Abstract
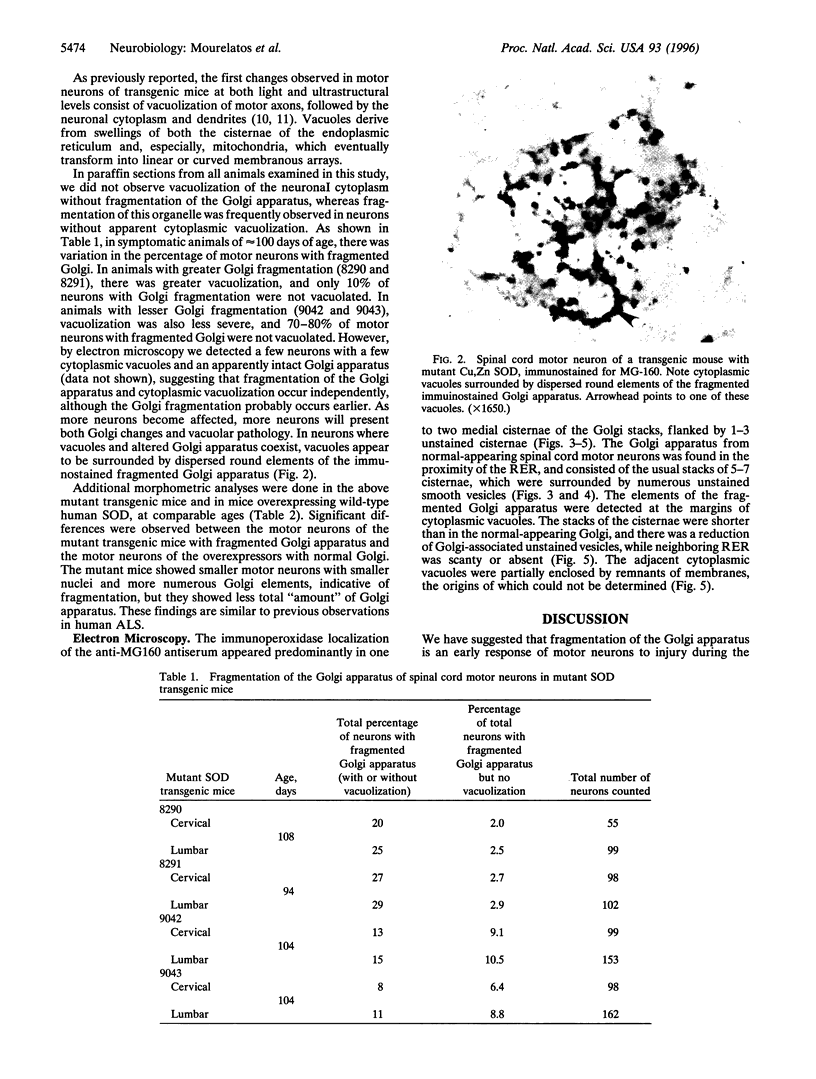
Dominant mutations of the SOD1 gene encoding Cu,Zn superoxide dismutase have been found in members of certain families with familial amyotrophic lateral sclerosis (ALS). To better understand the contribution of SOD1 mutations in the pathogenesis of familial ALS, we developed transgenic mice expressing one of the mutations found in familial ALS. These animals display clinical and pathological features closely resembling human ALS. Early changes observed in these animals were intra-axonal and dendritic vacuoles due to dilatation of the endoplasmic reticulum and vacuolar degeneration of mitochondria. We have reported that the Golgi apparatus of spinal cord motor neurons in patients with sporadic ALS is fragmented and atrophic. In this study we show that spinal cord motor neurons of transgenic mice for an SOD1 mutation display a lesion of the Golgi apparatus identical to that found in humans with sporadic ALS. In these mice, the stacks of the cisternae of the fragmented Golgi apparatus are shorter than in the normal organelle, and there is a reduction in Golgi-associated vesicles and adjacent cisternae of the rough endoplasmic reticulum. Furthermore, the fragmentation of the Golgi apparatus occurs in an early, presymptomatic stage and usually precedes the development of the vacuolar changes. Transgenic mice overexpressing the wild-type human superoxide dismutase are normal. In familial ALS, an early lesion of the Golgi apparatus of motor neurons may have adverse functional effects, because newly synthesized proteins destined for fast axoplasmic transport pass through the Golgi apparatus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine J. C., Maurice M., Feldmann G., Avrameas S. In vivo and in vitro effects of colchicine and vinblastine on the secretory process of antibody-producing cells. J Immunol. 1980 Nov;125(5):1939–1949. [PubMed] [Google Scholar]
- Beckman J. S., Carson M., Smith C. D., Koppenol W. H. ALS, SOD and peroxynitrite. Nature. 1993 Aug 12;364(6438):584–584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 1989;31:553–569. doi: 10.1016/s0091-679x(08)61626-x. [DOI] [PubMed] [Google Scholar]
- Chiu A. Y., Zhai P., Dal Canto M. C., Peters T. M., Kwon Y. W., Prattis S. M., Gurney M. E. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995 Aug;6(4):349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L. Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croul S., Mezitis S. G., Stieber A., Chen Y. J., Gonatas J. O., Goud B., Gonatas N. K. Immunocytochemical visualization of the Golgi apparatus in several species, including human, and tissues with an antiserum against MG-160, a sialoglycoprotein of rat Golgi apparatus. J Histochem Cytochem. 1990 Jul;38(7):957–963. doi: 10.1177/38.7.2355176. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Gurney M. E. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS). Brain Res. 1995 Apr 3;676(1):25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas J. O., Mourelatos Z., Stieber A., Lane W. S., Brosius J., Gonatas N. K. MG-160, a membrane sialoglycoprotein of the medial cisternae of the rat Golgi apparatus, binds basic fibroblast growth factor and exhibits a high level of sequence identity to a chicken fibroblast growth factor receptor. J Cell Sci. 1995 Feb;108(Pt 2):457–467. doi: 10.1242/jcs.108.2.457. