Abstract
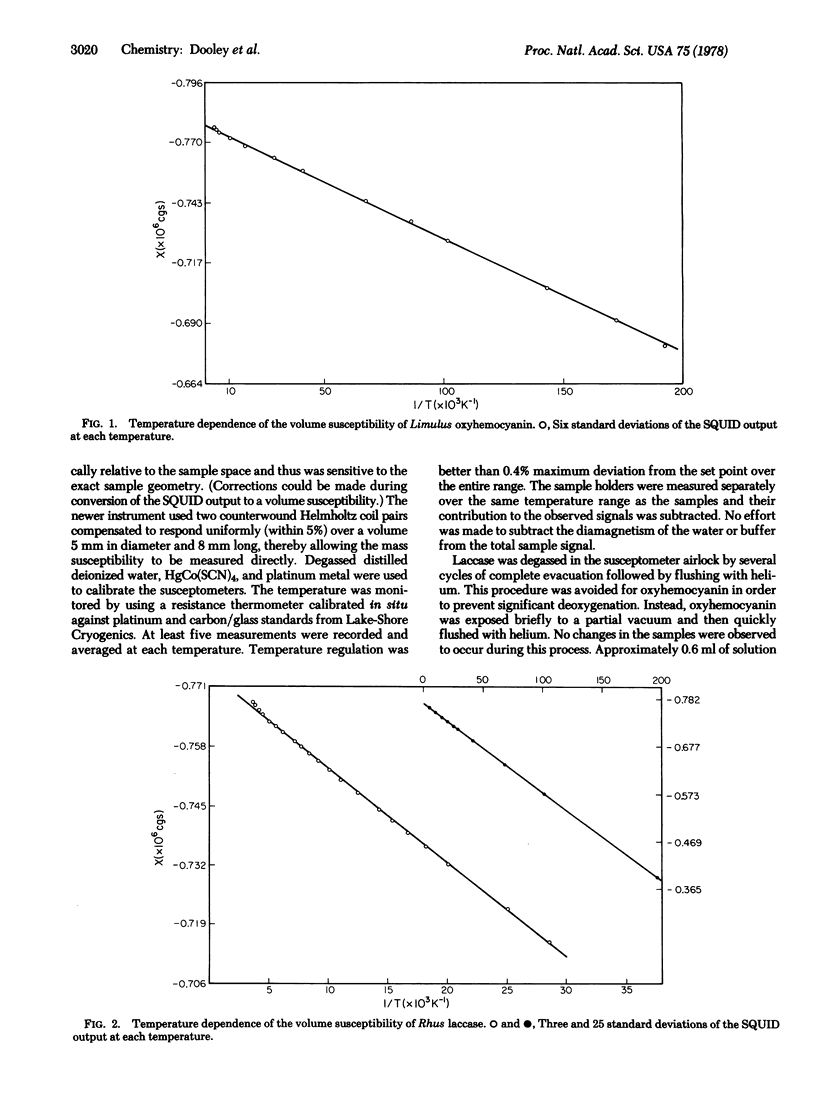
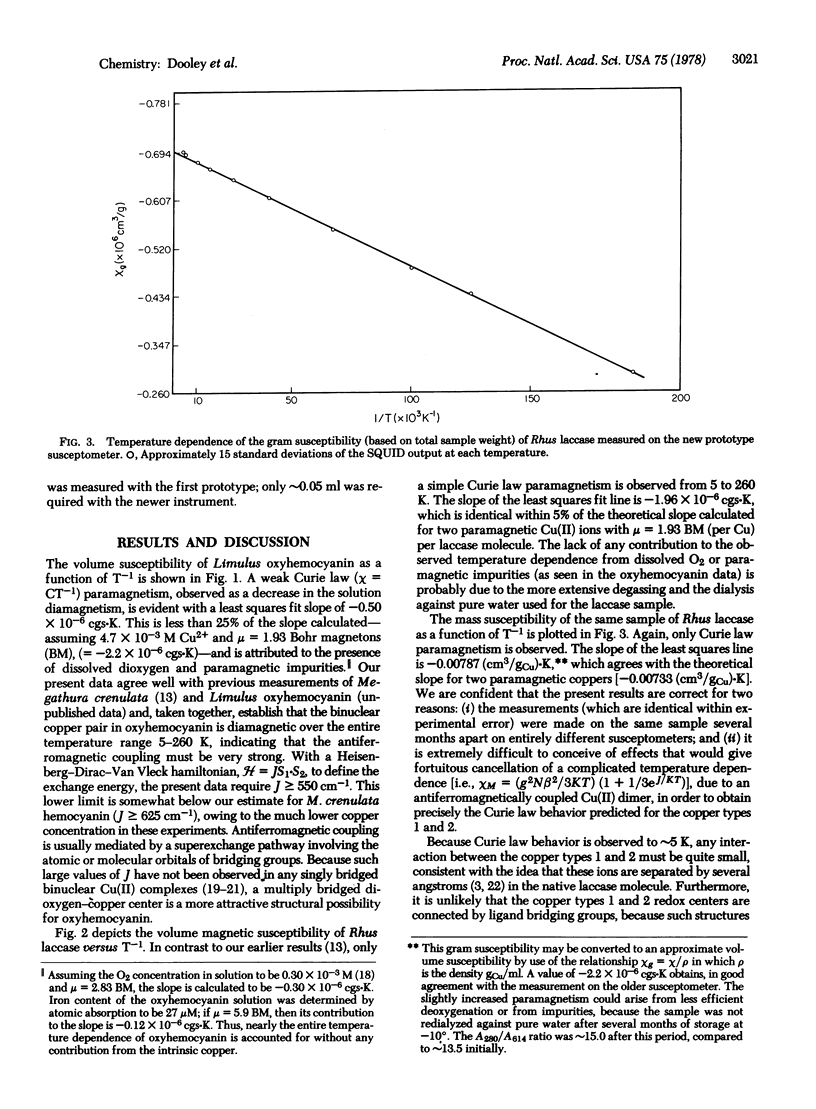
The magnetic susceptibility of Rhus vernicifera laccase has been remeasured over the temperature range 5-260 K. In contrast to our previous results [Solomon, E.I., Dooley, D. M., Wang R.-H., Gray, H.B., Cerdonio, M., Mogno, F. & Romani, G. L. (1975) J. Am. Chem. Soc. 98, 1029-1031] linear chi versus T-1 behavior was observed. The susceptibility of Limulus polyphemus oxyhemocyanin has also been measured in the range 5-260 K. Only weak paramagnetism, attributable to dissolved oxygen and a small amount of paramagnetic impurities, was observed. Analysis of the data establishes a lower limit of 550 cm-1 for J, consistent with our earlier work. The temperature dependence of the susceptibility of laccase is quantitatively accounted for by the presence of two paramagnetic copper ions (types 1 and 2) per enzyme molecule. Curie law behavior at low temperatures rules out significant interaction between the two coppper types, indicating that these redox centers are well separated (several angstroms) and are not connected by bridging ligands. Formulation of the type 3 site as binuclear Cu(II) requires J greater than or equal to 500 cm-1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Fee J. A., Briggs R. G. Studies on the reconstitution of bovine erythrocyte superoxide dismutase. V. Preparation and properties of derivatives in which both zinc and copper sites contain copper. Biochim Biophys Acta. 1975 Aug 19;400(2):439–450. doi: 10.1016/0005-2795(75)90200-7. [DOI] [PubMed] [Google Scholar]
- Fee J. A., Malkin R., Malmström B. G., Vänngård T. Anaerobic oxidation-reduction titrations of fungal laccase. Evidence for several high potential electron-accepting sites. J Biol Chem. 1969 Aug 10;244(15):4200–4207. [PubMed] [Google Scholar]
- Himmelwright R. S., Eickman N. C., Solomon E. I. Spectroscopic studies of ligand perturbation effects on the half oxidized action site of Busycon canaliculatum hemocyanin. Biochem Biophys Res Commun. 1978 Mar 15;81(1):237–242. doi: 10.1016/0006-291x(78)91655-8. [DOI] [PubMed] [Google Scholar]
