Hepatic steatosis and injury (↓ TAG, NEFA, cholesterol) ↓ Hepatic inflammation (↓TNF-α) ↓ Hepatic lipid peroxidation (↑ catalase and GPX) ↑ Hepatic enzyme antioxidant defenses ↓ mRNA of FAS, SCD-1, HSL in adipose |
| Park 2012, [46] |
Male Wistar rats (16-week-old) in a HF diet-induced obesity model. Groups including LF group, HF group, HF+1%GTE group, or HF+2%GTE group for 8 weeks. |
↓ ALT and hepatic lipid ↑ Glutathione ↓ Protein and mRNA levels of TNF-α and MCP-1, ↓NFκB binding activities in liver and adipose |
| Ramadan, 2009 [47] |
Adult male Wistar albino rats (120 g) in a cholesterol-rich diet. Groups including control group (500 mg distilled water) group, GTE50 group (50 mg/kg BW) or GTE100 group (100 mg/kg BW) for 4 weeks. |
↓ BW and liver weight:BW ratio ↓ Serum glucose, total lipids, TAG, phospholipids, and atherogenic index values ↓ Serum aminotransferase and ALP activities |
| Richard, 2009 [48] |
Male leptin-deficient (ob/ob) mice and their C57BL/6J lean littermates (4-week-old). Groups including control group (0.5% citric acid buffer) or GT group (decaffeinated green tea 2%, w/v) for 6 weeks. |
↓ BW in adult mice, not in lean littermates ↓ Plasma cholesterol, TG, and adiponectin ↔ Fecal lipid |
| Sae-tan, 2011 [49] |
Male C57BL/6J mice (5-week-old) in a HF diet-induced obesity model. Groups including LF group, HF group, or HF+EGCG (0.32%, w/w in diet) for 15 weeks. |
↓ BW, liver weight and TAG ↓ Blood glucose, plasma insulin, and insulin resistance ↑ Fat oxidation in skeletal muscle (MCAD, NRF1, UCP3, PPARα) ↑ Fecal lipid excretion |
| Sayama K 2000 [50] |
Female mice. Groups including control group, 1% (w/w in diet) GT, 2%GT, and 4%GT for 16 weeks. |
↓ BW and intraperitoneal adipose tissue ↓ Food intake in 4%GT group ↓ Liver cholesterol and TG ↓ Serum TG, NEFA, and leptin |
| Shen, 2012 [51] |
Female SD rats (3-month-old) in a HF diet-induced obesity model. Groups including LF group, HF group or HF+0.5% (w/v) GTP for 8 months (4 month with GTP). |
↓ BW and %FM and ↑ %FFM ↓ Serum IGF-I, leptin, adiponectin, and proinflammatory cytokines (IL-1α, IL-2, IL-4, IL-10, GM-CSF, IFN-γ, TNF-α) ↑ GPX protein expression in liver |
| Shimotoyodome, 2005 [52] |
Male C57BL/6J mice (8-week-old) in a HF diet-induced obesity model. Groups including LF group, HF group, HF+GTE (0.5%, w/w in diet) group, HF+EX (24m/min × 30 min/d × 3/wk) group, HF+GTE+EX group for 4 or 15 weeks. |
Compared to the HF group, the HF+GTE and HF+GTP+EX groups ↓ BW, visceral fat accumulation ↑ Energy expenditure and fat utilization ↑ FA oxidation in liver and skeletal muscle ↓ Plasma glucose, NEFA, leptin insulin levels ↓ RER and carbohydrate use |
| Tian, 2013 [53] |
Male Wistar rats in a HF diet-induced obesity model. Groups including HF group, HF+lowGTP group (0.8 g/L), HF+midGTP group (1.6 g/L), or HF+highGTP group (3.2 g/L) for 26 weeks. |
↓ BW, VAT accumulation ↓ Plasma glucose, HOMA-IR ↓ Lipid profiles (↓ TC, TG, LDL-C/HDL-C) ↑ Serum adiponectin and mRNA adiponectin ↓ Phosphorlation of PPARγ (↓ Protein and mRNA PPARγ) |
| Ueda, 2012 [54] |
Male C57BL/6J mice (5-week-old) in a HF diet-induced obese model. Groups including control group, control+0.5% (w/v) GTE group, HF diet group, or HF+0.5%GTE group in drinking water for 14 weeks. |
↓ BW and visceral adipose tissue weight ↓ Plasma glucose, insulin, and HOMA-IR ↑ IGFBP-1 in epididymal and mesenteric white adipose tissue |
Abbreviations: ABHD5, abhydrolase domain containing 5; ACC, acetyl-Coenzyme A carboxylase; ACO, acetyl-CoA oxidase; Adcyap1r1, adenylate cyclase activating polypeptide 1 receptor 1; Adrb1 Adrenergic, beta-1-, receptor; Agrp, agouti-related protein; ALT, alanine aminotransferase; ALP, alkaline phosphatase; Apoa4, apoliprotein (apo) A-IV; AST, aspartate transaminase; BW, body weight; C/EBP, CCAAT/enhancer-binding protein; Cntfr, ciliary neurotrophic factor receptor; COMT, Catechol-O-methyltransferase; CPT-1, carnitine palmitoyltransferase-1; CRP, C-reactive protein; EGCG, epigallocatechin gallate; EX, exercise; FA, fatty acids; FASN (FAS), fatty acid synthase; FATP4, fatty acid transport protein 4; FFA, free fatty acid; FM, fat mass; FFM, fat free mass; G6PD, glucose-6-phosphate dehydrogenase; G-CSF, granulocyte colony-stimulating factor; Ghr, growth hormone receptor; Ghrl, ghrelin/obestatin prepropeptide; GK, glucokinase; GLUT4, glucose transporter type 4; GM-CSF, granulocyte-macrophage colony-stimulating factor; GPX, glutathione peroxidase; GT, green tea; GTC, green tea catechins; GTE, green tea extract; GTP, green tea polyphenols; HDL-C, high density lipoprotein-cholesterol; HF, high fat; 4-HNE, 4-hydroxynonenal; HOMA-IR, homeostasis model assessment-insulin resistance; HSL, hormone sensitive lipase; HTR, HDL-cholesterol/total-cholesterol ratio; IFN, interferon; IGF-I, insulin like growth factor-I; IGFBP-1, insulin-like growth factor binding protein-1; IL, interleukin; iNOS, inducible nitric oxide synthase; Ins1, insulin 1; IR, insulin resistance; LDL-C, low density lipoprotein-cholesterol; Lepr, leptin receptor; LF, low fat; LPL, lipoprotein lipase; LPO, lactoperoxidase; LXR, liver X receptor; MCAD, medium chain acyl coA dehydrogenase; MCD, malonyl CoA decarboxylase; MCP-1, monocyte chemoattractant protein-1; ME, malic enzyme; MPO, myeloperoxidase; MyD88, myeloid differentiation primary response gene 88; NADPH, nicotinamide adenine dinucleotide phosphate; NEFA, non-esterified fatty acid; NF-κB, nuclear factor kappa B; NO, nitric oxide; Nr3c1, nuclear receptor subfamily 3, group C, member 1; NRF1, nuclear respiratory factor 1; PAP, phosphatidic acid phosphatase; PPAR, peroxisome proliferators-activated receptor; Pref-1, preadipocyte factor–1; RER, respiratory exchange ratio; RQ, Respiratory quotient; SCD1, stearoyl-CoA desaturase-1; SGLT1, sodium-dependent glucose transporter 1; SOD, superoxide dismutase; Sort1, Sortilin 1; SREBP-1, sterol regulatory element-binding protein-1; TAG, triacylglycerol; TC, total cholesterol; TG, triglyceride; TLR, toll like receptor; TNF-α, tumor necrosis factor alpha; TRAF6, TNF receptor-associated factor 6; UCP, uncoupling protein; ↑, increase; ↓, decrease; ↔, no change.
Although the etiology of obesity is still not well studied, emerging evidence has indicated the effect of chronic inflammation-induced excessive ROS or oxidative stress on the development of obesity [55]. Alvehus et al. reported that the human visceral fat depot proposes a unique inflammatory profile and it may directly contribute to chronic systemic inflammation in obese humans [55]. Therefore, lowering the levels of ROS or oxidative stress becomes an important strategy to deal with obesity-related alteration. More recently, it has been suggested that such anti-obesity benefit of green tea supplementation may be, in part, meditated through its ability to act as an antioxidant and singlet oxygen quencher, therefore inhibiting the destructive effects of ROS and suppressing inflammation in the development of obesity [38, 39, 43, 45, 51].
For instance, Shen et al. reported that compared to the female rats in low-fat diet, animals fed a high-fat diet for 8 months had increased percentage of fat mass (FM) and serum insulin-like growth factor-I (IGF-I) and leptin levels and reduced percentage of fat-free mass (FFM) and serum adiponectin levels. Supplementation of green tea polyphenols (GTP) in drinking water for 4 months in the obese rats decreased body weight (BW) and percentage of FM, increased percentage of FFM, bone mineral density and strength, and glutathione peroxidase (GPX) protein expression, and suppressed serum IGF-I, leptin, and proinflammatory cytokines in the obese rats. This study demonstrates GTP supplementation benefited body composition and bone properties in obese rats possibly through enhancing antioxidant capacity and suppressing inflammation [51]. Based on the obesity-related gene array analysis, the same research team further demonstrated that GTP supplementation has potent effects on increasing adiposity or fat accumulation in obese middle-aged female rats through enhancing obesity-related anorectic genes and anti-oxidative stress capacity, suppressing obesity-related orexigenic genes and pro-inflammation activity, and modulating estrogen-associated action [43].
The effect of green tea administration on obesity-related parameters has been reported by a number of human studies (Table 3) [56–76]. Green tea consumption, in the forms of EGCG or GTE with or without caffeine, by obese subjects decreased BW, body mass index (BMI), and waist and hip circumference, total body fat, and abdominal visceral and subcutaneous fat area. On the other hand, the impact of green tea supplementation on lipid profiles (total cholesterol, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), TAG, TG, or free fatty acids (FFA), glucose homeostasis (glucose, insulin sensitivity, or HOMA-IR), as well as blood pressure (systolic blood pressure (SBP) or diastolic blood pressure (DBP)) in obese subjects seem to be inconsistent by showing either an improvement or no effect (Table 3). The difference in lipid profiles, glucose homeostasis, and blood pressure may be related to different periods of weight management (weight loss period vs. weight maintenance period) and the health status of subjects. Nevertheless, authors commented that green tea may facilitate weight loss by enhancing energy expenditures and fatty acid oxidation, confirming the preclinical studies (Table 2).
Table 3.
