Abstract
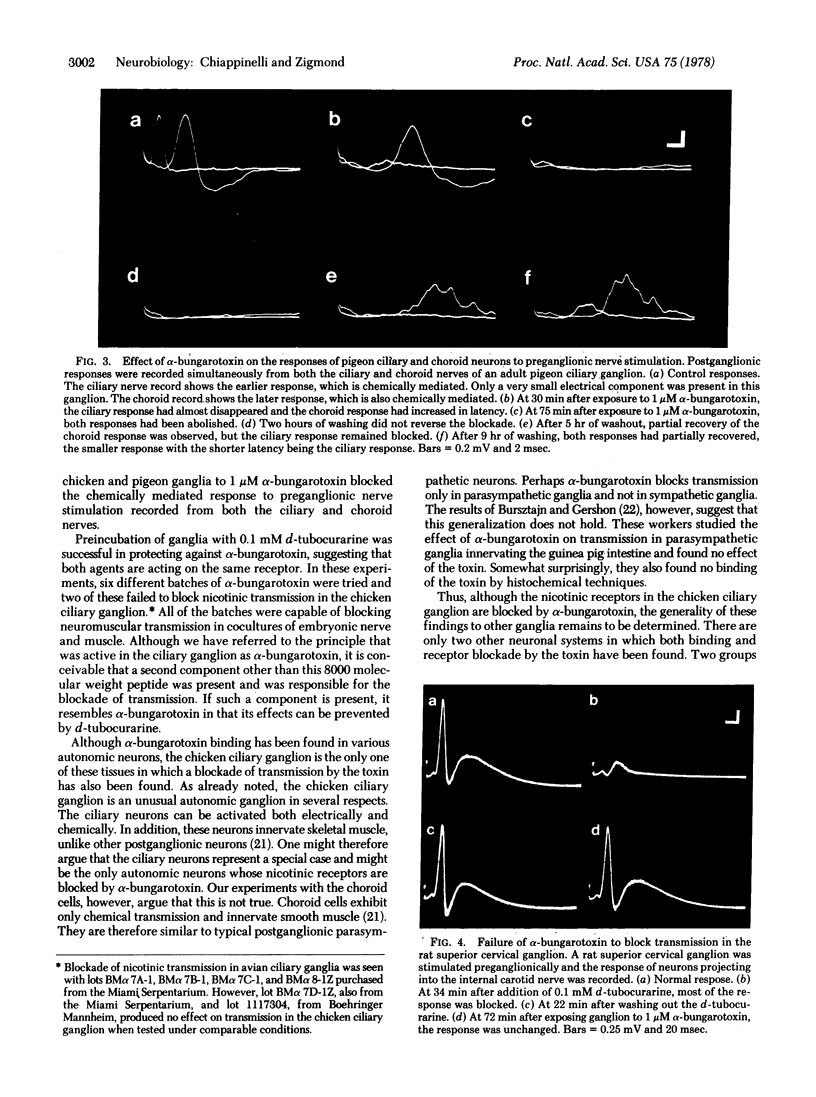
alpha-Bungarotoxin binds to nicotinic receptors in skeletal muscle, blocking neuromuscular transmission. Because this toxin has recently been shown to bind to chicken ciliary ganglia, an attempt has been made to determine whether it also blocks nicotinic transmission in this ganglion, alpha-Bungarotoxin (1 micrometer) completely blocked nicotinic transmission in both the ciliary and choroid neurons of chicken and pigeon ciliary ganglia. The effect of the toxin could be partially reversed by prolonged washing (2--8 hr). Incubation of ganglia with d-tubocurarine (0.1 mM) prior to the addition of alpha-bungarotoxin significantly decreased the duration of the washout period necessary to restore transmission. These results suggest that d-tubocurarine and alpha-bungarotoxin are interacting with the same receptor. Under similar conditions, alpha-bungarotoxin did not block nicotinic transmission in the rat superior cervical ganglion, in agreement with previous reports. The avian ciliary ganglion is the only vertebrate autonomic ganglion in which both alpha-bungarotoxin binding and alpha-bungarotoxin blockade of transmission have been shown to occur. This ganglion therefore provides a model system for using alpha-bungarotoxin to study neuronal nicotinic receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. K., Kelly R. B., Sargent P. B., Williamson P., Hall Z. W. Binding of -bungarotoxin to acetylcholine receptors in mammalian muscle (snake venom-denervated muscle-neonatal muscle-rat diaphragm-SDS-polyacrylamide gel electrophoresis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):147–151. doi: 10.1073/pnas.69.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Fumagalli L. Department of Pharmacology, The School of Pharmacy, University of London, London, Great Britain. Brain Res. 1977 Jun 24;129(1):165–168. doi: 10.1016/0006-8993(77)90981-7. [DOI] [PubMed] [Google Scholar]