- Jolley R. L., Jr, Evans L. H., Makino N., Mason H. S. Oxytyrosinase. J Biol Chem. 1974 Jan 25;249(2):335–345. [PubMed] [Google Scholar]
- Ke C. H., Schubert J., Lin C. I., Li N. C. Nuclear magnetic resonance study of the binding of glycine derivatives to hemocyanin. J Am Chem Soc. 1973 May 16;95(10):3375–3379. doi: 10.1021/ja00791a049. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Gould D. C., Ehrenberg A., Loehr J. S., Mason H. S. Magnetic properties of Cancer magister hemocyanin. Biochemistry. 1973 Jun 19;12(13):2444–2449. doi: 10.1021/bi00737a012. [DOI] [PubMed] [Google Scholar]
- Palmer G., Babcock G. T., Vickery L. E. A model for cytochrome oxidase. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2206–2210. doi: 10.1073/pnas.73.7.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhammar B. Purification and properties of laccase and stellacyanin from Rhus vernicifera. Biochim Biophys Acta. 1970 Apr 7;205(1):35–47. doi: 10.1016/0005-2728(70)90059-9. [DOI] [PubMed] [Google Scholar]
- Solomon E. I., Dooley D. M., Wang R. H., Gray H. B., Credonio M., Mogno F., Romani G. L. Letter: Susceptibility studies of laccase and oxyhemocyanin using an ultrasensitive magnetometer. Antiferromagnetic behavior of the type 3 copper in Rhus laccase. J Am Chem Soc. 1976 Feb 18;98(4):1029–1031. doi: 10.1021/ja00420a035. [DOI] [PubMed] [Google Scholar]
- Sullivan B., Bonaventura J., Bonaventura C. Functional differences in the multiple hemocyanins of the horseshoe crab, Limulus polyphemus L. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2558–2562. doi: 10.1073/pnas.71.6.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamann T. J., Loehr J. S., Loehr T. M. Resonance Raman study of oxyhemocyain with unsymmetrically labeled oxygen. J Am Chem Soc. 1977 Jun 8;99(12):4187–4189. doi: 10.1021/ja00454a063. [DOI] [PubMed] [Google Scholar]