Effect of green tea catechins on obesity in human studies
| First author, yr [ref] |
Experimental design and treatments | Results |
|---|---|---|
| Auvichayapat, 2008 [56] |
Randomized, controlled trial. Obese subjects (n=60) in Thailand. Groups including placebo group or GT group (44 mg total catechins, 28 mg caffeine) for 12 weeks. |
↓ BW ↑ REE, RQ, fat oxidation, urine VMA ↔ BMI, waist/hip circumference, food intake, physical activity, satiety |
| Basu, 2010 [58] |
Randomized, controlled trial. Obese subjects with metabolic syndrome (n=35, 42.5±1.7 y) in USA. Groups including the control group (4 cups water/d), GT group (928 mg total catechins in 4 cups/d) or GTE group (870 mg total catechins in 2 capsules/d) for 8 weeks. |
↓ BW, BMI ↓ Lipid peroxidation (↓ MDA, HNE) ↑ Plasma EGC, EGCG, ECG ↔ Lipid profiles (TG, total cholesterol, LDL-C, HDL-C), glucose, HbA1C, HOMA-IR |
| Basu, 2011 [57] |
Randomized controlled trial. Obese subjects with metabolic syndrome (n=35, 42.5±1.7 y). Groups including the control group (4 cups water/d), GT group (928 mg total catechins in 4 cups/d) or GTE group (870 mg total catechins in 2 capsules/d) for 8 weeks. |
↔ Waist circumference, SBP, DBP, TG, HDL, glucose ↔ Inflammatory markers (adiponectin, CRP, IL-6, IL-1β, sVCAM-1, sICAM-1, leptin, leptin:adiponectin ratio) ↔ Plasma AST, ALT, BUN, creatinine, Hb, platelets, WBC, electrolytes, albumin, total protein, TSH ↓ Plasma serum amyloid alpha |
| Bogdnanski, 2012 [59] |
Double-blinded, placebo-controlled trial. Obese, hyptertensive subjects (n=56, 30–60 yr). Groups including placebo group or GTE group (379 mg GTE/d) for 3 months. |
↔ BMI, waist circumference, creatinine, glucose ↓ SBP, DBP ↓ TCH, LDL-C, TG and ↑ HDL-C ↓ Serum insulin, HOMA-IR, ↓ Inflammation and oxidative stress (↓ TNF-α and CRP, ↑ total antioxidant status) |
| Boschmann, 2007 [60] |
Randomized double blinded placebo-controlled, cross-over pilot study. Overweight/obese male subjects (n=6, 40±1 y) in Switzerland. Groups including placebo group or EGCG group (300 mg EGCG/day) for 2 days. |
↓ RQ ↔ Energy expenditure |
| Brown, 2009 [61] |
Double-blinded, randomized and placebo-controlled study. Overweight/ obese men (n=88, 40–65 y) in UK. Groups including placebo group (800 mg lactose/day) or EGCG group (800 mg EGCG/day) for 8 weeks. |
↓ %Body fat ↔ Insulin sensitivity, insulin secretion, glucose tolerance ↓ DBP ↑ Positive mood feeling |
| Brown, 2011 [62] |
Double-blinded, randomized, placebo-controlled cross-over study. Overweight/obese men (n=137, 40–69 yr) in UK. Groups including placebo group or GTE group (800 mg total catechins/d) for 6 weeks. |
↔ SBP, DBP ↔ Total cholesterol, HDL-C, TAG, glucose, insulin ↑ Plasma EGCG and urinary EGC, 4’-O-methyl EGC ↓ LDL-C |
| Chantre, 2002 [63] |
Open uncontrolled design. Overweight/obese subjects (n=70, 20–69 yr) in France. Groups including placebo, caffeine, or GTE (375 mg catechins) for 3 months. |
↓ BW, waist circumference ↑ Total 24-h EE ↔ Plasma cholesterol, SBP, DBP |
| Di Pierro, 2009 [64] |
Controlled study. Obese adults (n=100, 25–60 yr) in Italy. Groups including control group or GTE group (300 mg) for 90 days. All subjects consumed a hypocaloric diet. Only n=10–30 from each group for outcome measures. |
↓ BMI, Total cholesterol, TG ↔ Waist circumference, LDL-C, HDL-C, glucose, insulin, IGF-I, GH, cortisol, leptin (n=10/group) |
| Diepvens, 2005 [65] |
A double-blinded, placebo-controlled, parallel design. Overweight women (n=23, 19–57 yr) in Netherlands. Groups including placebo group, LowGT (50 mg caffeine+250 mg catechins), or HighGT (75 mg caffeine+375 mg catechins) for 87 days. |
↔ Energy expenditure, substrate oxidation ↔ BW, BMI, waist and hip circumference, FM, FFM |
| Dulloo, 1999 [66] |
Placebo-controlled study. Healthy young men (n=10, 25±1 y) in France. Groups including placebo group, GTC group (50 mg caffeine and 90 mg EGCG), or caffeine group (50 mg) for 24 hours. |
↑ 24-h EE ↓ 24-h RQ |
| Hill, 2007 [67] |
Randomized, placebo-controlled study. Overweight/obese postmenopausal women (n=38, 45–70 yr) in USA. All subjects exercised at moderate intensity. Groups including placebo group or EGCG (300 mg/d) for 12 weeks. |
↔ BW, BMI, waist circumference, total body fat, abdominal fat, intra-abdominal adipose tissue ↓ Resting HR, plasma glucose |
| Hsu, 2008 [68] |
Randomized, double-blinded, placebo-controlled trial in Taiwan. Obese women (n=100, 16–60 yr). Groups including placebo group to GTE group (491 mg catechin/d) for 12 weeks. |
↔ BW, BMI, waist circumference |
| Kovacs, 2004 [69] |
Randomized, parallel, placebo-controlled design. Overweight and moderately obese men and women (n=104, 18–60 y) in Netherlands. All subjects were in very-low-energy diet intervention of 4 weeks followed by a weight-maintenance period of 13 weeks. Groups including placebo group (450 mg/d) or green tea group (104 mg caffeine/d, 573 mg catechins /d) for 17 weeks. |
After 4 weeks weight loss period: ↓ BW, FM, FFM, waist circumference ↓ REE & RQ ↓Plasma glucose, insulin, BHB, glycerol, NEFA, TG, leptin ↑ Fatty oxidation After 13 weeks weight-maintenance period: no changes in any parameters. |
| Maki, 2009 [70] |
Randomized, double-blinded, placebo-controlled trial. Overweight/obese adults (n=132, 16–60 y) in USA. Groups including control group (39 mg caffeine/day) or GTC group (625 mg GTC, 39 mg caffeine/day) for 12 weeks. All subjects engaged in moderate intensity exercise. |
↓ BW, total abdominal fat area, abdominal subcutaneous fat area ↓ Serum TG, FFA ↔ %body fat, waist circumference, intra-abdominal fat area, plasma hs-CRP, plasma MDA, glucose homeostasis (glucose, inclusin, HbA1c) |
| Matsuyama, 2008 [71] |
Double-blinded, randomized, controlled design. Obese children (n=40, 6–16 yr) in Japan. Groups including control group (75 mg catechins) or catechin group (576 mg catechins) for 24 weeks. |
↔ Body FA, FFA, glucose, TG, PAI-1, hsCRP, leptin, growth hormone, blood chemistry profile ↓ Waist circumference, LDL-C/HDL-C |
| Nagao, 2005 [73] |
Double-blinded study. Normal to overweight male (n=17–18, 24–46 y) in Japan. Groups including control group (22 mg GTC/d) or GTC group (690 mg GTC/d) for 12 weeks. |
↓ BW, BMI, body FM, subcutaneous fat area, waist circumference ↑ Skin fold thickness ↓ MDA-modified LDL |
| Nagao, 2007 [72] |
Randomized double-blinded controlled parallel multicenter trial. Obese subjects (n=240, 25–55 y) in Japan. Groups including control group (96 mg catechins/d) or catechin group (583 mg catechins/d) for 12 weeks. |
↓ BW, BMI, body fat ratio, body FM, waist and hip circumference, visceral fat area, and subcutaneous fat area ↓ SBP ↓ LDL-C |
| Suliburska, 2012 [74] |
Prospective, randomized, double-blind design. Obese subjects (n=46, 30–60 yr) in Poland. Groups including placebo group or GT group (379 mg GTE, 208 mg EGCG daily) for 3 months. |
↓ BMI, waist circumference, glucose ↔ SBP, DBP, HDL-C, glucose ↔ Serum Fe, Cu, Ca, Mg ↓ Total cholesterol, LDL -C, TG ↑ Total antioxidant level (↑ serum Zn) |
| Thielecke, 2010 [75] |
Randomzied, double-blinded crossover study. Healthy overweight/obese men (n=10, 23– 40 y) in Germany. Groups including placebo group, low EGCG group (300 mg daily), high EGCG group (600 mg daily), caffeine group (200 mg daily), or EGCG/caffeine group (300 mg/200 mg daily) for 3 days. |
↔ Energy expenditure ↓ RQ ↑ Postprandial fat oxidation rate (EGCG/caffeine group only) ↓ Carbohydrate oxidation rate (EGCG/caffeine group only) |
| Wang, 2010 [76] |
Randomized, placebo-controlled trial. Moderately overweight Chinese subjects (n=182, 18–55 y). Groups including control group (30 mg catechins, 10 mg caffeine/day), GT1 group (458 mg catechins, 104 mg caffeine/day), GT2 group (468 mg catechins, 126 mg caffeine/day), or GT3 group (886 mg catechins, 198mg caffeine/day) for 90 days. |
↓ Total body fat and fat % in a dose-dependent manner ↓ Intra-abdominal fat area, waist circumference in GT3 group |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; BHB, beta-hydroxybutyrate; BMI, body mass index; BUN, blood urea nitrogen; BW, body weight; Ca, calcium; CRP, C-reactive protein; Cu, cooper; DBP, diastolic blood pressure; EGC, epigallocatechin; EGCG, epigallocatechin gallate; FFA, free fatty acids; FA, fatty acids; Fe, iron; FFA, free fatty acid; FM, fat mass; FFM, fat free mass; GH, growth hormone; GT, green tea; GTC, green tea catechins; GTE, green tea extract; Hb, hemoglobin; HbA1C, glycated hemoglobin, HDL-C, high density lipoprotein-cholesterol; HNE, hydroxynonenal; HOMA-IR, homeostasis model assessment insulin resistance; hs-CRP, high sensitivity C-reactive protein; IGF-I, insulin like growth factor-I; IL, interleukin; LDL, low density lipoprotein; LDL-C, low density lipoprotein-cholesterol; MDA, malondialdehyde; Mg, magnesium; NEFA, non-esterified fatty acid; PAI-1, plasminogen activator inhibitor type I; REE, resting energy expenditure; RQ, respiratory quotient; SBP, systolic blood pressure; sICAM-I, soluble intercellular adhesion molecule I; sVCAM-1, soluble vascular cell adhesion molecule-1; TAG, triacylglycerol; TG, triglyceride; TSH, thyroid-stimulating hormone; VMA, vanilmandelic acid; WBC, white blood cell; Zn, zinc; ↑, increase; ↓, decrease; ↔, no change.
In human studies, not all studies have found positive results for obesity-related measures. Green tea administration has also shown no influence on BW, BMI, FM, and waist and hip circumference. For example, Diepvens et al. reported that 12-week green tea supplementation to a low-energy diet, independent of habitual caffeine intake, had no effect on measures of BW or body composition in overweight women at 4 weeks or 3 months [65]. Similar non-significant findings was observed in Hill’s study that there was no difference in BW, BMI, waist circumference, abdominal fat and intra abdominal adipose tissue between the placebo group and the EGCG-supplemented group in overweight/obese postmenopausal women who attended regular aerobic exercise [67]. In a randomized, double-blinded, placebo-controlled trial in Taiwan, Hsu et al. reported there was no statistical difference in % reduction in BW, BMI, and waist circumference between the placebo and the GTE-supplemented (491 mg catechins) groups after 12 weeks of treatment [68]. Unlike Diepvens’ or Hill’s studies, there was no other weight control maneuver in Hsu’s study. Furthermore, lack of beneficial impact of GTE on obesity was also reported by Matsuyama et al. in obese children [71], Bogdnanski et al. in obese with hypertensive subjects [59], and Basu et al. in obese subjects with metabolic syndrome [57].
Several systemic reviews have been published in the past four years. Based on a meta-analysis of 11 studies, Hursel et al. concluded that catechin or an EGCG-caffeine mixture contained in green tea had a small effect on weight loss and weight maintenance [77]. Phung et al. reported that participants in intervention groups who received green tea catechins (GTC) in combination with caffeine had a decreased BW and waist circumference, compared with the control groups containing similar amounts of caffeine [78]. However, in a recent meta-analysis analyzing randomized controlled trials (RCT) (n=14) with at least 12 week’s duration, Jurgens et al. reported that green tea administration appears to induce a small, statistically non-significant weight loss in overweight or obese adults. Because the amount of weight loss is small, Jurgen et al. commented that it is not likely to be clinical important. In addition, green tea had no significant effect on the maintenance of weight loss [79].
The discrepancies among these clinical studies employing green tea may be due to the varieties of study designs (uncontrolled design, RCT, crossover), the length of study (24 hours - 24 weeks), age and gender of subjects, the ethnicity of subjects, the formulations of green tea supplement (EGCG, GTC, GTE), and the presence or absence of weight control factors (i.e., caffeine, exercise, low-caloric diet), etc.
In summary, cellular and animal studies have shown that dietary supplementation with EGCG or GTE is a potentially viable nutritional strategy for the prevention of obesity. However, the low bioavailability of GTC along with potential confounders (i.e., ethnicity and genetic effects, habitual caffeine or GTC intake, etc.) may have contributed to the inconsistent outcome of human studies. More well-controlled, long-term human studies using green tea supplementation are warranted.
3. Resveratrol
Resveratrol (3, 4’, 5-trihydroxystilbene), a phytoalexin, is a naturally occurring polyphenolic compound. It is produced by some plants in response to strenuous conditions such as fungal infection, injuries or UV irradiation [80]. Resveratrol is found in grapes, red wine and some berries. Resveratrol exists in two isomeric forms: cis- and trans-resveratrol. Trans-resveratrol is naturally found in grape, mainly in the skin but not in flesh; it is also present in the leaf epidermis of the grape vine [81]. Trans-resveratrol is the main form of resveratrol found in red grape juice (3.38 mg/L) [82]. Other than grapes, trans-resveratrol is found in 72 other plant species and in foods like mulberries, peanuts and in small amounts in cocoa [83, 84]. Cis-resveratrol is present in red wine but not grapes; trans-resveratrol is converted to cis- form by yeast during fermentation or is released from trans-resveratrol polymer vinferins. Cis-resveratrol can also be formed by exposure of trans-resveratrol to UV irradiation [85]. Both trans- and cis- forms of resveratrol have similar biological and antioxidant activities. Since cis- resveratrol is not commonly found in foods and is unstable, trans-resveratrol has been studied more extensively [86] and is the subject of this review.
Many studies using adipocytes have demonstrated that resveratrol has an anti-obesity potential by inhibiting preadipocyte differentiation, decreasing adipocyte proliferation, inducing adipocyte apoptosis, decreasing lipogenesis, and promoting lipolysis and fatty acid β-oxidation (Table 4) [87–100]. These effects of resveratrol may be mediated by central regulators of adipogenesis, lipogenesis, and fatty acid β-oxidation including the aforementioned AMPK, sirtuin 1 (SIRT1), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α). Resveratrol increases the intracellular cyclic adenosine monophosphate (cAMP) concentration by inhibiting cAMP phosphodiesterases (PDEs), which degrade cAMP [101, 102]. Increased cAMP concentrations activate AMPK, an important enzyme in regulating cellular energy homeostasis. AMPK binds to the promoter of PGC-1α, a transcriptional coactivator and a regulator of mitochondrial biogenesis and function [103]. Resveratrol is an indirect activator of SIRT1, a deacetylase [104, 105]. Activated SIRT1 can further deacetylate and activate PGC-1α via transcriptional and post-translational mechanisms [106].
Table 4.
