Abstract
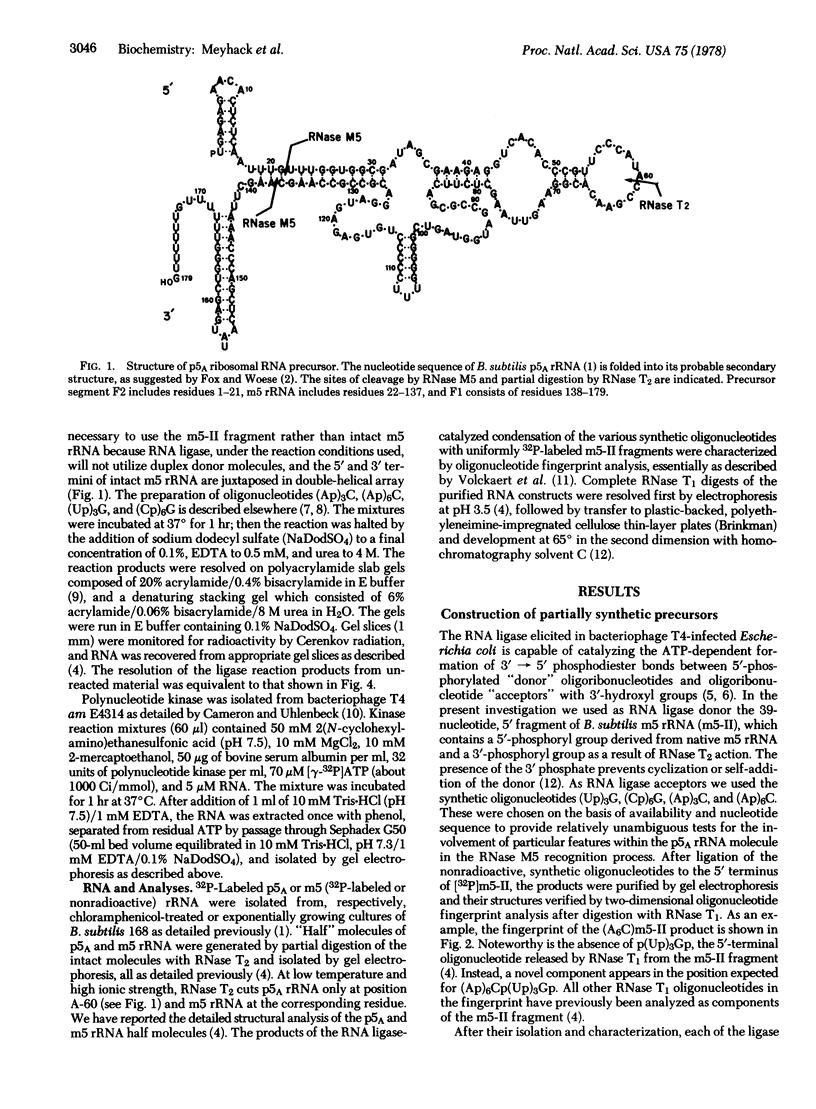
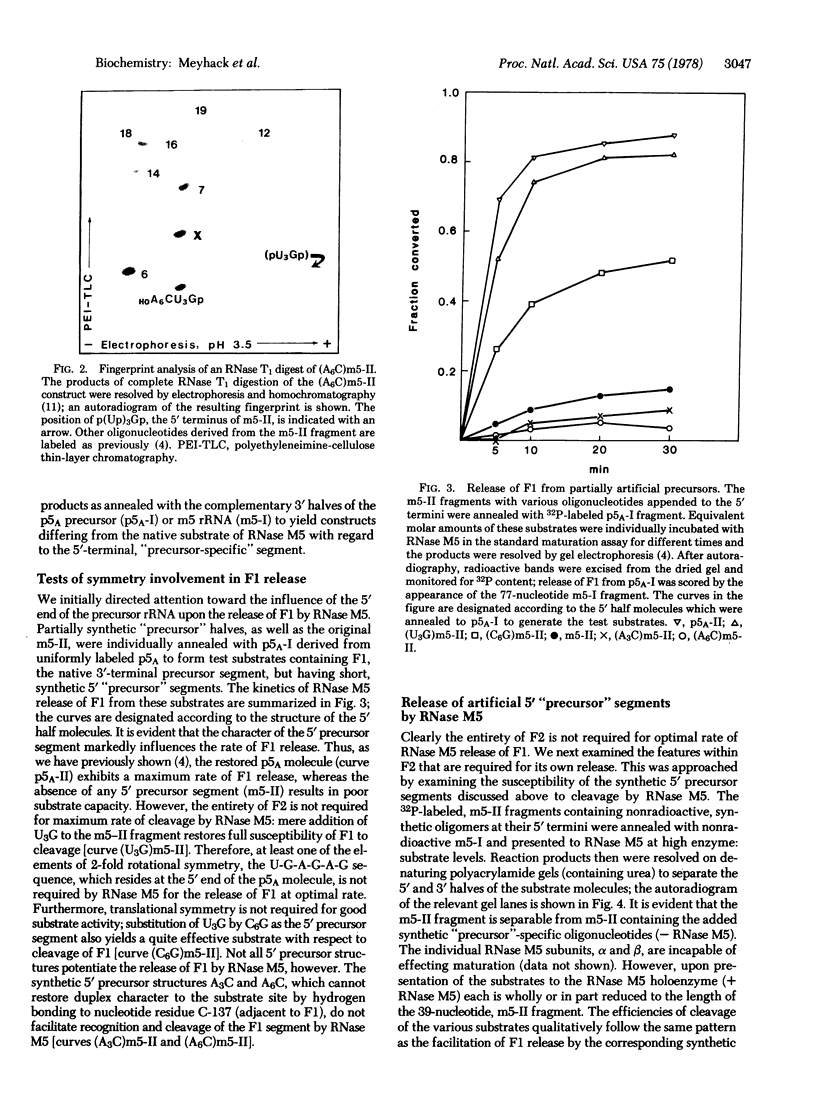
RNase M5 of Bacillus subtilis specifically cleaves a 179-nucleotide precursor 5S rRNA to yield mature 5S rRNA (116 nucleotides) and two fragments derived from the termini. Possible recognition elements for RNase M5 within the precursor structure include nucleotide sequences arranged with 2-fold rotational and translational symmetry about the substrate bonds. We have used bacteriophage T4 RNA ligase to construct, from synthetic oligonucleotides and mature or precursor 5S rRNA fragments, test substrates lacking these symmetry elements. The susceptibilities of the artificial substrates to RNase M5 demonstrate that the symmetrically arranged sequences are not used in the RNase M5 interaction with the precursor. Additionally, the synthetic protocols permitted the invention of an acid-soluble assay for RNase M5 and, potentially, other specific endoribonucleases.
Keywords: ribosome synthesis, Bacillus subtilis, nucleotide sequence symmetry
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron V., Uhlenbeck O. C. 3'-Phosphatase activity in T4 polynucleotide kinase. Biochemistry. 1977 Nov 15;16(23):5120–5126. doi: 10.1021/bi00642a027. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyhack B., Pace B., Pace N. R. Involvement of precursor-specific segments in the in vitro maturation of Bacillus subtilis precursor 5S ribosomal RNA. Biochemistry. 1977 Nov 15;16(23):5009–5015. doi: 10.1021/bi00642a011. [DOI] [PubMed] [Google Scholar]
- Silber R., Malathi V. G., Hurwitz J. Purification and properties of bacteriophage T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3009–3013. doi: 10.1073/pnas.69.10.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Pace B., Pace N. R. Partial purification and properties of a ribosomal RNA maturation endonuclease from Bacillus subtilis. J Biol Chem. 1977 Feb 25;252(4):1350–1357. [PubMed] [Google Scholar]
- Sogin M. L., Pace N. R. Nucleotide sequence of 5 S ribosomal RNA precursor from Bacillus subtilis. J Biol Chem. 1976 Jun 10;251(11):3480–3488. [PubMed] [Google Scholar]
- Uhlenbeck O. C., Cameron V. Equimolar addition of oligoribonucleotides with T4 RNA ligase. Nucleic Acids Res. 1977 Jan;4(1):85–98. doi: 10.1093/nar/4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- Walker G. C., Uhlenbeck O. C., Bedows E., Gumport R. I. T4-induced RNA ligase joins single-stranded oligoribonucleotides. Proc Natl Acad Sci U S A. 1975 Jan;72(1):122–126. doi: 10.1073/pnas.72.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]