- Bursztajn S., Gershon M. D. Discrimination between nicotinic receptors in vertebrate ganglia and skeletal muscle by alpha-bungarotoxin and cobra venoms. J Physiol. 1977 Jul;269(1):17–31. doi: 10.1113/jphysiol.1977.sp011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976 Apr;69(1):144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. A. Possible regulatory function of acetylcholine receptor in maintenance of retinotectal synapses. Nature. 1977 Sep 15;269(5625):218–222. doi: 10.1038/269218a0. [DOI] [PubMed] [Google Scholar]
- Fumagalli L., De Renzis G., Miani N. Acetylcholine receptors: number and distribution in intact and deafferented superior cervical ganglion of the rat. J Neurochem. 1976 Jul;27(1):47–52. doi: 10.1111/j.1471-4159.1976.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Greene L. A. Binding of alpha-bungarotoxin to chick sympathetic ganglia: properties of the receptor and its rate of appearance during developement. Brain Res. 1976 Jul 23;111(1):135–145. doi: 10.1016/0006-8993(76)91054-4. [DOI] [PubMed] [Google Scholar]
- Kehoe J., Sealock R., Bon C. Effects of alpha-toxins from Bungarus multicinctus and Bungarus caeruleus on cholinergic responses in Aplysia neurons. Brain Res. 1976 May 14;107(3):527–540. doi: 10.1016/0006-8993(76)90142-6. [DOI] [PubMed] [Google Scholar]
- Kouvelas E. D., Greene L. A. The binding properties and regional ontogeny of receptors for alpha-bungarotoxin in chick brain. Brain Res. 1976 Aug 20;113(1):111–126. doi: 10.1016/0006-8993(76)90010-x. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Chemistry and pharmacology of polypeptide toxins in snake venoms. Annu Rev Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Tseng L. F., Chiu T. H. Influence of denervation on localization of neurotoxins from clapid venoms in rat diaphragm. Nature. 1967 Sep 9;215(5106):1177–1178. doi: 10.1038/2151177a0. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. TRANSMISSION THROUGH THE CILIARY GANGLION OF THE CHICK. J Physiol. 1963 Sep;168:464–475. doi: 10.1113/jphysiol.1963.sp007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