Effect of resveratrol on obesity in in vitro, animals, and humans
| First author, yr [ref] |
Experimental design and treatments | Results |
|---|---|---|
| In vitro study | ||
| Ahn, 2007 [87] |
3T3-L1 cells treated with RSV (0, 5, 25, 50, and 100 µM) for 6 hours. | ↓ TNF-α-induced PAI-1 and IL-6 production in a dose-dependent manner ↑ Adipokines and adiponectin |
| Chen, 2011 [88] |
3T3-L1 cells treated with RSV (0, 10, 20, 40, and 80 µM) for 0–8 days. | ↓ Phosphorylation of AMPK ↓ Adipocyte differentiation ↓ PPARγ, C/EBPα, SREBP-1c, adiponectin, and leptin in a dose-dependent manner ↓ Fat accumulation in a dose-dependent manner |
| Chen, 2012 [89] |
3T3-L1 cells treated with RSV (0–80 µM) for 0–96 hours. |
↓ Cell proliferation ↑ Apoptosis ↑ Lactate dehydrogenase leaking ratio ↑ SIRT1 and AMPK ↓ AKT |
| Costa, 2011 [90] |
Human visceral adipocytes treated with RSV (1 µM) for 1 hour. |
↑ SIRT1, FOXO1, adiponectin, mRNA expression ↓ PPARγ1-3, and PPARβ/δ mRNA expression |
| Kang, 2010 [91] |
RAW264.7 cells and 3T3-L1 cells treated with RSV (0.1, 1, and 10 µM) for 1 hour. | RAW264.7 cells: ↓ LPS-stimulated IL-6 and TNF-α production 3T3-L1 cells: ↓ Proinflammatory factors production and adipokine mRNA expression ↓NF-κB activation and ERK1/2 phosphorylation ↓ Phosphorylation of IRS-1 and AKT ↑ Insulin-stimulated glucose consumption |
| Kang, 2012 [92] |
3T3-L1 cells treated with RSV (0–40 µM) for 0–6 days. |
↓ TG concentration ↓ C/EBPβ, PPARγ and FABP4 ↓ MMP-2 and MMP-9 |
| Kwon, 2012 [93] |
3T3-L1 cells treated with RSV (0, 25, and 50 µM) for 0–6 days. | ↓ Fat accumulation in a dose-dependent manner ↓ C/EBPαand PPARγ ↓ Adipogenesis ↓ MCE and insulin signaling pathway ↑ Cell cycle arrest |
| Lasa, 2011 [94] |
3T3-L1 cells treated with RSV (0, and 100 µM) for 12, 24, and 48 hours. | ↑ FFA release ↑ ATGL gene and protein expressions ↓ TG content |
| Lasa, 2012 [95] |
3T3-L1 cells treated with RSV (1, 10, and 25 µM) for 24 hours for mature adipocytes or 0–8 days for premature adipocytes. | ↓ Intracellular TAG content ↓ C/EBPβ and PPARγ mRNA levels, ↑ ATGL, CPT-1, SIRT-1, and PGC1-α expression ↓ FASN |
| Rayalam, 2008 [96] |
3T3-L1 cells treated with RSV (0, 10, 25, 50, 100, 200 and 400 µM) for 48 hours. | ↓ Cell viability at >100 µM ↑ Apoptosis at >100 µM ↓ Lipid accumulation at 25 and 50 µM ↓ PPARγ, C/EBPα, SREBP1 and FAS, LPL mRNA expression ↑ SIRT3, UCP1 and Mfn2 mRNA expression ↑ Mitochondria activity |
| Wang, 2011 [97] |
3T3-L1 cells treated with RSV (0, 12.5, 25, 50, and100 µM) for 0–24 hours. | ↑ DsbA-L and adiponectin ↓ PI3K pathway ↑ FOXO1 and AMPK |
| Zhang, 2012 [98] |
3T3-L1 cells treated with RSV (0–50 µM) for 8 hours. | ↑ Adiponectin synthesis ↑ PPARc DNA-binding activity |
| Zhang 2012 [99] |
3T3-L1 cells treated with RSV (0, 30, 60, 120, 250, 500, and 1,000 µg/ml) for 24 or 48 hours. |
↔ Cell viability ↓ Fat accumulation in a dose-dependent manner ↓ LPL, SCD1, and FAS ↓ FABP4, C/EBPα, and PPARγ ↓ Cell differentiation |
| Zhu, 2008 [100] |
3T3-L1 cells treated with RSV (0, 10, 25, and 50 µM) for 6 hours. | ↓ TNF α-induced IL-6 and MCP-1 production in a dose-dependent manner ↓ TNF α-induced NF-κB activation |
| Animal | ||
| Alberdi, 2011 [112] |
Male Sprague-Dawley rats (180 g) in a obesogenic diet (high fat and high sucrose). Groups including control group or RSV group (30 mg/kg/d) for 6 weeks. |
↔ BW ↓ Adipose tissues ↓ Activities of lipogenic enzymes (FAS, ACC) ↓ Heparin-releasable LPL ↓ Hormone-sensitive lipase mRNA levels |
| Alberdi, 2013 [111] |
Male Sprague-Dawley rats in obesogenic diet (high fat and high sucrose). Groups including HF group and HF+RSV (30 mg/kg BW/d). |
↓ Hepatic fat accumulation and ACC activities ↑ Hepatic CPT-I, ACO, AMPK and PGC-1α ↔ Lipogenesic enzymes (FAS, ME) ↔SRBP-1c, PPAR-α, SIRT1, PGC-1α, HNF-4α, TFAM, and COX2 |
| Baur, 2006 [113] |
Male C57BL/6NIA mice (1 year old). Groups including SD group, HF group, or HF+RSV group for 110 weeks. |
↑ Survival and mitochondrial number ↓ Increased size and weight of liver by high-calorie diet ↓ IGF-I levels ↑ Insulin sensitivity, AMPK and PGC-1α activity |
| Bujanda, 2008 [114] |
Wistar CRL: Wi (Han) male rats (225 g). Groups including control group (regular chow), steatosis group (a high carbohydrate-fat free modified diet), or Steatosis+RSV group (10 mg/d by oral) for 4 weeks. |
↓ BW and fat deposition ↓ TNF-α and MDA in liver ↑ SOD, GPX, catalase in liver ↓ NOS in liver |
| Dal-Pan, 2010 [115] |
Non-human primate, the grey mouse lemur (3-year-old). Groups including control group or RSV group (200 mg/kg/day) for 4 weeks. |
↓ Seasonal body-mass gain ↓ Energy intake ↑ Resting metabolic rate ↓ Depth of daily torpor (energy-saving process) ↑ Mobilization of fat stores (↑ glucose-dependent insulinotropic polypeptide) |
| Franco, 2012 [116] |
Lactating Wistar rats with early weaning (EW) stage. Groups including EW group (vehicle) or EW+RSV (30 mg/kg/day by gavage) group for 30 days. |
↓ Liver steatosis and TG ↓ Oxidative stress (↓ TBARS, MDA) ↑ Defense capacities (↑ SOD, GPX) |
| Gomez-Zorita, 2012 [117] |
Male obese fa/fa Zucker rats (6-week-old) in a model of steatosis. Groups including control group, RSV15 group (15 mg/kg BW/d), RSV45 group (45 mg/kg BW/d) for 6 weeks. |
↓ Liver weight and TAG content ↑ CPT-Iα and ACO activities ↓ NEFA and ALP ↓ Liver oxidative stress (TBARS, GSSG) ↔ Lipogenic enzymes, ALT, TAG, and adiponectin |
| Kim, 2011 [118] |
Male leptin-deficient ob/ob obese mice (9-week-old) in a high-fat diet-induced obesity model. Groups including control group, HF group, or HF+RSV group (0.4% RSV) for 10 weeks. |
↓ BW gain and visceral fat-pad weights ↓ Plasma TG, FFA, total cholesterol, glucose, TNF-α, and MCP1 ↓ Adipogenic genes expression (PPARg2, C/EBPα, SREBP-1c, FAS, LPL, aP2, leptin) ↓ Galanin-mediated signaling molecules (GalR1/2, PKCd, Cyc-D, E2F1, p-ERK) ↓ Pro-inflammatory cytokines (TNF-α, IFN-α, IFN-β, IL-6) and signaling molecules (TLR2/4, MyD88, Tirap, TRIF, TRAF6, IRF5, p-IRF3, NF-κB) |
| Macarulla, 2009 [119] |
Male Sprague-Dawley rats (180 g) in a HC diet. Groups including the HC group, HC+RSV1 group (6 mg/kg/day in diet), HC+RSV2 group (30 mg/kg/day) or HC+RSV3 group (60 mg/kg/day) for 6 weeks. |
↓ Size of white adipose tissue ↔ Cholesterol, TG, free FFA, glucose |
| Nagao, 2013 [120] |
Male OLETF rats (6-week-old) in a OLETF model. Groups including control group and RSV (0.5%, w/w diet) group for 4 weeks. |
↔ Lipid parameters (serum and liver cholesterol and TG) ↑ Fat metabolism and sparing actions for carbohydrate and protein ↑ Adipose mRNA levels of CPT |
| Poulsen, 2012 [121] |
Male Wistar rats (8-week-old) in a high-fat diet-induced obesity model. Groups including control diet group, HF group, and HF+RSV (100 mg) for 8 weeks. |
↑ Hepatic TG and mitochondria content (↑ UCP-2 expression) |
| Rivera, 2009 [122] |
Male obese (fa/fa) Zucker rats and lean heterozygous littermates (13-week-old). Groups including lean-control group, lean-RSV (10 mg/kg BW orally) group, obese-control group, or obese-RSV group for 8 weeks. |
↓ Hepatic lipid content, TG, total cholesterol, FFA, insulin ↓ Systolic blood pressure ↓ Leptin and ↑ adiponectin ↑ Phosphorylation of AMPK and ACC ↓ TNF-α and NO production ↑ eNOS expression in VAT and aortic ring |
| Human | ||
| Poulsen, 2013 [123] |
Randomized, double-blinded, placebo-controlled trial. Healthy obese men (n=24). Groups including placebo group or RSV group for 4 weeks. |
↔ Blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or inflammatory and metabolic biomarkers |
| Timmers, 2011 [124] |
Randomized, placebo-controlled, double-blinded crossover study. Healthy, obese men subjects (n=11). Groups including placebo group or RSV group (150 mg/d) for 30 days. |
↓ Sleeping and resting metabolic rate ↓ Glucose, insulin, HOMA-IR, leptin ↑ Muscle mitochondrial respiration (↑ AMPK, SIRT1 and PGC-1α protein levels in muscle) ↑ Intramyocellular lipid levels ↓ Adipose tissue lipolysis, plasma FA and TG, intrahepatic lipid content, ALT ↑ Citrate synthase activity ↓ Inflammation markers (TNF-α, leukocytes, ALAT) |
| Tome-Carneiro, 2012 [125] |
Triple-blinded, randomized, parallel, dose-response, placebo-controlled, 6-month follow-up trial. Patients (n=75) on statin and at high CVD risk status. Groups including placebo, RSV (RSV-rich grape supplement, 8 mg RSV), or grape supplement group (lacking RSV) for 6 months. |
↔ Hepatic, thyroid, and renal function. ↓ LDL-cholesterol, ApoB, LDLox, and LDLox/ApoB ↑ non-HDLc/ApoB |
| Tome-Carneiro, 2013 [126] |
Triple-blinded, randomized, parallel, dose-response, placebo-controlled, 1-year follow-up trial. Patients (n=75) on statin and at high CVD risk status. Groups including placebo, RSV (RSV-rich grape supplement, 8 mg RSV), or grape supplement group (lacking RSV) for the first 6 months and a double dose for the next 6 months. |
↓ Inflammatory and fibrinolytic biomarkers (↓ PAI-1) ↑ Serum adiponectin ↑ Inflammation- related transcription factor (↑ KLF2) ↓ Inflammation-related transcription factor (↓ NF-κB, Ap-1,JUN, ATF-2, CREBBP) ↓ 27 extracellular-space acting genes involved in inflammation, cell migration and T-cell interaction signals in PBMCs |
| Tome-Carneiro, 2013 [127] |
Triple-blinded, randomized, parallel, dose-response, placebo-controlled, 1-year follow-up trial. Patients (n=75) on statin and at high CVD risk status. Groups including placebo, RSV (RSV-rich grape supplement, 8 mg RSV), or grape supplement group (lacking RSV) for the first 6 months and a double dose for the next 6 months. |
↓ Inflammation (↓ hs-CRP, TNF-α, PAI-1, IL-6/IL-10 ratio,sICAM-1 ) ↑ Anti-inflammation (↑ IL-10) ↑ Adiponectin |
Abbreviations: ACC, acetyl-Coenzyme A carboxylase; ACO, acetyl-CoA oxidase; AKT, serine/threonine protein kinase; ALAT (ALT), alanine aminotransferase; ALP, alkaline phosphatase; AMPK, AMP-activated protein kinase; Ap-1, activator protein 1; aP2, adipocyte protein 2; ATF-2, activating transcription factor 2; ATGL, adipose tissue triglyceride lipase; BW, body weight; C/EBP, CCAAT/enhancer-binding protein; COX2, cyclooxygenase 2; CPT-1, Carnitine palmitoyltransferase-1; CREBBP, CREB-binding protein; CVD, cardiovascular disease; Cyc-D, cyclin D; DsbA-L, disulfide-bond A oxidoreductase-like protein; E2F1, E2F transcription factor 1; eNOS, endothelial nitric oxide synthase; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; FABP4, fatty acid binding protein 4; FASN (FAS), fatty acid synthase; FFA, free fatty acid; FOXO1, forkhead box protein O1; GalR, galanin receptor ; GPX, glutathione peroxidase; GSSG, glutathione disulfide; HF, high fat; HNF-4 α , hepatocyte nuclear factor receptor-4α; HOMA-IR, homeostasis model assessment-insulin resistance; hs-CRP, high sensitivity C-reactive protein; IFN, interferon; IL, interleukin; JNK, Jun NH2-terminal kinase; IGF-I, insulin like growth factor-I; IRF, interferon regulatory factor ; IRS-1, insulin receptor substrate-1; KLF2, Kruppel-like factor 2; LPL, lipoprotein lipase; LPS, lipopolysaccharide; MCE, mitotic clonal expansion; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; ME, malic enzyme; Mfn2, mitofusin 2; MMP, matrix metalloproteinase; MyD88, myeloid differentiation primary response gene 88; NEFA, non-esterified fatty acid; NF-κB, nuclear factor kappa B; NO, nitric oxide; NOS, nitric oxide synthase; OLETF, Otsuka Long Evans Tokushima Fatty; PAI1, plasminogen activator inhibitor-1; PGC-1α, peroxisome proliferator-activated receptor -coactivator 1a; PI3K, phosphatidylinositide 3-kinase; PKCd, protein kinase C delta; PMBC, peripheral blood mononuclear cell; PPAR, peroxisome proliferators-activated receptor; RSV, resveratrol; SCD1, stearoyl-CoA desaturase-1; sICAM-1: soluble intercellular adhesion molecule-1; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1; SOD, superoxide dismutase; SREBP-1, sterol regulatory element-binding protein-1; TAG, triacylglycerol; TBARS, thiobarbituric acid reactive substances; TFAM, mitochondrial transcription factor A; TG, triglyceride; Tirap, toll-interleukin 1 receptor (TIR) domain-containing adaptor protein; TLR, toll like receptor; TNF-α, tumor necrosis factor alpha; TRAF6, TNF receptor-associated factor 6; TRIF, TIR-domain-containing a; UCP, uncoupling protein; VAT, visceral adipose tissue; ↑, increase; ↓, decrease; ↔, no change.
