Abstract
Context:
Hyperhomocysteinemia has been associated with psychiatric diseases in non-Indian populations.
Objectives:
We aimed to determine if total plasma Homocysteine (Hcys) is associated with schizophrenia or depression in South Indian Tamil patients and if so, to correlate their severity and phenomenology to Hcys levels.
Settings and Design:
40 patients each with schizophrenia and depression and 40 healthy controls were recruited from the psychiatry department of a quaternary referral centre. Association between Hcys and psychiatric disorders was determined using a Case- control design. Hcys levels were correlated with age, gender and severity and duration of the disease by appropriate statistical methods using SPSS17.
Materials and Methods:
Schizophrenia and depression were defined using ICD10 DCR version. Severity of depression was assessed by Hamilton Depression Rating Scale and that of schizophrenia using Positive and Negative Schizophrenia scales (PANSS). Hcys levels were determined using automated chemiluminiscence immunoassay (74-76).
Statistical Analysis:
Differences between the mean values of plasma homocysteine levels among schizophrenia, depression and control groups were compared using analysis of variants. The association between the severity and duration of schizophrenia and depression and the plasma homocysteine levels were determine using Pearson correlation.
Conclusions:
In Tamilian population, schizophrenia and depression are associated with total plasma Hcys levels which correlated with the duration and severity of psychosis.
Keywords: Depression, plasma homocysteine, schizophrenia
INTRODUCTION
Schizophrenia and depression are leading causes of the morbidity and mortality all over the world. In developing countries like India, they affect large number of people especially adolescents and young adults who form the backbone of the society. According to WHO report on Global Burden of Disease in 2004, unipolar depression is the leading cause of years lost due to disability (21.7 YLD) affecting 65.3 million, while schizophrenia accounted for 16.3 YLD.[1] In India, the national prevalence rate for depression was 0.5-53/1000 population with a median of 31.2/1000.[2] Investigations in both these disorders have revealed functional disturbances in the neurons of central nervous system though the morphological abnormalities were minimal or insignificant.
Since the discovery of the neurotransmitters, it had been postulated that the alteration in tissue levels of several of them could cause abnormal psychomotor activity. Simple amino acids which form the building blocks or metabolic regulators of neurotransmitters have been under intensive study because genetic alterations in enzymes which regulate the metabolism of these aminoacids can in turn affect the tissue concentration and functional effectiveness of neurotransmitters too.[3] Extraneous factors like vitamin intake and lifestyle factors such as smoking and alcohol use also alter the level of the amino acids involved in the metabolism of the neurotransmitters. Hcys, a nonessential amino acid, is one of those important molecules under intense evaluation and has been proven to be a riskfactor for the development of atherosclerosis with substantial evidence.[4] Hyperhomocysteinemia was also associated with other nervous system disorders, first documented in patients with severe cystathionine β synthase deficiency, causing homocystinuria characterized by mental retardation, cerebral atrophy, and seizures.[5] Since then, several studies had been carried out to elucidate its role in the neurological and psychiatric disorders.
Hcys is directly toxic to neurons and blood vessels by inducing DNA strand breakage, oxidative stress, and apoptosis. Concentrations of Hcys above 11.9 μmol/L were associated with approximately three-fold higher risk for white matter damage when compared to concentrations below 8.6 μmol/L.[6] Hcys has multiple dose-dependent effects on brain function, including interaction with N-methyl-D- aspartate (NMDA) receptors. At high concentrations, Hcys may activate the NMDA receptor glutamate site, leading to increased susceptibility of neurons to excitotoxicity. However, at lower concentrations Hcys also has the ability to act as a competitive antagonist at the NMDA receptor co agonist glycine site. Lipton et al.,[7] showed that Hcys acts as a partial antagonist at the glycine site of the NMDA receptor and therefore inhibits NMDA receptor-mediated activity in the presence of normal concentrations of glycine. It has been postulated that this could be a molecular mechanism for the cognitive changes seen in the neuropsychiatric disorders in general.