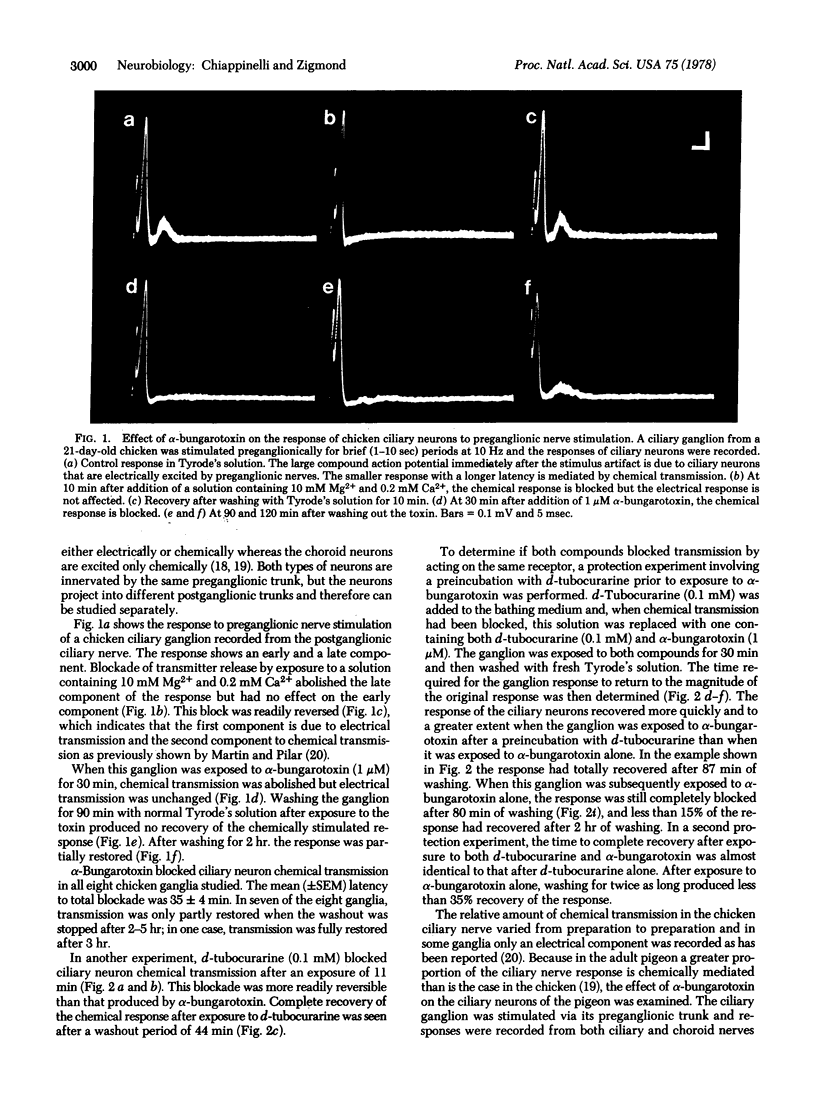
- Marwitt R., Pilar G., Weakly J. N. Characterization of two ganglion cell populations in avian ciliary ganglia. Brain Res. 1971 Jan 22;25(2):317–334. doi: 10.1016/0006-8993(71)90441-0. [DOI] [PubMed] [Google Scholar]
- Moore W. M., Brady R. N. Studies of nicotinic acetylcholine receptor protein from rat brain. Biochim Biophys Acta. 1976 Aug 24;444(1):252–260. doi: 10.1016/0304-4165(76)90242-7. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., O'Lague P. H. Formation of cholinergic synapses between dissociated sympathetic neurons and skeletal myotubes of the rat in cell culture. Proc Natl Acad Sci U S A. 1975 May;72(5):1955–1959. doi: 10.1073/pnas.72.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K. Transmitter sensitivities of some nerve and muscle cells in culture. Brain Res. 1974 Jun 14;73(1):71–88. doi: 10.1016/0006-8993(74)91008-7. [DOI] [PubMed] [Google Scholar]
- Patrick J., Stallcup B. alpha-Bungarotoxin binding and cholinergic receptor function on a rat sympathetic nerve line. J Biol Chem. 1977 Dec 10;252(23):8629–8633. [PubMed] [Google Scholar]
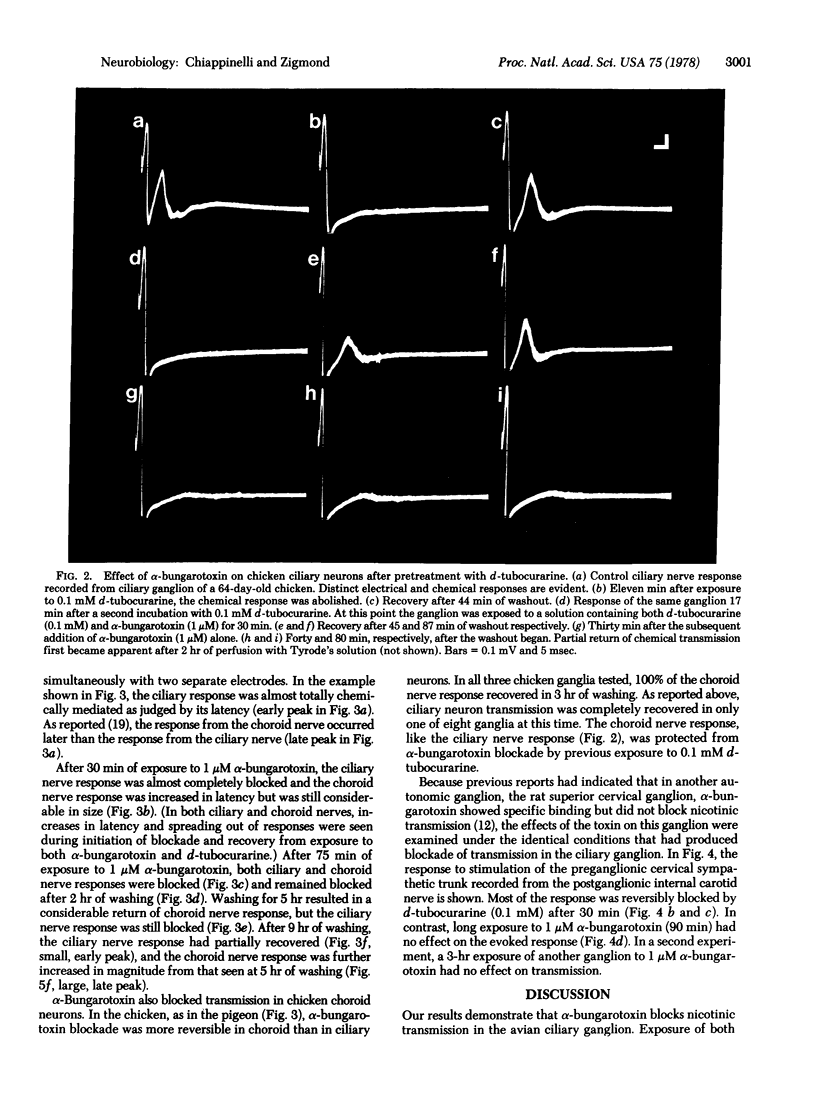
- Patrick J., Stallcup W. B. Immunological distinction between acetylcholine receptor and the alpha-bungarotoxin-binding component on sympathetic neurons. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4689–4692. doi: 10.1073/pnas.74.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain W., Greene L. A., Carpenter D. O., Sytkowski A. J., Vogel Z. Aplysia acetylcholine receptors: blockade by and binding of alpha-bungarotoxin. Brain Res. 1974 Jun 7;72(2):225–240. doi: 10.1016/0006-8993(74)90861-0. [DOI] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. The acetylcholine receptor of the adrenal medulla. J Neurochem. 1977 Apr;28(4):687–695. doi: 10.1111/j.1471-4159.1977.tb10615.x. [DOI] [PubMed] [Google Scholar]