Similar to EGCG, resveratrol induces apoptosis of adipocytes, decreases their proliferation, and causes cell cycle arrest at a concentration range 10 to 100 µM [93]. At these concentrations resveratrol also inhibits preadipocyte differentiation in a dose-dependent manner with a concomitant suppression of expression of PPARγ, C/EBPα, and fatty acid binding protein 4 (FABP4), and other biomarker proteins (e.g., AMPK, SIRT1, FOXO1) for adipocytes [88, 90, 92, 93]. Down-regulation of PPARγ and C/EBPα expression is attributed to resveratrol-mediated activation of AMPK and SIRT1; the latter deacetylates and activates forkhead box protein O1 (FOXO1), a protein that binds to the promoter sites of PPARγ and further prevents its transcription [107].
Resveratrol also decreases lipogenesis in adipocytes. In 3T3-L1 cells resveratrol (10–100 µM) dose-dependently decreases intracellular triglyceride (TG) accumulation [88, 92, 93, 96] with concomitant down-regulation of lipogenic genes including fatty acid synthase (FAS), lipoprotein lipase (LPL), SREBP1c, and stearoyl-CoA desaturase-1 (SCD1) [96, 99]. Resveratrol-activated AMPK phosphorylates and further inactivates acetyl-CoA carboxylase [108] and consequently, blocks the production of malonyl-CoA, a stimulator of lipogenesis.
The anti-adipogenic activity of resveratrol is furhther enhanced by resveratrol-stimulated lipolysis. In mature 3T3-L1 and human adipocytes resveratrol (100 µM) significantly increased free fatty acid (FFA) release concomitant with 5- and 12-fold rises in the mRNA and protein levels of adipose triglyceride lipase (ATGL), respectively [95]. An AMPK inhibitor reversed these effects, suggesting that AMPK activation mediates the action of resveratrol. Cell culture studies have demonstrated that resveratrol increases fatty acid β-oxidation, mitochondrial biogenesis and activity [96, 109], and mitochondrion-related genes including UCP-1, SIRT3 [96] and PGC-1α; the latter up-regulates the expression of UCP-1 and UCP-2 [110]. Resveratrol also reduces the level of malonyl-CoA, an inhibitor of fatty acid β-oxidation [108].
The anti-obesity property of resveratrol may also stem from its effect on antiinflammatory response in adipose tissue. Resveratrol (0.1–10 µM) significantly reduced nuclear factor-κB (NF-κB) activation and the expression and release of interleukin (IL)-6 and tumor necrosis factor-alpha (TNF-α) in RAW 264.7 macrophages and 3T3-L1 cells [91]. Resveratrol at up to 50 µM also suppressed the expression of matrix metalloproteinase (MMP)-2 and MMP-9 [92] and TNF-α-induced release of IL-6, monocyte chemoattractant protein-1 (MCP-1) [100] and other adipokines [87] in 3T3-L1 cells.
The impact of resveratrol on obesity in animal studies was mainly based on a diet-induced obese animal model (Table 4) [106, 111–122]. In general, authors reported that resveratrol supplementation has shown to decrease body weight [106, 114], various adipose tissue weights [112, 119], and hepatic fat accumulation and deposition [112, 113] in a dose-dependent manner [112, 119], resulting in improvement of blood lipid profiles [116] and glucose homeostasis [113]. These observations of the anti-obesity effects of resveratrol seem to be consistent with findings from other animal models, including leptin-deficient ob/ob mice (a model of peripheral neuropathy of type II diabetes and obesity) [118] or obese (fa/fa) Zucker rats (a spontaneous genetic obesity model) [117, 122]. On the other hand, Nagao et al. recently reported that the short-term resveratrol supplementation in a diet enhances fat metabolism (by sharing metabolism for carbohydrate and protein) along with unchanged lipid parameters on male OLETE rats (a model of type II diabetes with obesity) [120]. Dal-Pan et al. also confirmed that dietary resveratrol prevented weight gain in non-human primate, as evidenced in a marked reduction of energy intake, an elevation of resting metabolic rate and fat mobilization [115]. There were few exceptions where resveratrol supplementation failed to induce changes in body weight [112], lipogenesic enzymes [111], and blood lipid profiles and glucose [119]. The discrepancy could be due to the duration of study, dosage of resveratrol, and age of animals.
The anti-obesity effect of resveratrol in animals seems to be mediated through stimulation of fat oxidation and metabolism [106, 113, 120, 122] or suppression of adipogenic gene expression (i.e., PPAR, C/EBPα, SREBP-1c, FAS, LPS, fatty acid binding protine (aP2), leptin) [112, 118]. In addition, resveratrol administration not only inhibits obesity-induced chronic inflammation (i.e., expression of TNF-α, interferon (IFN)-γ, IFN-β, and IL-6 along with downstream signaling molecules) [114, 118, 122] and oxidative stress (decreased thiobarbituric acid reactive substances (TBARS), malondialdehyde (MDA), and glutathione disulfide (GSSG)) [116, 117], but also enhances anti-oxidant defense capacities (increased liver superoxide dismutase (SOD), GPX, catalase)) [114, 116].
Several clinical studies examined the effects of resveratrol on obesity-related energy expenditure, fat oxidation, plasma lipid profiles, glucose hemostasis, and inflammation (Table 4) [123–127]. In a randomized, placebo-controlled, double-blinded crossover study, Timmers et al. reported beneficial effects of 30-day resveratrol supplementation on the metabolic profiles in healthy obese men, which appears to mimic the effects observed during calorie restriction. The modest metabolic changes included improvement in glucose hemostasis (decreased glucose, insulin, HOMA-IR and leptin) and lipid profile (decreased plasma FA, glycerol, and TG) [124].
In a triple-blinded, randomized, parallel, dose-response, placebo-controlled study, Tome-Carneiro and colleagues reported that the intake of one capsule/day of Stilvid® (a resveratrol-rich grape supplement containing grape polyphenols including 8 mg resveratrol) significantly reduced LDL-cholesterol, oxidized LDL, and ApoB in patients undergoing primary prevention of CVD with gold-standard medication [118]. In a follow-up one-year study, Tome-Carneiro et al. found a resveratrol-rich grape supplement improved the inflammatory and fibrinolytic status in patients who were on statins for primary prevention of CVD and at high CVD risk [125]. In a recent study by the same team, authors further demonstrated that resveratrol supplementation to patients resulted in increased serum adiponectin, an anti-inflammatory cytokine, and decreased thrombogenic plasminogen activator inhibitor type 1 (PAI-1) by modulating six key inflammation-related transcription factors along with 27 extracellular-space acting genes involved in inflammation, cell migration, and T-cell interaction signals [126]. However, in all of the studies by Tome-Carneiro and colleagues, the authors were not able to rule out the potential synergistic effect of resveratrol with the rest of grape polyphenols and/or statins in patients with coronary artery disease [118–120].
A recent randomized, placebo-controlled clinical trial by Poulsen et al. did not seem to support the anti-obesity potential of resveratrol in healthy obese men; no effect was seen on blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or inflammatory and metabolic biomarkers [123]. The lack of effect may be due to a relative short-term study intervention.
In summary, evidence from these limited clinical studies combined with the results from in vitro and animal studies indicate that potential anti-obesity effects of resveratrol may be achieved through dietary supplementation. However, the optimal doses and study period for the anti-obesity potential of resveratrol remain to be determined.
4. Curcumin
Curcumin, a yellow-colored hydrophobic polyphenol, is the principal curcuminoid of the spice turmeric, the ground rhizome of the herb Curcuma longa [128]. Curminn comprises 2–8% of turmeric [129]. Turmeric known for its anti-inflammation, anti-carcinogenesis, anti-obesity, anti-angiogenesis, and anti-oxidant activities [130] has been used as a dietary spice, coloring agent in foods and textiles. The Asian Indian population has consumed curcumin at doses up to 100 mg/day for many years [131, 132]. Recent human studies indicate that consumption of 8 g/day doesn’t show side effects [133].
Many recent studies [134–144] have demonstrated that, similar to EGCG and resveratrol, curcumin has an anti-obesity property through inhibiting preadipocyte differentiation, suppressing lipogenesis, promoting fatty acid oxidation, and reducing inflammatory response in adipose tissue [130]. Incubating 3T3-L1 cells with 0–25 µM curcumin does not change the viability of cells. However, curcumin significantly inhibited the differentiation of 3T3-L1 cells with concomitant decreases in the expression of adipocyte differentiation biomarkers, such as FABP4 and C/EBPα [134, 136, 138].
Consistent with the inhibition of curcumin-mediated differentiation, curcumin at 10–20 µM decreases fat accumulation in a dose-dependent manner in 3T3-L1 cells [141, 144]. Concurrently, curcumin deceased the mRNA level of CD36, a fatty acid transporter located on the adipocyte membrane [144]. In addition, curcumin (5–20 µM) dose-dependently increased the phosphorylation and activation of AMPK [136] and consequently, the phosphorylation and inactivation of ACC [136, 139] in 3T3-L1 cells. Other studies using 3T3-L1 cells also reported that curcumin can decrease the expression of fatty acid synthase (FASN) [134, 144], a key enzyme in the de novo long-chain fatty acid synthesis pathway, and glycerol-3-phosphate acyltransferase-1 (GPAT-1) [136], an important enzyme for triglyceride synthesis. As compared to control 3T3-L1 cells, 10 and 20 µM of curcumin decreased both protein levels and activity of FAS by 1.5 and 4 folds, respectively [144]. When 3T3-L1 adipocytes were treated with 5, 10 and 20 µM of curcumin, the mRNA levels of GPAT-1 were 0.8-, 0.5-, and 0.1-fold lower than them in control 3T3-L1 cells, respectively [136].
Moreover, curcumin enhances fatty acid β-oxidation in adipocytes with a concomitant increase in the expression of carnitine palmitoyltransferase 1 (CPT-1), a key enzyme in transferring acyl-CoA into mitochondria for β-oxidation [136]. When 3T3-L1 adipocytes were treated with 5, 10 and 20 µM of curcumin, the palmitic acid oxidation was 1.1-, 1.3-, and 2.0-fold higher than it in control 3T3-L1 cells, respectively; and mRNA expression levels of CPT-1 were 1.8-, 3.4-, and 3.8-fold higher than them in control 3T3-L1 cells, respectively [136]. In addition, Dong et al. demonstrated that curcumin increases cholesterol efflux by activating and up-regulating the expression of liver X receptor (LXR) and ATP-binding cassette A1 (ABCA1) in subcutaneous adipocytes isolated from rabbits [135].
Curcumin also exerts anti-inflammatory effects on macrophages and adipocytes [141, 142]. Recruitment and infiltration of macrophages into expanded adipose tissue can increased its local inflammatory response. Inhibitory effect of curcumin on chemotaxis and release of MCP-1 from adipocytes [130, 135] may partially contribute to its anti-inflammatory activity. When RAW 264.7 macrophages were treated with adipose tissue-conditioned medium containing 0.1 to 10 µM curcumin, the migration of macrophages was inhibited in a dose-dependent manner [142]. In addition, curcumin decreases the expression and release of inflammatory factors from adipocytes through inhibiting NF-κB activity and its nuclear translocation [141]. Shao et al fed male C57BL6J mice with high fat diets in combination with or without curcumin (4 g/ kg diet) for 28 weeks [130]. After isolating primary adipocytes from epididymal fat pads, they demonstrated that NF-κB expression levels in both whole cell lysates and nuclear extract were significantly lowered by curcumin in mice fed high fat diet. As a result of NF-kB down-regulation, curcumin at 1 to 20 µM significantly decreased the expression and release of downstream IL-6, TNF-α, MCP-1 from 3T3-L1 cells [128, 137, 142].
Several preclinical studies have shown the beneficial effects of dietary curcumin in obesity-related metabolic disorders in animals. For example, curcumin supplementation was shown to lower serum and liver cholesterol levels in rats fed with a cholesterol-rich diet [145]. In a rat model of high fat diet-induced obesity, authors reported curcumin supplementation significantly decrease plasma FFA [146] and TAG [147], and liver TG [148], TAG and cholesterol [147]. Evidence from high-fat-fed wide-type or ob/bo mice demonstrated that curcumin supplementation can increase the basal metabolic rate, thereby contributing to increased energy expenditure and weight loss [136, 140, 149]. Loss of body weight and increase in the percentage of lean mass are extremely beneficial toward improved insulin sensitivity, as shown decreased serum glucose, increased glucose disposal, decreased HMOA-IR in these obese animals supplemented with curcumin [136, 149–152]].
In addition to the rodent models, other types of animals have also been used to examine the effect of curcumin on obesity-related metabolic disorders (Table 5) [136, 140, 145–156]]. Jang et al. [154] reported the beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Compared to the control, hamsters fed with curcumin for 10 weeks had significantly lower FFA, total cholesterol, TG, leptin, and HOMA-IR but increased plasma HDL-C, Apo A-1, and paraoxonase (PON). In addition, relative to the control group, curcumin-supplemented group had lower hepatic cholesterol and TG levels, FFS, HMG-CoA reductase, and acyl-coenzyme A: cholesterol acyltransferase (ACAT) activities along with increased fatty acid β-oxidation activity [154]. Mesa and colleagues [157] demonstrated that oral administration of turmeric extract (which primary contain curcumin) prevented the oxidation of red blood cell membranes and lipid peroxidation in liver microsome in rabbits fed an atherosclerotic diet [157]. Similarly, Leray et al. concluded that highly bioavailable curcumin extract lowered plasma aminoalkyl glucosaminide 4-phosphate (AGP) concentration as well as obesity-related inflammatory cytokines [156].
Table 5.