Since many Asian and Indian populations have consanguinity as a common cultural practice and are also likely to differ in their genetic and epigenetic profile from these populations, there is a need to investigate any such important associations of Homocysteine in Indian population.[8] Several clinical studies associated the role of Hcys to psychiatric disorders such as depression and schizophrenia.[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] However, other studies have questioned these reports.[27,28,29,30,31,32] These studies have all been on Caucasian and other races from abroad. In India, susceptibility to vitamin B deficiency, associated with vegetarianism as well as consanguinity-related predisposition to recessively inherited inborn errors of its metabolism can all potentially lead to elevation in plasma Hcys levels in the population.[8,33,34,35] This adds to the importance of studies on any relationship of hyperhomocysteinemia with psychiatric disorders in this population.
We aimed to determine if patients with depression or schizophrenia have higher level of plasma Hcys than age- and gender-matched control subjects and if so to correlate the total plasma Hcys level with the severity and phenomenology of these psychiatric disorders.
MATERIALS AND METHODS
Study was conducted in collaboration between the departments of psychiatry, neurology, pharmacology and biochemistry. Patients attending this quaternary care teaching institute mainly came from urban and suburban areas of Puducherry and the rural areas of the neighbouring districts of the Arcot region of Tamil Nadu. Subjects 18-45 years, presenting with symptoms suggestive of depression or psychosis who attend the psychiatry outpatient department were initially screened by a Psychiatry senior resident followed by detailed interview of patients and their family members to confirm the diagnosis of schizophrenia or depression based on International Classification of Diseases (ICD)-10 criteria. Among them drug naïve patients were further evaluated with ICD-10 DCR version[36] and all subjects with confirmed diagnosis who gave informed consent by themselves or from one of the close family members (spouse, if married or one of the parents) were recruited for this study and appropriate rating scales applied. The exclusion criteria were major comorbid medical illnesses often associated with hyperhomocysteinemia namely hypertension, diabetes, chronic renal, or hepatic failure, patients on medications which would alter plasma Hcys level and those with other psychiatric illnesses like substance abuse.
Patients diagnosed as having depression of moderate to severe degree were assessed by Hamilton Depression Rating Scale (HDRS).[37] Those diagnosed as having schizophrenia were assessed by Positive and Negative Syndrome Scale (PANSS).[38] Diagnoses and scores were confirmed by an independent observer (another senior resident or faculty in-charge of a particular patient) in the department of psychiatry. Age- and sex-matched control subjects were identified at random and 40 healthy volunteers were selected after getting informed consent.
Clinical and psychiatric evaluation
A semistructured performa comprising of sociodemographic factors like age, sex, residence, marital status, and other relevant information.
ICD-10 DCR: The Diagnostic Criteria for Research accompanying the ICD-10 (DCR-10) translated and administered in the local language (Tamil). The criteria were 1. The depressive episode should last for at least 2 weeks. 2. There have been no hypomanic or manic symptoms sufficient to meet the criteria for hypomanic or manic episode at any time in the individual's life. 3. Most commonly used exclusion clause. The episode is not attributable to psychoactive substance use or to any organic mental disorder.