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K. Rous-Whipple Award Lecture. Contributions to the physiology and pathology of the Golgi apparatus. Am J Pathol. 1994 Oct;145(4):751–761. [PMC free article] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Mourelatos Z., Chen Y., Gonatas J. O., Appel S. H., Hays A. P., Hickey W. F., Hauw J. J. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am J Pathol. 1992 Mar;140(3):731–737. [PMC free article] [PubMed] [Google Scholar]
- Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994 Jun 17;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hammerschlag R., Stone G. C., Bolen F. A., Lindsey J. D., Ellisman M. H. Evidence that all newly synthesized proteins destined for fast axonal transport pass through the Golgi apparatus. J Cell Biol. 1982 Jun;93(3):568–575. doi: 10.1083/jcb.93.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Kurland L. T., Sayre G. P. Familial amyotrophic lateral sclerosis. A subgroup characterized by posterior and spinocerebellar tract involvement and hyaline inclusions in the anterior horn cells. Arch Neurol. 1967 Mar;16(3):232–243. doi: 10.1001/archneur.1967.00470210008002. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Chen J., Tsai M., Martin J. C., Smith C. D., Beckman J. S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992 Nov 1;298(2):431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- Johnston P. A., Stieber A., Gonatas N. K. A hypothesis on the traffic of MG160, a medial Golgi sialoglycoprotein, from the trans-Golgi network to the Golgi cisternae. J Cell Sci. 1994 Mar;107(Pt 3):529–537. doi: 10.1242/jcs.107.3.529. [DOI] [PubMed] [Google Scholar]
- Karecla P. I., Kreis T. E. Interaction of membranes of the Golgi complex with microtubules in vitro. Eur J Cell Biol. 1992 Apr;57(2):139–146. [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Cole N. B., Marotta A., Conrad P. A., Bloom G. S. Kinesin is the motor for microtubule-mediated Golgi-to-ER membrane traffic. J Cell Biol. 1995 Feb;128(3):293–306. doi: 10.1083/jcb.128.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O., Schmidt A., Salaméro J., Hoflack B., Roa M., Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994 Dec;127(6 Pt 1):1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Goto S., Kusaka H., Imai T., Murakami N., Hashizume Y., Okazaki H., Hirano A. Ubiquitin-positive inclusion in anterior horn cells in subgroups of motor neuron diseases: a comparative study of adult-onset amyotrophic lateral sclerosis, juvenile amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. J Neurol Sci. 1993 Apr;115(2):208–213. doi: 10.1016/0022-510x(93)90226-o. [DOI] [PubMed] [Google Scholar]
- Miesenböck G., Rothman J. E. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995 Apr;129(2):309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misteli T., Warren G. Mitotic disassembly of the Golgi apparatus in vivo. J Cell Sci. 1995 Jul;108(Pt 7):2715–2727. doi: 10.1242/jcs.108.7.2715. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z., Adler H., Hirano A., Donnenfeld H., Gonatas J. O., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis revealed by organelle-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4393–4395. doi: 10.1073/pnas.87.