Effect of curcumin on obesity in cell, animal, and human
| First author, yr [ref] |
Experimental design and treatments | Results |
|---|---|---|
| In vitro study | ||
| Ahn, 2010 [134] |
3T3-L1 cells treated with CUR (0, 10 and 25 µM) for 48 hours. | ↓ Adipocyte differentiation and lipid accumulation ↓ C/EBPα and PPARγ, SREBP-1 and FASN ↓ MAPK phosphorylation ↑ β-catenin translocation ↑ Wnt signaling |
| Dong, 2011 [135] |
Rabbit subcutaneous adipocytes treated with CUR (0–20 µg/mL) for 24 hours. |
↑ Cholesterol efflux from adipocytes ↑ PPARγ, LXRα, ABCA1 |
| Ejaz, 2009 [136] |
3T3-L1 cells treated with CUR (0, 5,10 and 20 µM) for 24 hours. | ↓ Adipocyte differentiation, fat accumulation, and adipogenesis ↑ CPT-1 and fat oxidation ↓ ACC, GPAT-1 ↑ AMPK activation ↑ Apoptosis |
| Gonzales, 2008 [137] |
3T3-L1 cells treated with CUR (0–20 µM) for 24 or 62 hours. |
↓NF-κB level and nuclear translocation ↓ Expression of IL-6, TNF-a and COX2 |
| Kim, 2011 [138] |
3T3-L1 cells treated with CUR (0–30 µM) for 18 hours, 24 hours, 48 hours, and 6 days. |
↓ Adipocyte differentiation and fat accumulation ↓ Cell viability ↓ MCE process ↓ C/EBPβ, PPARγ and C/EBPα |
| Lee, 2009 [139] |
3T3-L1 cells treated with CUR (0–50 µM) for 8 days. |
↑ AMPK ↓ PPARγ ↑ Phosphorylation of ACC ↓ Fat accumulation |
| Shao, 2012 [140] |
Primary cell culture from epididymal fat pads treated with CUR (0–20 µM) for 0–60 minutes. |
↔ Wnt signaling ↓ Inflammatory and oxidative pathway ↑ Insulin-stimulated PKB phosphorylation ↓NF-κB signaling |
| Wang, 2009 [141] |
3T3-L1 cells treated with CUR (0–20 µM) for 24 or 48 hours. |
↑ Insulin-stimulated glucose uptake ↓ TNF-α and IL-6, NF-κB p65 ↓ JNK, ERK1/2, p38MAPK |
| Woo, 2007 [142] |
Raw 264.7 (murine macrophage cell line) and 3T3-L1 cells treated with CUR (0.1, 1, 10 µM) for 24 hours. |
↓ Migration of macrophages ↓ Nitric oxide ↓ MCP-1 and TNF-α |
| Xie, 2012 [143] |
3T3-L1 cells treated with CUR (0–30 µM) for 2 and 24 hours. | ↑ Apoptosis at 30 µM ↓ Glycerol release ↓ MAPK phosphorylation ↓ HSL recovered |
| Zhao, 2011 [144] |
3T3-L1 cells treated with CUR (0–100 µM) for 0–8 days. |
↓ Adipocyte differentiation and lipid accumulation ↓ FAS, PPARγ, CD36 |
| Animal | ||
| Asai, 1999 [153] |
ddY mice (9-week-old). Groups including control group or turmeric group (>99% curcuminoid) group for 1 week. |
↓ TAG and cholesterol levels in liver ↓ α-tocopherol levels in RBC ↓ Lipid peroxidation (↓TBARS in liver and ↓ PLOOH in RBC) |
| Asai, 2001 [147] |
Male Sprague-Dawley rats (7-week-old) in a HF diet-induced obesity model. Groups including HF group, HF+0.2%CUR group (w/w curcuminoids in diet), or HF+1%CUR group for 2 weeks. |
In HF+1%CUR group: ↓ Epididymal adipose tissue weight ↓ Liver TAG and cholesterol ↓ Plasma TAG in the VLDL fraction ↑ Hepatic acyl-CoA oxidase activity |
| Ejaz, 2009 [136] |
Male C57BL/6 mice (4-week-old) in a HF diet-induced obesity model. Groups including control group, HF group, or HF+CUR group (500 mg/kg of diet) for 12 weeks. |
↓ BW, fat, microvessel density in adipose tissue. ↓ Liver weights and hepatic steatosis ↓ Serum glucose and total cholesterol ↓ Angiogenesis (↓ VEGF and VEGFR-2 mRNA) ↑ Fatty acid and energy metabolism (↑ P-AMPK, P-ACC, and CPT-1 mRNA expression; ↓ GPAT-1, PPARγ and C/EBPα mRNA expression) |
| He, 2012 [151] |
Male C57BL/6J mice (8-week-old) in a HF diet-induced obesity and insulin resistance model. Groups including LF group, HF group, and HF+CUR (50 mg/kg BW by gavage) for 15 days. |
↔ BW ↑ Glucose disposal and insulin sensitivity (↓ insulin, HMOA-IR) ↓ Oxidative stress (↓ MDA and ROS in skeletal muscle and mitochondria) ↑ NrF2 signaling in skeletal muscle) |
| Jang, 2008 [154] |
Male Golden-Syrian hamsters (4-week-old) in a HF diet-induced obesity model. Groups including HF group or HF+CUR (0.05% in diet) for 10 weeks. |
↔ BW, food intake, fat pad mass, plasma glucose ↓ Plasma FFA, total cholesterol, TG, leptin, insulin, HOMA-IR ↑ Plasma HDL-C, apo A-I, PON ↓ Hepatic cholesterol and TG ↑ FA β-oxidation activity ↓ FAS, HMG-CoA reductase, ACAT activities ↓ Lipid peroxide levels (↓ RBC and hepatic TBARS) |
| Kempaiah, 2006 [155] |
Female Wistar rats in a cholesterol-rich diet. Groups including cholesterol control group or cholesterol+0.2%CUR (w/w in diet) group for 8 weeks. |
↓ Activity of Ca2+, Mg2+-ATPase in RBC membranes |
| Leray, 2011 [156] |
European obese cats (6.5-year-old). Groups including control group, citrus group, or CUR group (0.09%) for 8 weeks. |
↓ Plasma AGP concentration ↓IFN-γ and IL-2 mRNA levels ↔ mRNA expression of TNF-α, IL-1β, IL-4, IL-5, IL-10, IL-12, IL-18, TGF-β |
| Mesa, 2003 [157] |
Male New Zealand rabbits in a cholesterol-rich diet. Groups including cholesterol control group or cholesterol+turmeric group (1.66 mg/kg BW) for 10 days and 30 days. |
↓ Lipid peroxidation (↓ TBARS in RBC membranes, ↓ H2O2 and TBARS in liver microsomes) |
| Oner-Iyidogan, 2013 [148] |
Male Sprague-Dawley rats in a HF diet-induced obesity model. Groups including control group, CUR100 group (100 mg/kg/BW/d), CUR400 group (400 mg/kg/BW/d), HF group, HF+CUR100, or HF+CUR400 for 8 weeks. |
↓ Liver TG ↓ Serum fetuin-A |
| Pongchaidecha, 2009 [146] |
Male Wistar rats (100–120 g) in a HF diet-induced obesity model. Groups including control group, HF control group, HF+30mg/kg BW curcuminoid group, HF+60 mg/kg BW curcuminoid group, or HF+90mg/kg BW curcuminoid group for 12 weeks. |
↓ Plasma FFA and glucose levels ↔ Insulin, HOMA-IR Improved cardiac autonomic disturbance |
| Rao, 1970 [145] |
Female Wistar rats (45-day-old) in a cholesterol-rich diet. Groups including control group, 0.5% (w/w in diet) CUR group, cholesterol group, cholesterol+0.1%CUR group, cholesterol+0.25%CUR group, or cholesterol+0.5%CUR group for 7 weeks. |
In cholesterol-supplemented groups: ↓ Liver weight ↓ Free cholesterol and cholesterol levels in serum and liver ↑ Cholic acid, deoxycholic acid, and free cholesterol excretion in feces ↓ β-lipoprotein and ↑ α-lipoprotein in serum |
| Shao, 2012 [140] |
Male C57BL/6J mice (5-week-old) in a HF diet induced-obesity model. Groups including LF group, HF group, or HF+CUR (4 g/kg diet, added 2 days/week) group for 28 weeks. |
↓ BW and fat ↓ Hepatic lipogenic gene expression (↓ NF-κB, SREBP-1c, ChREBP and LPK) ↑ Glucose disposal and insulin sensitivity ↔ Wnt signaling in mature adipocytes ↓ Inflammatory and oxidative pathway in adipocytes |
| Weisberg, 2008 [149] | Male wild-type C57BL/6J mice (8-10-week-old) in a diet-induced-obesity (DIO) model. Male ob/ob C57BL/6J mice (3-5-week-old). At age of 20 weeks, groups including DIO control group, DIO+3%CUR (w/w in diet), ob/ob control group, or ob/ob+3%CUR for 60 days. |
↓ BW and body fat ↑ Glycemic status and insulin sensitivity ↑ ER stress response (↑ mRNA expression of SIRT1, HSP70, HSP90, FOXO1α in white adipose tissue) ↓ Adipose, hepatic, and systemic inflammation (↓ MCP-1) ↑ mRNA expression of adiponectin ↓ Hepatic NF-κB activity |
| Yekollu, 2011 [152] |
Male C57BL/6J, ob/ob mice and nonobese littermates in a model of steatosis. Groups including ob/ob control group, lipo group, ob/ob+CUR group, nonobese control group, or nonobese+CUR group for 24 or 72 hours. |
↓NF-κB pathway ↓ Proinflammatory cytokine (↓ TNF, IL-6; ↑ IL-4) ↑ Insulin sensitivity (↓ fasting glucose and insulin, HOMA-IR) ↑ Serum adiponectin |
| Yu, 2008 [150] |
Male Sprague-Dawley rats in a HF diet-induced obesity model. Groups including control group, highCUR (5.00 g/kg BW, HF group, HF+lowCUR (1.25 g/kg diet) group, or HF+highCUR (5.00 g/kg diet) for 4 weeks. |
↓ BW, blood glucose, insulin, leptin, TNF-α ↓ Insulin resistance and leptin resistance |
| Human | ||
| Baum, 2007 [160] |
Randomized, double-blinded, placebo-controlled trial. Elderly (n=36, > 50 y) in Hong Kong. Groups including placebo group, 1 g/d CUR group, or 4 g/d CUR group for 6 months. |
↔ Serum lipid profile (TAG, total, LDL-C, HDL-C) |
| Ramirez-Bosca, 2000 [158] |
A pre-post study. Healthy adults (n=8, 43–70 yr) in Spain. All subjects received oral administration of CUR (10 mg/d) for 30 days. |
↓ Serum LDL, apo B, apo B/apo A ↑ Serum HDL and apo A |
| Ramirez-Bosca, 2000 [159] |
A pre-post study. Healthy adults (n=8, 43–70 yr) in Spain. All subjects received oral administration of CUR (10 mg/d, twice a day) for 15 days. |
↓ Plasma fibringen |
Abbreviations: ABCA1, ATP binding cassette transporter A1; ACAT, Acyl CoA:cholesterol acyltransferase; ACC, acetyl-Coenzyme A carboxylase; AGP, a1-acid glycoprotein; AMPK, AMP-activated protein kinase; BW, body weight; Ca, calcium; C/EBP, CCAAT/enhancer-binding protein; ChREBP, carbohydrate-responsive-element-binding protein; CPT-1, Carnitine palmitoyltransferase-1; COX2, cyclooxygenase 2; CUR, curcumin; ER, endoplasmic reticulum; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; FA, fatty acids; FASN (FAS), fatty acid synthase; FFA, free fatty acid; FOXO1, forkhead box protein O1; GPAT-1, glycerol-3-phosphate acyl transferase-1; HDL-C, high density lipoprotein-cholesterol; HF, high fat; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA; HOMA-IR, homeostasis model assessment-insulin resistance; HSL, hormone sensitive lipase; HSP, heat shock protein; IFN, interferon; IL, interleukin; JNK, Jun NH2-terminal kinase; LDL-C, low density lipoprotein-cholesterol; LPK; liver-type pyruvate kinase; LXR, liver X receptor; MAPK, mitogen-activated protein kinase; MCE, mitotic clonal expansion; MCP-1, monocyte chemoattractant protein-1; MDA, malondialdehyde; Mg, magnesium; NF-kB, nuclear factor kappa B; Nrf2: nuclear factor erythroid-2-related factor-2; PKB, protein kinase B; PLOOH, phospholipid hydroperoxides; PON, paraoxonase; PPAR, peroxisome proliferators-activated receptor; RBC, red blood cell; ROS, reactive oxygen species; SIRT1, sirtuin (silent mating type information regulation 2 homolog) 1; SREBP-1, sterol regulatory element-binding protein-1; TAG, triacylglycerol; TBARS, thiobarbituric acid reactive substances; TG, triglyceride; TGF-β, transforming growth factor- β; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor-receptor 2; VLDL, very low density lipoprotein; ↑, increase; ↓, decrease; ↔, no change.
There are limited clinical trials to investigate the effect of curcumin on obesity-related parameters. In a pre-post study, Ramı´rez-Bosca´ reported that oral administration of 10 mg curcumin extract per day for 30 days to 8 human subjects elevated HDL-C and apo A as well as lowered LDL-C, apo B and apo B/apo A ratio [158]. The same research team also found that 10 mg curcumin (twice/day) for 15 days significantly suppressed the levels of plasma fibrinogen in study subjects, which may reduce the risk for coronary disease [159]. In a randomized, double-blinded, placebo-controlled trial, Baum et al. [160] investigated the effects of curcumin consumption on the serum lipid profile in elderly men and women. Authors concluded that neither dose of curcumin (1g/day or 4 g/day) significantly affected blood TAG, total LDL, or HDL-C over 1 month or 6 months. However, the concentrations of plasma curcumin and serum cholesterol were positively and significantly correlated. Such insignificant findings of curcumin in Baum’s study may be due to the following reasons: (i) a relative small sample size, (ii) the relatively low absorption efficiency of curcumin, or (iii) subjects not suffering hypercholesterolemia or provided a special diet (a ceiling effect) [160].
Evidence from cellular and animal studies suggest the beneficial effects of curcumin on obesity and related metabolic disorders. These studies show that curcumin reduces BW, lowers TG synthesis, increases basal metabolic rate and FA oxidation, and improves insulin sensitivity, through its ability to act as an antioxidant or antiinflammatory mediator, therefore suppressing the destructive effects of oxidative stress or inflammation. However, translational studies from animal observation to human clinical trials are needed to verify such anti-obesity benefits of cucurmin.