Hamilton Depression Rating Scale (HDRS): A four-point, 24-item questionnaire (whose strengths include excellent validation and research base and ease of administration. Interrater reliability for the total score ranged from 0.87 to 0.95.[37]
Positive and Negative Scale (PANSS): A 30-item rating scale developed to assess the individuals with schizophrenia and is used very widely in research settings. Consisting of a semistructured interview and available supporting clinical information. The assessment provides separate scores in nine clinical domains including a positive syndrome, a negative syndrome, depression, a composite index, and general psychopathology with ratings generally based upon the information relating to the past week. Alpha-coefficient for internal reliability and homogeneity ranged from 0.73 to 0.83 (P<0.001) for each of the scales. The split-half reliability of the General Psychopathology Scale was demonstrated to be 0.80 (P<0.001).[39]
Estimation of homocysteine
For all patients and control subjects, 5 mL of morning fasting blood was collected from the ante cubital vein in sterile EDTA vials. The blood sample was immediately centrifuged, plasma separated, and transferred to another fresh, clean vial, and stored in deep freezer at -40°C in the neurobiology division of the department of neurology. Estimation of total plasma Hcys was performedusing the chemiluminescence method using Seamen's Centaur autoanalyzer. This method had a detection limit <0.9 μmol/L and was linear for total plasma Hcys between 2.4 and 58.8 μmol/L. The within and between assay imprecision was <6% and <7%, respectively. The analytical recovery ranged from 93.5% to 109.7%. The normal range of plasma Hcys level is 5-15 μmol/L. Hyperhomocysteinemia was defined as a plasma Hcys level >15 μmol/L and was classified as moderate (15-30 μmol/L), intermediate (30-100 μmol/L), or severe (>100 μmol/L).[18]
Statistical analysis
Sample size was calculated for each category as per standard guidelines. Baseline variables were explored for normality of distribution using Shapiro-Wilk test. We calculated mean and range for continuous variables and frequency and proportions for categorical variables. Association of depression and schizophrenia for total plasma Hcys levels were analyzed by comparing the means of total plasma Hcys levels while in remission with that of age-matched controls. Further, the Hcys levels were correlated to the duration of the disease and the severity using Pearson's correlation.
RESULTS
Demographic profile
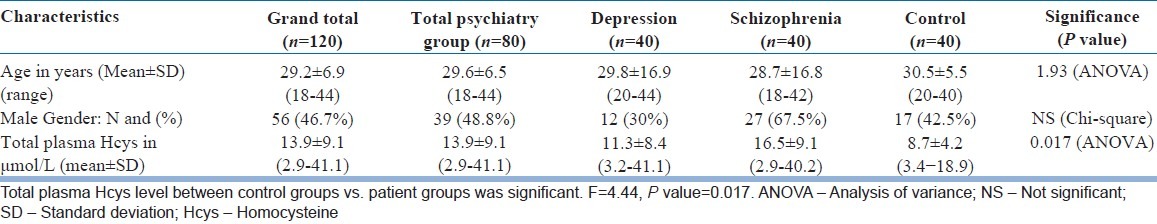
During a period of two years, well-defined consecutive cases, 40 each of depression, schizophrenia, and age-gender matched controls who fulfilled strict predefined inclusion and exclusion criteria were recruited into the study as outlined above. Baseline characteristics of the recruited subjects are shown in Table 1. Age and gender distributions were similar between the control group and the two psychiatric patient groups, though there was a male preponderance in the schizophrenia group (67.5%). Total plasma Hcys level showed a linear trend with increase in age in all three groups. However, the correlation between the Hcys levels and age was not significant in the control or schizophrenia groups but there was a significantly positive correlation in the depression group. The mean total plasma Hcys level was significantly higher in the psychiatry patients groups compared to the controls. (For men: P=0.017, F=4.44, For women: P=0.003, F=6.5).
Table 1.
Age, gender, and total plasma homocysteine values in patients and controls
Homocysteine versus schizophrenia
Significant differences in plasma Hcys levels existed for schizophrenia (P=0.002) and depression (P=0.043) groups compared to the controls among the men, but for women, such a difference was only for the schizophrenia group (P=0.001). There was also a linear increase in plasma Hcys level with increase in duration of schizophrenia (R=0.8; P<0.001) [Figure 1]. There was no significant difference in the Hcys values among the subtypes of schizophrenia. The mean and standard deviation for the paranoid (n=23), catatonic (n=5), and undifferentiated (n=11) groups were 16.5±9.5, 18.4±12, and 15.4±7.7 μmol/L respectively (P=0.8). The positive symptoms score (PANSS item P score) of schizophrenia was not significantly correlated with plasma Hcys level (R=0.007, P=0.9) [Figure 2]. However, the negative scores (N score) had a significant correlation with plasma Hcys levels (R=0.7; P<0.001) [Figure 3]. Plasma Hcys levels was linear with the general psychopathology scores in the schizophrenia group, though the correlation was not statistically significant.