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Gonatas J. O., Nycum L. M., Gonatas N. K., Biegel J. A. Assignment of the GLG1 gene for MGF-160, a fibroblast growth factor and E-selectin binding membrane sialoglycoprotein of the Golgi apparatus, to chromosome 16q22-q23 by fluorescence in situ hybridization. Genomics. 1995 Jul 20;28(2):354–355. doi: 10.1006/geno.1995.1156. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z., Hirano A., Rosenquist A. C., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis (ALS). Clinical studies in ALS of Guam and experimental studies in deafferented neurons and in beta,beta'-iminodipropionitrile axonopathy. Am J Pathol. 1994 Jun;144(6):1288–1300. [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Yachnis A., Rorke L., Mikol J., Gonatas N. K. The Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 1993 Jun;33(6):608–615. doi: 10.1002/ana.410330609. [DOI] [PubMed] [Google Scholar]
- Pavelka M., Ellinger A. Effect of colchicine on the Golgi complex of rat pancreatic acinar cells. J Cell Biol. 1983 Sep;97(3):737–748. doi: 10.1083/jcb.97.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Siddique T., Figlewicz D. A., Pericak-Vance M. A., Haines J. L., Rouleau G., Jeffers A. J., Sapp P., Hung W. Y., Bebout J., McKenna-Yasek D. Linkage of a gene causing familial amyotrophic lateral sclerosis to chromosome 21 and evidence of genetic-locus heterogeneity. N Engl J Med. 1991 May 16;324(20):1381–1384. doi: 10.1056/NEJM199105163242001. [DOI] [PubMed] [Google Scholar]
- Siddique T., Pericak-Vance M. A., Brooks B. R., Roos R. P., Hung W. Y., Antel J. P., Munsat T. L., Phillips K., Warner K., Speer M. Linkage analysis in familial amyotrophic lateral sclerosis. Neurology. 1989 Jul;39(7):919–925. doi: 10.1212/wnl.39.7.919. [DOI] [PubMed] [Google Scholar]
- Stieber A., Mourelatos Z., Chen Y. J., Le Douarin N., Gonatas N. K. MG160, a membrane protein of the Golgi apparatus which is homologous to a fibroblast growth factor receptor and to a ligand for E-selectin, is found only in the Golgi apparatus and appears early in chicken embryo development. Exp Cell Res. 1995 Aug;219(2):562–570. doi: 10.1006/excr.1995.1265. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Beem K. M., Richardson J. S., Richardson D. C. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181–217. doi: 10.1016/0022-2836(82)90174-7. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Tartakoff A. M. The response of the Golgi complex to microtubule alterations: the roles of metabolic energy and membrane traffic in Golgi complex organization. J Cell Biol. 1989 Nov;109(5):2081–2088. doi: 10.1083/jcb.109.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C., Pardo C. A., Borchelt D. R., Lee M. K., Copeland N. G., Jenkins N. A., Sisodia S. S., Cleveland D. W., Price D. L. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995 Jun;14(6):1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Yim M. B., Chock P. B., Stadtman E. R. Copper, zinc superoxide dismutase catalyzes hydroxyl radical production from hydrogen peroxide. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5006–5010. doi: 10.1073/pnas.87.13.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]