5. Potential mechanisms of action
Figure 1 illulstrates the potential mechanisms of dietary polyphenols (GTC, resveratrol, and curcumin) on anti-obesity. Many lines of research indicate that dietary polyphenols prevent obesity development through the following possible mechanisms: 1) lower food intake; 2) decrease lipogenesis; 3) increase lipolysis; 4) stimulate fatty acid β-oxidation; 5) inhibit adipocyte differentiation and growth; and 6) attenuate inflammatory responses and suppress oxidative stress. GTC, especially EGCG, may lower food intake by increasing the production and release of cholecystokinin with hunger-suppressive effects [28]. EGCG can decrease lipid digestion and absorption by decreasing activities of digestive enzymes and lipid emulsification [14, 161]. Green tea catechins, resveratrol and curcumin can decrease fat accumulation in adipocytes through activating AMPK, inhibiting ACC activation, and down-regulating the expression of lipogenic genes such as FAS, SCD1, and SREBP-1c [20, 28, 96, 99].
These polyphenols also increase lipolysis and stimulate fatty acid β-oxidation through up-regulating hormone sensitive lipase (HSL), UCPs, CPT-1 and PGC-1α [24, 25, 27, 95, 136]. These dietary polyphenols can decrease the proliferation of preadipocytes, induce their apoptosis, and promote cell cycle arrest. Green tea catechins, resveratrol and curcumin down-regulate the expression of key genes involved in adipocyte differentiation including C/EBPα, PPARγ and aP2 [17, 88, 90, 134, 136]. They also induce G0/G1 and G2/M cell cycle arrest [17, 19, 93] and apoptosis of adipocytes as shown in increase of DNA fragmentation and caspase-3 activation. Inflammatory responses are suppressed by these polyphenols. The expression and secretion of pro-inflammatory resistin, PAI-1, IL-6, TNF-α, MCP-1, MMPs are inhibited by green tea catechins, resveratrol and curcumin, which may be partially attributed to inactivated NF-κB and MAPK [43, 51, 91, 100, 128, 137, 141, 142]. These polyphenols can also decrese oxidative stress and increase anti-oxidant capacity in adipose tissue.
Although the underlying mechanism for the polyphenol effects are still unclear, limited studies have shown that such effects may be mediated by a receptor or intracellular Ca, at least in the case of EGCG. For example, EGCG is incorporated into cytoplasma and further transported into nuclei in L-929 cells [162]. Uptake of EGCG by HT-29 cells is via a passive diffusion process [163]. EGCG can bind on its receptor, 67 kDa laminin receptor, on cancer cells and activate subsequent signaling molecules and anti-cancer activities including decreasing cell viability, inducing apoptosis and altering intracellular Ca2+ [164, 165]. Few studies have investigated the initial mechanisms of EGCG in regulating lipid metabolism in adipocytes. Hsiech and colleagues reported that 67 kDa laminin receptor was expressed in 3T3-L1 and C3H10T1/2 adipocytes. EGCG binding to this receptor could inhibit insulin stimulation of adipocyte glucose uptake; the inhibitory effect was blocked by the receptor antibody [166]. In terms of resveratrol, many studies have demonstrated that resveratrol inhibits adipocyte differentiation and de novo lipogenesis, and stimulates lipolysis in adipocyte in a Sirt1-dependent manner or via activation of AMPK [88, 167, 168]. However, receptors for binding resveratrol to adipocytes and mechanisms for adipocytes uptake of resveratrol are unknown. More studies are required to investigate the specific mechanisms mediating initial resveratrol effects in adipocytes. Regarding curcumin, increased curcumin content in adipocytes was positively correlated with enhanced anti-adipogenic ability [169]. However, few studies have investigated the binding receptors, uptake or signaling mechanisms of curcumin in adipocytes.
We noted that the 3T3-L1 cells have been used in the majority of the in vitro studies presented here to study the effects of polyphenols on obesity. Use of 3T3-L1 cells has been used for decades as a good model for adipose tissue development. They present, however, some limitations. In some instances, the 3T3-L1 cells do not express adequate levels of certain adipocyte-specific genes and some other metabolic genes at the degree expressed in whole adipose tissue. Also their embryonic nature makes them more suitable to study adipose tissue development rather than adult or postnatal adipose tissue metabolism. Finally, these cells do not represent the complex and diverse cell type populations, the microvascular system or the extracellular matrix of the whole adipose tissue.
6. Summary and future research
Obesity is a complex and multifactorial condition of chronic inflammation and oxidative stress. Various strategies including lifestyle modifications (caloric restriction, increased physical activity, consumption of whole foods and/or dietary supplements), use of anti-obesity drugs and in extreme cases, bariatric surgery are used for weight loss. Weight loss results in decreased fat mass, reduced local and systemic inflammation or reduced production and secretion of pro-inflammatory cytokines into the bloodstream, along with an elevation of antioxidant defense mechanism. In vitro and in vivo studies reviewed above demonstrate that green tea catechins and some of its components (especially EGCG), resveratrol and curcumin all exert anti-inflammatory and/or anti-oxidant effects in obesity-related metabolic alterations. These effects lead to one or more of the following outcomes: weight loss, decreased cellular and circulating lipids, increased basal metabolic rate, fatty acid oxidation, energy expenditure, and improved insulin sensitivity. Thus, the dietary polyphenols described in this review are potential nutritional strategies for the prevention of obesity and associated inflammation.
Published studies show that obesity-associated inflammation, a major contributor to metabolic disorders in humans, can be prevented or partially reversed by dietary polyphenols such as those reviewed herein. Mechanistically, these dietary polyphenols exert their additive and/or synergistic effects through one or more signaling and transcriptional mechanisms including those mediated by NF-κB, AMPK, PPARγ, and PGC-1α. However, most dietary polyphenols have relatively short half-lives once ingested due to rapid metabolism, so it is important that their consumption is maintained throughout the life span.
It was noted that these polyphenols have a low level of oral bioavailability in humans and research animals [170–175]. After eating polyphenol-rich foods or taking dietary supplement, humans achieve peak plasma polyphenol concentrations at less than 10 µM [172, 174–177]. In addition, polyphenols in human bodies are quickly modified and metabolized by tissue enzymes, especially hepatic enzymes [178, 179]. As a result, the circulating concentrations of free polyphenols are even lower [175]. Most in vitro studies used polyphenol concentration range between 10 and 200 µM, which is much higher than the physiological concentrations in humans [21, 144]. On the other hand, after giving animals high doses of polyphenols, the body weight and fat mass were significantly decreased [34, 136]. However, we did not observe dramatic reduction in body weight and fat mass in humans with high-dose polyphenols intake. Since the stability and bioavailability of most of these dietary polyphenols are affected by their cooking, processing and storage methods, additional studies are needed to further understand their optimal preparation as well as their bioavailability and mechanism of action within the whole body.
In addition to the relative low bioavailability of dietary polyphenols in humans, other factors, such as ethnicity, genetics, and lifestyles also need to be taken into account in designing clinical trials. Recent studies using nutrigenomic approach showed heterogeneity in individual responses to nutrients due to genetic factors such as single nucleotide polymorphisms. More importantly, we conclude that translational studies from animal observations to human clinical trials and ultimately community interventions are needed to further confirm the anti-obesity and/or anti-diabetic benefits of these polyphenols and foods rich in these bioactive compounds.
ACKNOWLEDGMENTS
This work was supported by NIH/NCCAM grant (U01AT006691) and Laura W. Bush Institute for Women’s Health (CLS), Texas Department of Agriculture Food and Fiber Research Program and Texas Woman's University Research Enhancement Program (HM), and National Research Foundation of Korea (NRF, grant No. NRF-2008-220F00013 & NRF-2011-0014535) (ISK). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the National Institutes of Health.
Abbreviation
- ABCA1
ATP-binding cassette A1
- ACAT
acyl-coenzyme A, cholesterol acytransferase
- ACC
acetyl-coenzyme A carboxylase
- AGP
aminoalkyl glucosaminide 4-phosphate
- AMPK
AMP-activated protein kinase
- aP2
adipocyte P2 protein, which is also know as aFABP, the adipocyte fatty acid binding protein or FAPB-4
- ATGL
adipose triglyceride lipase
- BMI
body mass index
- BW
body weight
- cAMP
cyclic adenosine monophosphate
- C/EBPα
CCAAT/enhancer binding protein α
- CPT-1
carnitine palmitoyltransferase-1
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- EC
epicatechin
- ECG
epicatechin gallate
- EGC
epigallocatechin
- EGCG
epigallocatechin gallate
- FABP4
fatty acid binding protein 4
- FAS
fatty acid synthase
- FASN
fatty acid synthase
- FFA
free fatty acids
- FFM
fat-free mass
- FM
fat mass
- FOXO1
forkhead box protein O1
- GPAT
glycerol-3-phosphate acyltransferase
- GPX
glutathione peroxidase
- GSSG
glutathione disulfide
- GTC
green tea catechins
- GTE
green tea extracts
- GTP
green tea polyphenols
- HDL-C
high density lipoprotein-cholesterol
- HOMA-IR
homeostasis model assessment-insulin resistance
- HSL
hormone sensitive lipase
- IFN
interferon
- IGF-I
insulin-like growth factor-I
- IL
interleukin
- LDL-C
low density lipoprotein-cholesterol
- LPL
lipoprotein lipase
- LXR
liver X receptor
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor-κB
- PAI-1
plasminogen activator inhibitor type 1
- PDEs
phosphodiesterases
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PON
paraoxonase
- PPARγ
peroxisome proliferator activator receptor γ
- RCT
randomized controlled trials
- ROS
reactive oxygen speices
- SBP
systolic blood pressure
- SCD1
stearoyl-CoA desaturase-1
- SIRT1
sirtuin 1
- SOD
superoxide dismutase
- SREBP-1c
sterol regulatory element-binding protein 1c
- TAG
triacylglycerol
- TBARS
thiobarbituric acid reactive substances
- TG
triglyceride
- TNF-α
tumor necrosis factor-alpha
- UCP
uncoupling protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:50–61. doi: 10.1111/j.1467-789X.2009.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchard C. Defining the genetic architecture of the predisposition to obesity: a challenging but not insurmountable task. The American journal of clinical nutrition. 2010;91:5–6. doi: 10.3945/ajcn.2009.28933. [DOI] [PubMed] [Google Scholar]
- 3.Heber D. An integrative view of obesity. The American journal of clinical nutrition. 2010;91:280S–3S. doi: 10.3945/ajcn.2009.28473B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siriwardhana N, Kalupahana NS, Cekanova M, Lemieux M, Greer B, Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. The Journal of nutritional biochemistry. 2013;24:613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Molecular aspects of medicine. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Goran MI, Alderete TL. Targeting adipose tissue inflammation to treat the underlying basis of the metabolic complications of obesity. Nestle Nutrition Institute workshop series. 2012;73:49–60. doi: 10.1159/000341287. discussion p1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Archives of internal medicine. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 8.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA, Tracy RP. Association between physical activity and markers of inflammation in a healthy elderly population. American journal of epidemiology. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure-time physical activity and reduced plasma levels of obesity-related inflammatory markers. Obesity research. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 10.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: mechanistic insights. Advances in nutrition. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalupahana NS, Moustaid-Moussa N. The adipose tissue renin-angiotensin system and metabolic disorders: a review of molecular mechanisms. Critical reviews in biochemistry and molecular biology. 2012;47:379–390. doi: 10.3109/10409238.2012.694843. [DOI] [PubMed] [Google Scholar]
- 12.Esfahani A, Wong JM, Truan J, Villa CR, Mirrahimi A, Srichaikul K, et al. Health effects of mixed fruit and vegetable concentrates: a systematic review of the clinical interventions. Journal of the American College of Nutrition. 2011;30:285–294. doi: 10.1080/07315724.2011.10719971. [DOI] [PubMed] [Google Scholar]
- 13.Dietary Guidelines for Americans. [Accessed on July 4]; http://www.cnpp.usda.gov/dgas2010 policydocument.htm.