Figure 1.
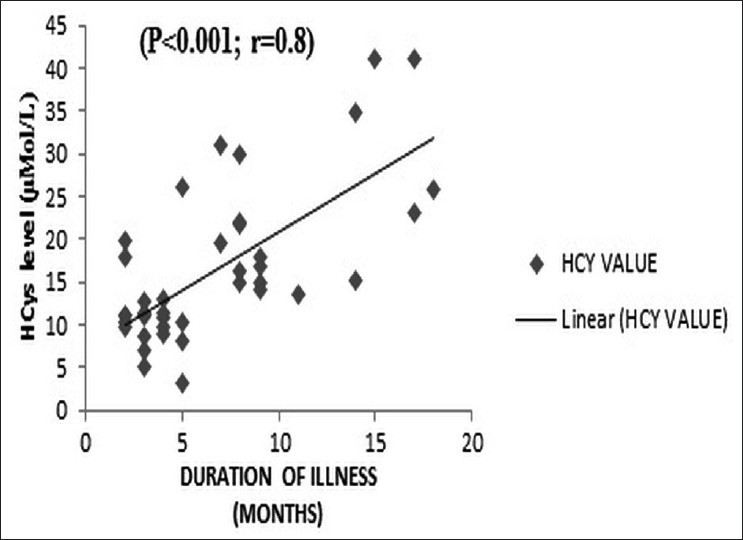
Correlation of total plasma Hcys with duration of schizophrenia. Thereis a linear increase in plasma Hcys level with increase in duration of schizophrenia
Figure 2.
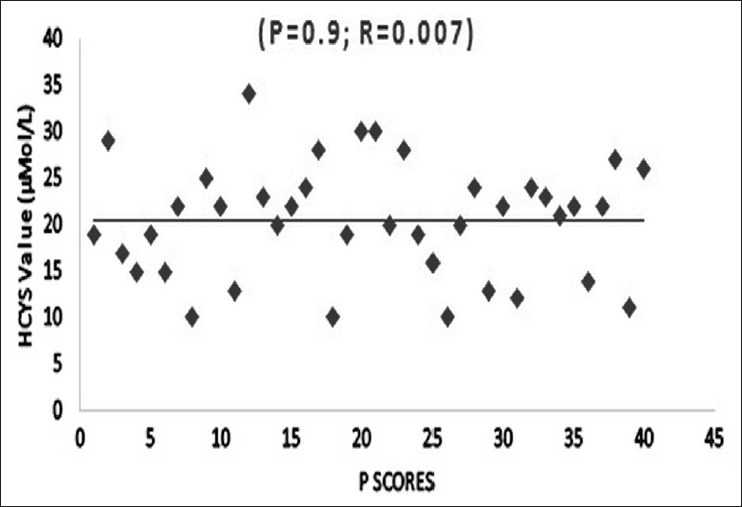
Correlation of total plasma Hcys with positive and negative syndrome scale scores. The correlation coefficient is 4.2(R2), P value is 0.9. The positive symptoms score is not significantly associated with plasma Hcys level
Figure 3.
