Abstract
A Sprague-Dawley (SD-1) rat embryo culture, at low passage level, released an endogenous ecotropic type C virus (SD-RaLV) and after about 20 further passages it underwent spontaneous transformation. The SD-RaLV, released from the transformed cells, did not cause rapid transformation of other rat embryo cells. However, when the transformed cells were repeatedly cocultivated with three different chemically transformed and serially transplanted rat tumor cell lines (sarcoma, carcinoma, and hepatoma), rapidly fibroblast-transforming “sarcoma” viruses (RaSV) were recovered after each attempt. RaSV was not recovered from one of these tumor cell lines before transplantation, nor could focus-forming virus be rescued from these same tumor cells by cocultivation with other cells releasing heterologous type C viruses. Foci were induced on normal rat kidney and several other rat embryo cell strains within 7-15 days and both productive and nonproductive NRK clones were derived. The productive clones were positive for rat specific p30 antigen and the RaSVs released were serially transmitted to other rat embryo cells. RaSV genome was rescued from the nonproductive clones by superinfection with SD-RaLV, wild rat type C virus, and several heterologous type C viruses. These observations appear to represent naturally occurring transformation-specific (src) genes being recovered in vitro in the form of stable “sarcoma” viruses. These viruses differ from the Kirsten and Harvey strains of murine sarcoma virus in that they apparently contain no MuLV sequences and are of purely rat origin.
Keywords: transforming type C viruses, chemical carcinogenesis, endogenous transforming (src) genes, viral recombination, tumor antigens
Full text
PDF

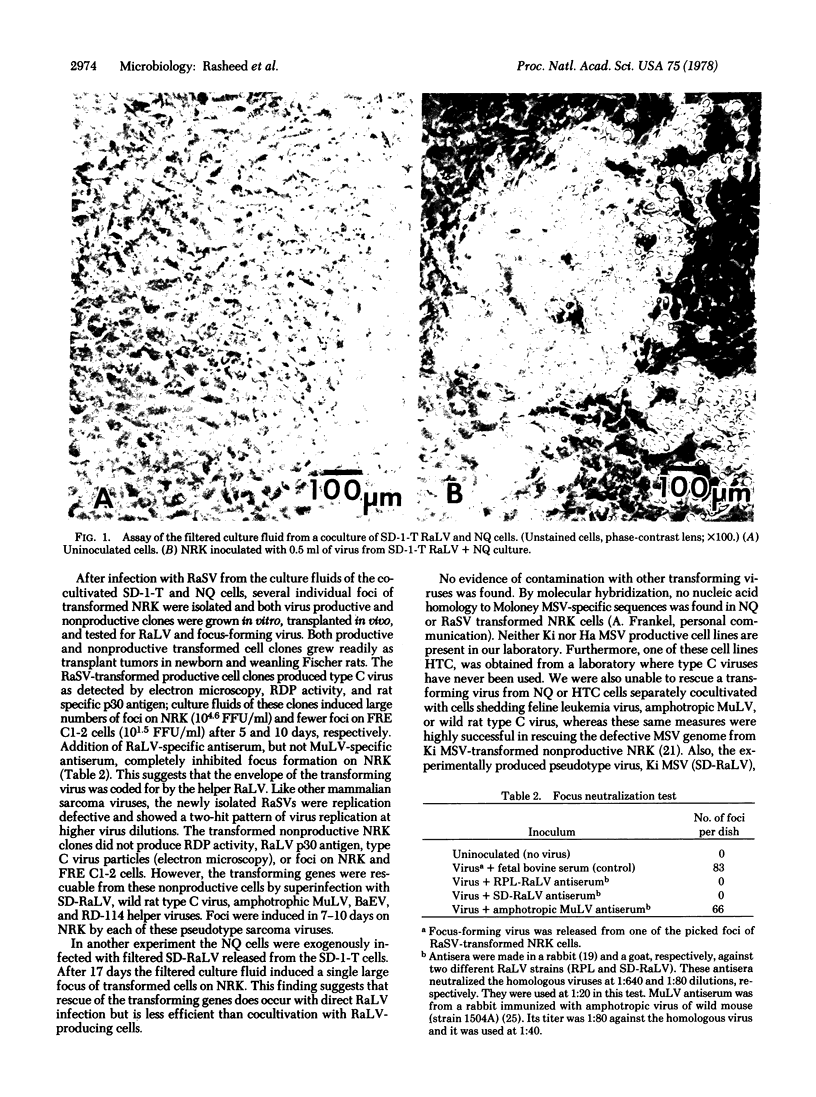


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Biologic characterization of mammalian cells transformed by a primate sarcoma virus. Virology. 1973 Apr;52(2):562–567. doi: 10.1016/0042-6822(73)90351-6. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Matovcik L. M. Expression of murine sarcoma virus genes in uninfected rat cells subjected to anaerobic sress. Science. 1977 Sep 30;197(4311):1371–1374. doi: 10.1126/science.197602. [DOI] [PubMed] [Google Scholar]
- Anderson G. R., Robbins K. C. Rat sequences of the Kirsten and Harvey murine sarcoma virus genomes: nature, origin, and expression in rat tumor RNA. J Virol. 1976 Feb;17(2):335–351. doi: 10.1128/jvi.17.2.335-351.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Tumor viruses: 1974. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1187–1200. doi: 10.1101/sqb.1974.039.01.137. [DOI] [PubMed] [Google Scholar]
- Chapman H. P., White M. H., Rahman R., Gilden R. V. Species and interspecies radioimmunoassays for rat type C virus p30: interviral comparisons and assay of human tumor extracts. J Virol. 1975 Jan;17(1):51–59. doi: 10.1128/jvi.17.1.51-59.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Rongey R. W., Arnstein P., Estes J. D., Sarma P., Huebner R. J., Rickard C. G. Experimental transmission of feline fibrosarcoma to cats and dogs. Nature. 1970 May 30;226(5248):807–809. doi: 10.1038/226807a0. [DOI] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS, II. SPECIFICATION OF RSV ANTIGENICITY BY HELPER VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:41–48. doi: 10.1073/pnas.51.