- 14.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A(2) and intestinal absorption of lipids in ovariectomized rats. The Journal of nutritional biochemistry. 2006;17:492–498. doi: 10.1016/j.jnutbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutrition reviews. 2007;65:361–375. doi: 10.1301/nr.2007.aug.361-375. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Zhu QY, Tsang D, Huang Y. Degradation of green tea catechins in tea drinks. Journal of agricultural and food chemistry. 2001;49:477–482. doi: 10.1021/jf000877h. [DOI] [PubMed] [Google Scholar]
- 17.Chan CY, Wei L, Castro-Munozledo F, Koo WL. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life sciences. 2011;89:779–785. doi: 10.1016/j.lfs.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Furuyashiki T, Nagayasu H, Aoki Y, Bessho H, Hashimoto T, Kanazawa K, et al. Tea catechin suppresses adipocyte differentiation accompanied by down-regulation of PPARgamma2 and C/EBPalpha in 3T3-L1 cells. Bioscience, biotechnology, and biochemistry. 2004;68:2353–2359. doi: 10.1271/bbb.68.2353. [DOI] [PubMed] [Google Scholar]
- 19.Hung PF, Wu BT, Chen HC, Chen YH, Chen CL, Wu MH, et al. Antimitogenic effect of green tea (−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. American journal of physiology Cell physiology. 2005;288:C1094–C1108. doi: 10.1152/ajpcell.00569.2004. [DOI] [PubMed] [Google Scholar]
- 20.Hwang JT, Park IJ, Shin JI, Lee YK, Lee SK, Baik HW, et al. Genistein, EGCG, and capsaicin inhibit adipocyte differentiation process via activating AMP-activated protein kinase. Biochemical and biophysical research communications. 2005;338:694–699. doi: 10.1016/j.bbrc.2005.09.195. [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Hiraishi A, Tsuchiya K, Sakamoto K. (−) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010;62:245–255. doi: 10.1007/s10616-010-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku HC, Chang HH, Liu HC, Hsiao CH, Lee MJ, Hu YJ, et al. Green tea (−)-epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. American journal of physiology Cell physiology. 2009;297:C121–C132. doi: 10.1152/ajpcell.00272.2008. [DOI] [PubMed] [Google Scholar]
- 23.Ku HC, Liu HS, Hung PF, Chen CL, Liu HC, Chang HH, et al. Green tea (−)-epigallocatechin gallate inhibits IGF-I and IGF-II stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor, but not AMP-activated protein kinase pathway. Molecular nutrition & food research. 2012;56:580–592. doi: 10.1002/mnfr.201100438. [DOI] [PubMed] [Google Scholar]
- 24.Lee MS, Kim CT, Kim IH, Kim Y. Inhibitory effects of green tea catechin on the lipid accumulation in 3T3-L1 adipocytes. Phytotherapy research : PTR. 2009;23:1088–1091. doi: 10.1002/ptr.2737. [DOI] [PubMed] [Google Scholar]
- 25.Lee MS, Kim Y. (−)-Epigallocatechin-3-gallate enhances uncoupling protein 2 gene expression in 3T3-L1 adipocytes. Bioscience, biotechnology, and biochemistry. 2009;73:434–436. doi: 10.1271/bbb.80563. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obesity research. 2005;13:982–990. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 27.Liu HS, Chen YH, Hung PF, Kao YH. Inhibitory effect of green tea (−)-epigallocatechin gallate on resistin gene expression in 3T3-L1 adipocytes depends on the ERK pathway. American journal of physiology Endocrinology and metabolism. 2006;290:E273–E281. doi: 10.1152/ajpendo.00325.2005. [DOI] [PubMed] [Google Scholar]
- 28.Moon HS, Chung CS, Lee HG, Kim TG, Choi YJ, Cho CS. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3T3-L1 cells. Obesity. 2007;15:2571–2582. doi: 10.1038/oby.2007.309. [DOI] [PubMed] [Google Scholar]
- 29.Wu BT, Hung PF, Chen HC, Huang RN, Chang HH, Kao YH. The apoptotic effect of green tea (−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the Cdk2 pathway. Journal of agricultural and food chemistry. 2005;53:5695–5701. doi: 10.1021/jf050045p. [DOI] [PubMed] [Google Scholar]
- 30.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. (50 Suppl).Journal of lipid research. 2009:S138–S143. doi: 10.1194/jlr.R800079-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazar MA. Resistin- and Obesity-associated metabolic diseases. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:710–716. doi: 10.1055/s-2007-985897. [DOI] [PubMed] [Google Scholar]
- 32.Ashida H, Furuyashiki T, Nagayasu H, Bessho H, Sakakibara H, Hashimoto T, et al. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors. BioFactors. 2004;22:135–140. doi: 10.1002/biof.5520220126. [DOI] [PubMed] [Google Scholar]
- 33.Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Strom K, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutrition & metabolism. 2012;9:105. doi: 10.1186/1743-7075-9-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. The Journal of nutrition. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. The Journal of nutrition. 2008;138:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 36.Chen N, Bezzina R, Hinch E, Lewandowski PA, Cameron-Smith D, Mathai ML, et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutrition research. 2009;29:784–793. doi: 10.1016/j.nutres.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Chen YK, Cheung C, Reuhl KR, Liu AB, Lee MJ, Lu YP, et al. Effects of green tea polyphenol (−)-epigallocatechin-3-gallate on newly developed high-fat/Western-style diet-induced obesity and metabolic syndrome in mice. Journal of agricultural and food chemistry. 2011;59:11862–11871. doi: 10.1021/jf2029016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung MY, Park HJ, Manautou JE, Koo SI, Bruno RS. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. The Journal of nutritional biochemistry. 2012;23:361–367. doi: 10.1016/j.jnutbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunha CA, Lira FS, Rosa Neto JC, Pimentel GD, Souza GI, da Silva CM, et al. Green tea extract supplementation induces the lipolytic pathway, attenuates obesity, and reduces low-grade inflammation in mice fed a high-fat diet. Mediators of inflammation. 2013;2013:635470. doi: 10.1155/2013/635470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedrich M, Petzke KJ, Raederstorff D, Wolfram S, Klaus S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. International journal of obesity. 2012;36:735–743. doi: 10.1038/ijo.2011.136. [DOI] [PubMed] [Google Scholar]
- 41.Kim HJ, Jeon SM, Lee MK, Jung UJ, Shin SK, Choi MS. Antilipogenic effect of green tea extract in C57BL/6J-Lep ob/ob mice. Phytotherapy research : PTR. 2009;23:467–471. doi: 10.1002/ptr.2647. [DOI] [PubMed] [Google Scholar]
- 42.Klaus S, Pultz S, Thone-Reineke C, Wolfram S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. International journal of obesity. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Zhu W, Shen CL, Gao W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PloS one. 2012;7:e38332. doi: 10.1371/journal.pone.0038332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monteiro R, Assuncao M, Andrade JP, Neves D, Calhau C, Azevedo I. Chronic green tea consumption decreases body mass, induces aromatase expression, and changes proliferation and apoptosis in adult male rat adipose tissue. The Journal of nutrition. 2008;138:2156–2163. doi: 10.1093/jn/138.11.2156. [DOI] [PubMed] [Google Scholar]
- 45.Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, et al. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. The Journal of nutritional biochemistry. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Park HJ, Lee JY, Chung MY, Park YK, Bower AM, Koo SI, et al. Green tea extract suppresses NFkappaB activation and inflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. The Journal of nutrition. 2012;142:57–63. doi: 10.3945/jn.111.148544. [DOI] [PubMed] [Google Scholar]
- 47.Ramadan G, El-Beih NM, Abd El-Ghffar EA. Modulatory effects of black v. green tea aqueous extract on hyperglycaemia, hyperlipidaemia and liver dysfunction in diabetic and obese rat models. The British journal of nutrition. 2009;102:1611–1619. doi: 10.1017/S000711450999208X. [DOI] [PubMed] [Google Scholar]
- 48.Richard D, Kefi K, Barbe U, Poli A, Bausero P, Visioli F. Weight and plasma lipid control by decaffeinated green tea. Pharmacological research : the official journal of the Italian Pharmacological Society. 2009;59:351–354. doi: 10.1016/j.phrs.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Sae-tan S, Grove KA, Lambert JD. Weight control and prevention of metabolic syndrome by green tea. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;64:146–154. doi: 10.1016/j.phrs.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayama K, Lin S, Zheng G, Oguni I. Effects of green tea on growth, food utilization and lipid metabolism in mice. In vivo. 2000;14:481–484. [PubMed] [Google Scholar]
- 51.Shen CL, Cao JJ, Dagda RY, Chanjaplammootil S, Lu C, Chyu MC, et al. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutrition research. 2012;32:448–457. doi: 10.1016/j.nutres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Shimotoyodome A, Haramizu S, Inaba M, Murase T, Tokimitsu I. Exercise and green tea extract stimulate fat oxidation and prevent obesity in mice. Medicine and science in sports and exercise. 2005;37:1884–1892. doi: 10.1249/01.mss.0000178062.66981.a8. [DOI] [PubMed] [Google Scholar]
- 53.Tian C, Ye X, Zhang R, Long J, Ren W, Ding S, et al. Green tea polyphenols reduced fat deposits in high fat-fed rats via erk1/2-PPARgamma-adiponectin pathway. PloS one. 2013;8:e53796. doi: 10.1371/journal.pone.0053796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ueda M, Ashida H. Green tea prevents obesity by increasing expression of insulin-like growth factor binding protein-1 in adipose tissue of high-fat diet-fed mice. Journal of agricultural and food chemistry. 2012;60:8917–8923. doi: 10.1021/jf2053788. [DOI] [PubMed] [Google Scholar]
- 55.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity. 2010;18:879–883. doi: 10.1038/oby.2010.22. [DOI] [PubMed] [Google Scholar]
- 56.Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B, et al. Effectiveness of green tea on weight reduction in obese Thais: A randomized, controlled trial. Physiology & behavior. 2008;93:486–491. doi: 10.1016/j.physbeh.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Basu A, Du M, Sanchez K, Leyva MJ, Betts NM, Blevins S, et al. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011;27:206–213. doi: 10.1016/j.nut.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. Journal of the American College of Nutrition. 2010;29:31–40. doi: 10.1080/07315724.2010.10719814. [DOI] [PubMed] [Google Scholar]
- 59.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutrition research. 2012;32:421–427. doi: 10.1016/j.nutres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Boschmann M, Thielecke F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: a pilot study. Journal of the American College of Nutrition. 2007;26:389S–95S. doi: 10.1080/07315724.2007.10719627. [DOI] [PubMed] [Google Scholar]
- 61.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. The British journal of nutrition. 2009;101:886–894. doi: 10.1017/S0007114508047727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown AL, Lane J, Holyoak C, Nicol B, Mayes AE, Dadd T. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. The British journal of nutrition. 2011;106:1880–1889. doi: 10.1017/S0007114511002376. [DOI] [PubMed] [Google Scholar]
- 63.Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2002;9:3–8. doi: 10.1078/0944-7113-00078. [DOI] [PubMed] [Google Scholar]
- 64.Di Pierro F, Menghi AB, Barreca A, Lucarelli M, Calandrelli A. Greenselect Phytosome as an adjunct to a low-calorie diet for treatment of obesity: a clinical trial. Alternative medicine review : a journal of clinical therapeutic. 2009;14:154–160. [PubMed] [Google Scholar]
- 65.Diepvens K, Kovacs EM, Nijs IM, Vogels N, Westerterp-Plantenga MS. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. The British journal of nutrition. 2005;94:1026–1034. doi: 10.1079/bjn20051580. [DOI] [PubMed] [Google Scholar]
- 66.Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. The American journal of clinical nutrition. 1999;70:1040–1045. doi: 10.1093/ajcn/70.6.1040. [DOI] [PubMed] [Google Scholar]
- 67.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? Journal of the American College of Nutrition. 2007;26:396S–402S. doi: 10.1080/07315724.2007.10719628. [DOI] [PubMed] [Google Scholar]
- 68.Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clinical nutrition. 2008;27:363–370. doi: 10.1016/j.clnu.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Kovacs EM, Lejeune MP, Nijs I, Westerterp-Plantenga MS. Effects of green tea on weight maintenance after body-weight loss. The British journal of nutrition. 2004;91:431–437. doi: 10.1079/BJN20041061. [DOI] [PubMed] [Google Scholar]
- 70.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. The Journal of nutrition. 2009;139:264–270. doi: 10.3945/jn.108.098293. [DOI] [PubMed] [Google Scholar]
- 71.Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity. 2008;16:1338–1348. doi: 10.1038/oby.2008.60. [DOI] [PubMed] [Google Scholar]
- 72.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity. 2007;15:1473–1483. doi: 10.1038/oby.2007.176. [DOI] [PubMed] [Google Scholar]
- 73.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. The American journal of clinical nutrition. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 74.Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biological trace element research. 2012;149:315–322. doi: 10.1007/s12011-012-9448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thielecke F, Rahn G, Bohnke J, Adams F, Birkenfeld AL, Jordan J, et al. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study. European journal of clinical nutrition. 2010;64:704–713. doi: 10.1038/ejcn.2010.47. [DOI] [PubMed] [Google Scholar]
- 76.Wang M, Bai J, Chen WN, Ching CB. Metabolomic profiling of cellular responses to carvedilol enantiomers in vascular smooth muscle cells. PloS one. 2010;5:e15441. doi: 10.1371/journal.pone.0015441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. International journal of obesity. 2009;33:956–961. doi: 10.1038/ijo.2009.135. [DOI] [PubMed] [Google Scholar]
- 78.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. The American journal of clinical nutrition. 2010;91:73–81. doi: 10.3945/ajcn.2009.28157. [DOI] [PubMed] [Google Scholar]
- 79.Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane database of systematic reviews. 2012;12:CD008650. doi: 10.1002/14651858.CD008650.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dixon RA, Paiva NL. Stress-Induced Phenylpropanoid Metabolism. The Plant cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fremont L. Biological effects of resveratrol. Life sciences. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 82.Romero-Perez AI, Ibern-Gomez M, Lamuela-Raventos RM, de La Torre-Boronat MC. Piceid, the major resveratrol derivative in grape juices. Journal of agricultural and food chemistry. 1999;47:1533–1536. doi: 10.1021/jf981024g. [DOI] [PubMed] [Google Scholar]
- 83.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. Journal of agricultural and food chemistry. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 84.Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. Journal of agricultural and food chemistry. 2008;56:8374–8378. doi: 10.1021/jf801297w. [DOI] [PubMed] [Google Scholar]
- 85.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: a molecule whose time has come? And gone? Clinical biochemistry. 1997;30:91–113. doi: 10.1016/s0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 86.Orallo F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Current medicinal chemistry. 2006;13:87–98. [PubMed] [Google Scholar]
- 87.Ahn J, Lee H, Kim S, Ha T. Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochemical and biophysical research communications. 2007;364:972–977. doi: 10.1016/j.bbrc.2007.10.109. [DOI] [PubMed] [Google Scholar]
- 88.Chen S, Li Z, Li W, Shan Z, Zhu W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Canadian journal of physiology and pharmacology. 2011;89:793–799. doi: 10.1139/y11-077. [DOI] [PubMed] [Google Scholar]
- 89.Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, et al. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. The Journal of nutritional biochemistry. 2012;23:1100–1012. doi: 10.1016/j.jnutbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Costa Cdos S, Rohden F, Hammes TO, Margis R, Bortolotto JW, Padoin AV, et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARgamma1-3 mRNA expression in human visceral adipocytes. Obesity surgery. 2011;21:356–361. doi: 10.1007/s11695-010-0251-7. [DOI] [PubMed] [Google Scholar]