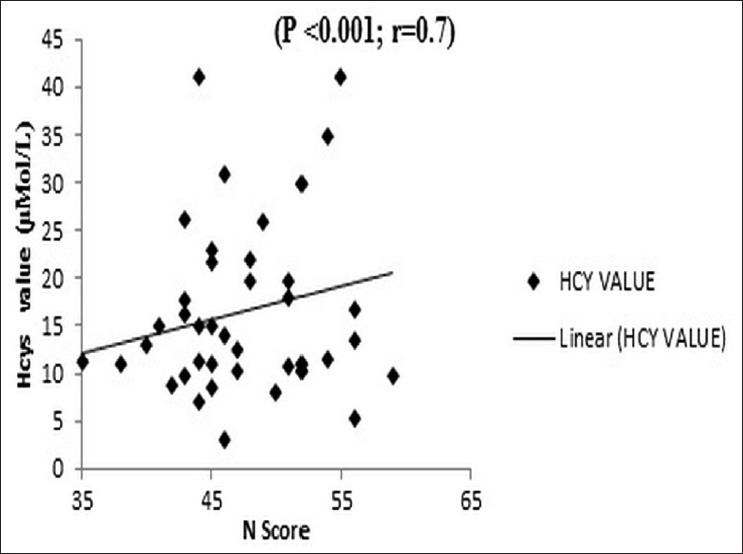
Correlation of total plasma Hcys with Positive and negative syndrome scale negative scores. The negative symptoms strongly correlate with the plasma Hcys levels. Correlation coefficient 0.7 and P<0.001
Homocysteine versus depression
The mean total plasma Hcys level was significantly higher in the depressed versus the control in men (P=0.04) but not in women (P=0.313). The plasma Hcys levels increased significantly with the Hamilton Depression Rating Score in a linear fashion (P<0.001) [Figure 4] and also with the duration of the depression (R=0.7; P<0.001) [Figure 5]. However, on subgroup analysis, the rise in Hcys levels and its linear trend with HDRS scores was confined to the severely depressed group (n=24, HDRS>24) (R=0.94; P<0.001) in contrast with the moderately depressed group (n=16, HDRS<25) (R=0.35; P=0.2).
Figure 4.
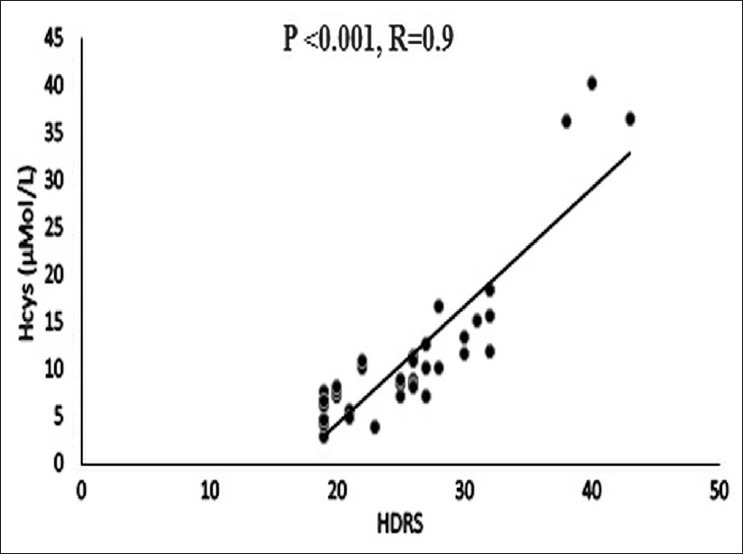
Correlation of total plasma Hcys with severity of depression HDRS (Hamilton Depression Rating Scale scores). There is a significant association between HDRS score and plasma Hcys level
Figure 5.