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Halpern C. C., Buchhagen D. L., Kawai S. Recovery of avian sarcoma virus from tumors induced by transformation-defective mutants. J Exp Med. 1977 Dec 1;146(6):1735–1747. doi: 10.1084/jem.146.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Hartley J. W., Rowe W. P., Lane W. T., Capps W. I. Rescue of the defective genome of Moloney sarcoma virus from a noninfectious hamster tumor and the production of pseudotype sarcoma viruses with various murine leukemia viruses. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Helmke R. J., Panigel M., Heberling R. L., Felsburg P. J., Axelrod L. R. Observations of apparent C-type particles in baboon (Papio cynocephalus) placentas. Science. 1973 Mar 30;179(4080):1332–1333. doi: 10.1126/science.179.4080.1332. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Bova D., Huebner R. J., Gilden R. V. Major group-specific protein of rat type C viruses. J Virol. 1972 Oct;10(4):746–750. doi: 10.1128/jvi.10.4.746-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles P. T., Scolnick E. M., Howk R. S. Increased sarcoma virus RNA in cells transformed by leukemia viruses: model for leukemogenesis. Science. 1976 Jun 11;192(4244):1143–1145. doi: 10.1126/science.179144. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Bruszewski J., Rongey R., Roy-Burman P., Charman H. P., Gardner M. B. Spontaneous release of endogenous ecotropic type C virus from rat embryo cultures. J Virol. 1976 May;18(2):799–803. doi: 10.1128/jvi.18.2.799-803.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Freeman A. E., Gardner M. B., Huebner R. J. Acceleration of transformation of rat embryo cells by rat type C virus. J Virol. 1976 May;18(2):776–782. doi: 10.1128/jvi.18.2.776-782.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Toth E., Gardner M. B. Characterization of purely ecotropic and amphotropic naturally occurring wild mouse leukemia viruses. Intervirology. 1977;8(6):323–335. doi: 10.1159/000148908. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Huebner R. J. Trans-species rescue of defective genomes of murine sarcoma virus from hamster tumor cells with helper feline leukemia virus. Proc Natl Acad Sci U S A. 1970 Jan;65(1):81–87. doi: 10.1073/pnas.65.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Goldberg R. J., Williams D. Characterizatiion of rat genetic sequences of Kirsten sarcoma virus: distinct class of endogenous rat type C viral sequences. J Virol. 1976 May;18(2):559–566. doi: 10.1128/jvi.18.2.559-566.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Maryak J., Vass W., Goldberg R. J., Parks W. P. Type C particle-positive and type C particle-negative rat cell lines: characterization of the coding capacity of endogenous sarcoma virus-specific RNA. J Virol. 1976 Dec;20(3):570–582. doi: 10.1128/jvi.20.3.570-582.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Williams D., Parks W. P. Purification and characterisation of viral RNA of a sarcoma virus isolated from a woolly monkey. Nature. 1976 Dec 23;264(5588):809–811. doi: 10.1038/264809a0. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H. Colon carcinogens: their metabolism and mode of action. Cancer. 1971 Jul;28(1):60–70. doi: 10.1002/1097-0142(197107)28:1<60::aid-cncr2820280113>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]