- 91.Kang L, Heng W, Yuan A, Baolin L, Fang H. Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: Relative to inhibition of inflammatory responses. Biochimie. 2010;92:789–796. doi: 10.1016/j.biochi.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 92.Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutrition research and practice. 2012;6:499–504. doi: 10.4162/nrp.2012.6.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kwon JY, Seo SG, Yue S, Cheng JX, Lee KW, Kim KH. An inhibitory effect of resveratrol in the mitotic clonal expansion and insulin signaling pathway in the early phase of adipogenesis. Nutrition research. 2012;32:607–616. doi: 10.1016/j.nutres.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 94.Lasa A, Miranda J, Churruca I, Simon E, Arias N, Milagro F, et al. The combination of resveratrol and CLA does not increase the delipidating effect of each molecule in 3T3-L1 adipocytes. Nutricion hospitalaria : organo oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2011;26:997–1003. doi: 10.1590/S0212-16112011000500012. [DOI] [PubMed] [Google Scholar]
- 95.Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simon E, Zechner R, et al. Resveratrol regulates lipolysis via adipose triglyceride lipase. The Journal of nutritional biochemistry. 2012;23:379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 96.Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytotherapy research : PTR. 2008;22:1367–1371. doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 97.Wang A, Liu M, Liu X, Dong LQ, Glickman RD, Slaga TJ, et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. The Journal of biological chemistry. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang HY, Du ZX, Meng X. Resveratrol prevents TNFalpha-induced suppression of adiponectin expression via PPARgamma activation in 3T3-L1 adipocytes. Clinical and experimental medicine. 2012 doi: 10.1007/s10238-012-0189-2. [DOI] [PubMed] [Google Scholar]
- 99.Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutrition research and practice. 2012;6:286–293. doi: 10.4162/nrp.2012.6.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhu J, Yong W, Wu X, Yu Y, Lv J, Liu C, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochemical and biophysical research communications. 2008;369:471–477. doi: 10.1016/j.bbrc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 101.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chung JH, et al. Using PDE inhibitors to harness the benefits of calorie restriction: lessons from resveratrol. Aging. 2012;4:144–145. doi: 10.18632/aging.100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 104.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. The Journal of biological chemistry. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chemical biology & drug design. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 106.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Harp JB. New insights into inhibitors of adipogenesis. Current opinion in lipidology. 2010;15:303–307. doi: 10.1097/00041433-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. European journal of pharmacology. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 109.Mercader J, Palou A, Bonet ML. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. The Journal of nutritional biochemistry. 2011;22:828–834. doi: 10.1016/j.jnutbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Crowley VE, Yeo GS, O'Rahilly S. Obesity therapy: altering the energy intake-and-expenditure balance sheet. Nature reviews Drug discovery. 2002;1:276–286. doi: 10.1038/nrd770. [DOI] [PubMed] [Google Scholar]
- 111.Alberdi G, Rodriguez VM, Macarulla MT, Miranda J, Churruca I, Portillo MP, et al. Hepatic lipid metabolic pathways modified by resveratrol in rats fed an obesogenic diet. Nutrition. 2013;29:562–567. doi: 10.1016/j.nut.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 112.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Arias N, Andres-Lacueva C, et al. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutrition & metabolism. 2006;8:29. doi: 10.1186/1743-7075-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, Garcia-Urkia N, et al. Resveratrol inhibits nonalcoholic fatty liver disease in rats. BMC gastroenterology. 2008;8:40. doi: 10.1186/1471-230X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dal-Pan A, Blanc S, Aujard F. Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC physiology. 2010;10:11. doi: 10.1186/1472-6793-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Franco JG, Lisboa PC, Lima NS, Amaral TA, Peixoto-Silva N, Resende AC, et al. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. The Journal of nutritional biochemistry. 2012 doi: 10.1016/j.jnutbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 117.Gomez-Zorita S, Fernandez-Quintela A, Macarulla MT, Aguirre L, Hijona E, Bujanda L, et al. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. The British journal of nutrition. 2012;107:202–210. doi: 10.1017/S0007114511002753. [DOI] [PubMed] [Google Scholar]
- 118.Kim S, Jin Y, Choi Y, Park T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochemical pharmacology. 2011;81:1343–1351. doi: 10.1016/j.bcp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 119.Macarulla MT, Alberdi G, Gomez S, Tueros I, Bald C, Rodriguez VM, et al. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. Journal of physiology and biochemistry. 2009;65:369–376. doi: 10.1007/BF03185932. [DOI] [PubMed] [Google Scholar]
- 120.Nagao K, Jinnouchi T, Kai S, Yanagita T. Effect of dietary resveratrol on the metabolic profile of nutrients in obese OLETF rats. Lipids in health and disease. 2013;12:8. doi: 10.1186/1476-511X-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poulsen MM, Larsen JO, Hamilton-Dutoit S, Clasen BF, Jessen N, Paulsen SK, et al. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutrition research. 2012;32:701–708. doi: 10.1016/j.nutres.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 122.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochemical pharmacology. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 123.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell metabolism. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tome-Carneiro J, Gonzalvez M, Larrosa M, Garcia-Almagro FJ, Aviles-Plaza F, Parra S, et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: a triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Molecular nutrition & food research. 2013;56:810–821. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 126.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, et al. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tome-Carneiro J, Larrosa M, Yanez-Gascon MJ, Davalos A, Gil-Zamorano J, Gonzalvez M, et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related microRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacological research : the official journal of the Italian Pharmacological Society. 2013 doi: 10.1016/j.phrs.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 128.Alappat L, Awad AB. Curcumin and obesity: evidence and mechanisms. Nutrition reviews. 2010;68:729–738. doi: 10.1111/j.1753-4887.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- 129.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Annals of the New York Academy of Sciences. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 130.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) Journal of alternative and complementary medicine. 2008;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 132.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 133.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer research. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 134.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. American journal of physiology Cell physiology. 2010;298:C1510–C1506. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 135.Dong SZ, Zhao SP, Wu ZH, Yang J, Xie XZ, Yu BL, et al. Curcumin promotes cholesterol efflux from adipocytes related to PPARgamma-LXRalpha-ABCA1 passway. Molecular and cellular biochemistry. 2011;358:281–285. doi: 10.1007/s11010-011-0978-z. [DOI] [PubMed] [Google Scholar]
- 136.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. The Journal of nutrition. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 137.Gonzales AM, Orlando RA. Curcumin and resveratrol inhibit nuclear factor-kappaB-mediated cytokine expression in adipocytes. Nutrition & metabolism. 2008;5:17. doi: 10.1186/1743-7075-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim CY, Le TT, Chen C, Cheng JX, Kim KH. Curcumin inhibits adipocyte differentiation through modulation of mitotic clonal expansion. The Journal of nutritional biochemistry. 2011;22:910–920. doi: 10.1016/j.jnutbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 139.Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, Park OJ. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. Journal of agricultural and food chemistry. 2009;57:305–310. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 140.Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, et al. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PloS one. 2012;7:e28784. doi: 10.1371/journal.pone.0028784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang SL, Li Y, Wen Y, Chen YF, Na LX, Li ST, et al. Curcumin, a potential inhibitor of up-regulation of TNF-alpha and IL-6 induced by palmitate in 3T3-L1 adipocytes through NF-kappaB and JNK pathway. Biomedical and environmental sciences : BES. 2009;22:32–39. doi: 10.1016/S0895-3988(09)60019-2. [DOI] [PubMed] [Google Scholar]
- 142.Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life sciences. 2007;80:926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 143.Xie XY, Kong PR, Wu JF, Li Y, Li YX. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-alpha or isoproterenol in 3T3-L1 adipocytes. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2012;20:3–8. doi: 10.1016/j.phymed.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 144.Zhao J, Sun XB, Ye F, Tian WX. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Molecular and cellular biochemistry. 2011;351:19–28. doi: 10.1007/s11010-010-0707-z. [DOI] [PubMed] [Google Scholar]
- 145.Rao DS, Sekhara NC, Satyanarayana MN, Srinivasan M. Effect of curcumin on serum and liver cholesterol levels in the rat. The Journal of nutrition. 1970;100:1307–1315. doi: 10.1093/jn/100.11.1307. [DOI] [PubMed] [Google Scholar]
- 146.Pongchaidecha A, Lailerd N, Boonprasert W, Chattipakorn N. Effects of curcuminoid supplement on cardiac autonomic status in high-fat-induced obese rats. Nutrition. 2009;25:870–878. doi: 10.1016/j.nut.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 147.Asai A, Miyazawa T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. The Journal of nutrition. 2001;131:2932–2935. doi: 10.1093/jn/131.11.2932. [DOI] [PubMed] [Google Scholar]
- 148.Oner-Iyidogan Y, Kocak H, Seyidhanoglu M, Gurdol F, Gulcubuk A, Yildirim F, et al. Curcumin prevents liver fat accumulation and serum fetuin-A increase in rats fed a high-fat diet. Journal of physiology and biochemistry. 2013 doi: 10.1007/s13105-013-0244-9. [DOI] [PubMed] [Google Scholar]
- 149.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu Y, Hu SK, Yan H. [The study of insulin resistance and leptin resistance on the model of simplicity obesity rats by curcumin] Zhonghua yu fang yi xue za zhi [Chinese journal of preventive medicine] 2008;42:818–822. [PubMed] [Google Scholar]
- 151.He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World journal of diabetes. 2012;3:94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yekollu SK, Thomas R, O'Sullivan B. Targeting curcusomes to inflammatory dendritic cells inhibits NF-kappaB and improves insulin resistance in obese mice. Diabetes. 2011;60:2928–2938. doi: 10.2337/db11-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Asai A, Nakagawa K, Miyazawa T. Antioxidative effects of turmeric, rosemary and capsicum extracts on membrane phospholipid peroxidation and liver lipid metabolism in mice. Bioscience, biotechnology, and biochemistry. 1999;63:2118–2122. doi: 10.1271/bbb.63.2118. [DOI] [PubMed] [Google Scholar]
- 154.Jang EM, Choi MS, Jung UJ, Kim MJ, Kim HJ, Jeon SM, et al. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism: clinical and experimental. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 155.Kempaiah RK, Srinivasan K. Beneficial influence of dietary curcumin, capsaicin and garlic on erythrocyte integrity in high-fat fed rats. The Journal of nutritional biochemistry. 2006;17:471–478. doi: 10.1016/j.jnutbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 156.Leray V, Freuchet B, Le Bloc'h J, Jeusette I, Torre C, Nguyen P. Effect of citrus polyphenol- and curcumin-supplemented diet on inflammatory state in obese cats. The British journal of nutrition. 2011;106(Suppl 1):S198–S201. doi: 10.1017/S0007114511002492. [DOI] [PubMed] [Google Scholar]
- 157.Mesa MD, Aguilera CM, Ramirez-Tortosa CL, Ramirez-Tortosa MC, Quiles JL, Baro L, et al. Oral administration of a turmeric extract inhibits erythrocyte and liver microsome membrane oxidation in rabbits fed with an atherogenic diet. Nutrition. 2003;19:800–804. doi: 10.1016/s0899-9007(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 158.Ramirez-Bosca A, Soler A, Carrion MA, Diaz-Alperi J, Bernd A, Quintanilla C, et al. An hydroalcoholic extract of curcuma longa lowers the apo B/apo A ratio. Implications for atherogenesis prevention. Mechanisms of ageing and development. 2000;119:41–47. doi: 10.1016/s0047-6374(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 159.Ramirez Bosca A, Soler A, Carrion-Gutierrez MA, Pamies Mira D, Pardo Zapata J, Diaz-Alperi J, et al. An hydroalcoholic extract of Curcuma longa lowers the abnormally high values of human-plasma fibrinogen. Mechanisms of ageing and development. 2000;114:207–210. doi: 10.1016/s0047-6374(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 160.Baum L, Cheung SK, Mok VC, Lam LC, Leung VP, Hui E, et al. Curcumin effects on blood lipid profile in a 6-month human study. Pharmacological research : the official journal of the Italian Pharmacological Society. 2007;56:509–514. doi: 10.1016/j.phrs.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 161.Wang S, Noh SK, Koo SI. Epigallocatechin gallate and caffeine differentially inhibit the intestinal absorption of cholesterol and fat in ovariectomized rats. The Journal of nutrition. 2006;136:2791–2796. doi: 10.1093/jn/136.11.2791. [DOI] [PubMed] [Google Scholar]
- 162.Han DW, Matsumura K, Kim B, Hyon SH. Time-dependent intracellular trafficking of FITC-conjugated epigallocatechin-3-O-gallate in L-929 cells. Bioorg Med Chem. 2008;16:9652–9659. doi: 10.1016/j.bmc.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 163.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 164.Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. The Journal of biological chemistry. 2008;283:3050–3058. doi: 10.1074/jbc.M707892200. [DOI] [PubMed] [Google Scholar]
- 165.Hsu YC, Liou YM. The anti-cancer effects of (−)-epigallocatechin-3-gallate on the signaling pathways associated with membrane receptors in MCF-7 cells. J Cell Physiol. 2011;226:2721–2730. doi: 10.1002/jcp.22623. [DOI] [PubMed] [Google Scholar]
- 166.Hsieh CF, Tsuei YW, Liu CW, Kao CC, Shih LJ, Ho LT, et al. Green tea epigallocatechin gallate inhibits insulin stimulation of adipocyte glucose uptake via the 67-kilodalton laminin receptor and AMP-activated protein kinase pathways. Planta Med. 2010;76:1694–1698. doi: 10.1055/s-0030-1249877. [DOI] [PubMed] [Google Scholar]
- 167.Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, et al. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. The American journal of clinical nutrition. 2010;92:5–15. doi: 10.3945/ajcn.2009.28435. [DOI] [PubMed] [Google Scholar]
- 168.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim CY, Bordenave N, Ferruzzi MG, Safavy A, Kim KH. Modification of curcumin with polyethylene glycol enhances the delivery of curcumin in preadipocytes and its antiadipogenic property. Journal of agricultural and food chemistry. 2011;59:1012–1019. doi: 10.1021/jf103873k. [DOI] [PubMed] [Google Scholar]
- 170.Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- 171.Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. The Journal of nutrition. 2001;131:1731–1737. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 172.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 173.Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 174.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 175.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews Drug discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 176.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. The Journal of nutrition. 1998;128:593–597. doi: 10.1093/jn/128.3.593. [DOI] [PubMed] [Google Scholar]
- 177.Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996;21:703–707. doi: 10.1016/0891-5849(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 178.Hong YJ, Mitchell AE. Metabolic profiling of flavonol metabolites in human urine by liquid chromatography and tandem mass spectrometry. Journal of agricultural and food chemistry. 2004;52:6794–6801. doi: 10.1021/jf040274w. [DOI] [PubMed] [Google Scholar]
- 179.Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51:454–463. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]