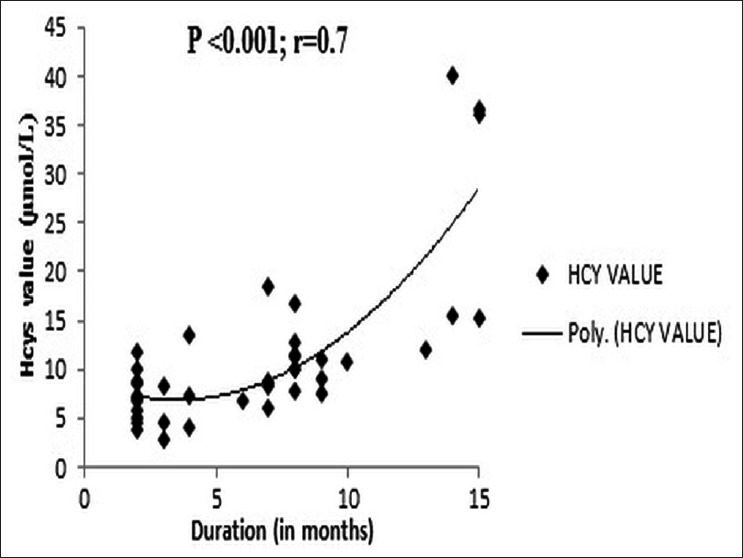
Correlation of total plasma Hcys with duration of depression. The duration of depression positively correlates with the plasma Hcys levels. The correlation coefficient is 0.7 and P<0.001
DISCUSSION
Homocysteineis implicated in the etiopathogenesis of psychiatric disorders and various mechanisms were postulated. We are still unclear how these proposed mechanisms acting alone or in concert contribute to the development of disorders of brain, but recent studies started to unravel the mystery at the molecular level.[40] In brief, Hcys is postulated to cause damage to the neurons and cause psychiatric disturbances: By direct neuronal toxicity, acting through specific receptors on the neurons thereby inducing cell death, damaging the cerebral vasculature especially the endothelium of small vessels. This may induce neuronal damage or death indirectly and also alter the central neurotransmitters which are involved in the basic functions of the brain, and thus altering the normal functioning of the mind of an individual.
Plasma Hcys levels has been investigated in depression fairly widely from over the western hemisphere. Hordoland Hcys study,[9] King's College study,[41] Rotterdam study,[10] and Kuopio Ischaemic Heart Disease risk factor study[11] had all found association between Hcys and depression in cross-sectional case-control studies without age limits. When Sachdev et al.,[42] (University of New South Wales, Sydney, Chen et al., (Taiwan),[12] Hin et al., London[43] and Almeida et al., Western Australia[44] carried out similar studies in the elderly population, the results were similar and logistic regression studies pointed against a vascular factor in the Sydney study, while Reif et al., Germany[13] showed in a study exclusively among women that depression among other psychiatric disorders was specifically associated with hyperhomocysteinemia. Fraguas et al.,[14] showed association of Hcys levels with the length of depressive episode. Severity of depression in multiple sclerosis also was reported to have association with multiple sclerosis. However, few cohorts have looked at association of Hcys with depression and in one such cohort study in Korean elderly community dwellers, Kim et al.,[45] found association of Hcys with depression at baseline and incident depression was more frequent in people with a relative increase in Hcys on follow-up. Thus, despite a few negative reports such as from Fava et al.,[28] Penning et al.,[29] and Morris et al.,[27] the overwhelming evidence from the Caucasian and two oriental populations has been in support of an association between total plasma Hcys levels and depression. Our data bred true for the expected gender predilections for depression (female 70%). Despite an obvious trend, the difference between total plasma Hcys levels between controls and depression was not significant for the whole group. Nevertheless our study, for the first time, demonstrated unequivocal association between total plasma Hcys and depression in ethnic Tamil male population from South India and a lack of any similar association for the more predisposed female gender. Thus, the majority of the depressed patients, who belong to the moderately depressed female groups, failed to show any association with the severity of depression but our study did show a correlation between severity of depression (HDRS scores) and Hcys levels in the severely depressed patients.
In schizophrenia, Levine et al.,[17] found that only in men younger than 50 years of age, the association with Hcys was significant. Applebaum et al.,[19] Nevo et al.,[46] Haidemenos et al.,[23] andAkanji et al.,[24] also found such an association is mainly in young males in Caucasian populations. Though Goff et al.,[20] too found that association with schizophrenia, and further a correlation with severity of extrapyramidal symptoms, this study had serious flaws in study design such as poor age matching and non-uniformity in Hcys assays. Kim and Moon,[47] Lee et al.,[21] and Feng et al.,[26] also reported this association irrespective of age, in oriental populations. Petronijevi et al.,[25] noted that the Hcys levels rise in schizophrenia subjects during exacerbations in both the groups of patients, those with predominantly positive or negative syndrome. But the Hcys levels correlated only with the negative PANSS scores and not with positive scores. Our study too interestingly showed correlation for Hcys levels with the PANSS severity scores for negative symptoms but not for positive symptoms of schizophrenia and with the general psychopathology scores. Since the negative symptoms are associated with a poor outcome, the effects of Hcys lowering interventions in these patients assume significance. In the global scenario, however, there were negative studies as well, such as those reported by Susser et al.,[48] Virgoset al.,[30] Muntjewerff et al.,[31] and Viella (2005). A robust evidence was provided through a metaanalysis later by Muntjewerff et al.,[16] which concluded that a 5 μmole/L higher Hcys level was associated a 70% higher risk of schizophrenia. Levine et al.,[22] added a final concrete evidence when a randomized double-bind placebo-controlled, crossover study on 42 Caucasian schizophrenia patients showed that both positive and negative symptoms declined significantly with active treatment with B6, B12, and folate vitamins compared with placebo. However, no Indian populations had been proven to have this association. Our study, for the first time, showed a significant association of Hcys with schizophrenia among both genders in South Indian Tamil population conforming to results from Caucasian data and also its association with negative PANSS scores. In this population, there were no significant differences in the Hcys levels between the modestly sized subtype groups of schizophrenia.
Among studies investigating plasma Hcys levels in psychiatric disorders, most were on people of older age group. The age range of subjects who participated in our study was 18-44 with mean ages of overall 29.4, controls 30.4, depression 29.8, and schizophrenia 28.7. Among the schizophrenics, there was no correlation between age and Hcys levels, but among the depressed, the Hcys levels correlated with the age. Since age is an important risk factor for elevated plasma Hcys values, our study provided added value in investigating the association of plasma Hcys with psychiatric diseases. Exclusion of comorbid medical conditions and confounding effects of obesity, renal failure, smoking and use of alcohol, and relevant medications which would affect the plasma Hcys levels from patients and controls in this young population of subjects further increased the strength of the study.[49]
Since methionine is the precursor of S-adenosylmethionine (SAM), which is the immediate methyl donor in many reactions involved in the synthesis of monoaminergeric neurotransmitters, deranged metabolism of Hcys might cause alteration in the levels of monoamine neurotransmitters. Bottiglieri and Hyland[50] reported the favorable effect of SAM on mood. It had been shown to be of having an augmenting value in depressed patients who were not improved with conventional antidepressants alone.
It has been suggested that a defect in methylation processes is central to the neuropsychiatric manifestations due to the deficiency of vitamins B6, B12, and folate. All these vitamins are closely connected with Hcys metabolism such that total plasma Hcys level is considered to be a sensitive marker of the functional deficiency of these vitamins.[16]
Homocysteine is involved in one-carbon metabolism, which is intricately related to the metabolism of monoamine neurotransmitters [Figure 1]. Hcys is a sulphur-containing, nonessential amino acid, derived from the essential amino acid methionine through demethylation. Hcys is remethylated to methionine via the 5-methyltetrahydrofolate pathway and this reaction is mediated by methionine synthase and vitamin B12 acts as a cofactor in this reaction. Methyl group is donated by 5-methyltetrahydrofolate (a derivative of folic acid) through 5-methyltetrahydrofolate reductase (MTHFR) enzyme. Hcys can be condensed with serine to form cystathionine in a reaction that uses vitamin B6. Methionine is subsequently metabolized into SAM. This metabolite is involved in numerous methylation reactions, involving proteins, phospholipids, DNA, and neurotransmitter metabolism. So it is not surprising to suspect the role of Hcys in the neurological and psychiatric disorders, where the central neurotransmitters are known to be altered. Alteration in the level of Hcys, either because of genetic abnormalities or due to the acquired/environmental influences like low intake of vitamins involved in the metabolism, would theoretically correlate with the neurochemical abnormalities seen in the various neuropsychiatric disorders. Current evidence suggests that Hcys can be imported from the plasma into the brain and vice versa, probably via specific, bidirectional cellular transporters.[51,52]
Furthermore, Hcys itself influences global and gene promoter-specific DNA methylation. Acute Hcys treatment had been shown to cause misregulation of different gene-specific promoters and changes of corresponding mRNA levels. These epigenetic alterations may directly influence monoaminergic neurotransmission by modifying promoter methylation of candidate genes such as COMT and 5-HTTLPR. It has been suggested that these molecular mechanisms play a substantial role in the pathogenesis of different psychiatric disorders associated with hyperhomocysteinemia including schizophrenia.[53]
Merits and demerits of the study
This is the first ever study to investigate the association between Hcys and psychiatric disorders in India. Only other studies from Asia have been from China, Korea, and Arab countries. The age group selected was much younger compared to similar other studies, which could alleviate other confounding factors seen in older age such as hypertension, renal disease, osteoarthritis, and so on, which are associated with hyperhomocysteinemia. The use of well-validated instruments for diagnosis such as ICD 10-DCR, HDRS, and PANSS is another important strength of this study. Hcys estimation method was automated and the specimen collected was processed with separation of plasma which ensured high quality of data, which added to the precision in estimation of the biological factor studied.
The sample size is modest though statistically robust. This case-control study can at best only prove an association between Hcys and psychiatric disease and no causative relationship, which would be possible only through a cohort study. We did not study the plasma levels of vitamin B12 and folate levels which would have given further insight into this interesting association since B12 band folate are well-known to affect Hcys levels as well as mental status. Genetic studies such as MTHFR genotypes in the same groups were not done; this would have been another robust method to investigate into such associations between Hcys and psychiatric disorders.
CONCLUSION
In a South Indian Tamilian population, we estimated total plasma Hcys using a high precision automated chemiluminescence technique, in schizophrenia and depression patients of the typical age groups as well as in age-matched controls using strict inclusion and exclusion criteria. We found significant association of total plasma Hcys levels with schizophrenia and younger women with depression and further specifically with duration of psychosis, severity of depression and negative symptom scales of schizophrenia. This interesting association now needs to be tested on longitudinal cohort studies to probe into whether this association is causative and how strong a biomarker it indeed is.
ACKNOWLEDGMENT
We acknowledge the academic section, JIPMER for the intramural grants toward the purchase of homocysteine kits, Mr. Venkatraman, technician, Department of biochemistry and Mr. Vadivelan, technician, Neurobiology lab for the Hcys chemiluminescence immunoassays. We acknowledge Dr. Nandisha, Assistant professor of Biochemistry in supervising the biochemical tests.
Footnotes
Source of Support: JIPMER Intramural Research Grant
Conflict of Interest: None declared
REFERENCES
- 1.Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–90. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 2.Madhav SM. Epidemiological study of prevalence of mental disorders in India. Indian J Commu Med. 2001;26:192–200. [Google Scholar]
- 3.Derkach KV, Shpakov AO, Uspenskaia ZI, Iudin AL. The study of molecular mechanisms of action of natural amino acids and serotonin on adenylyl and guanylyl cyclases of the ciliates. Tsitologiia. 2012;54:270–7. [PubMed] [Google Scholar]
- 4.McCully KS, Wilson RB. Homocysteine theory of arteriosclerosis. Atherosclerosis. 1975;22:215–27. doi: 10.1016/0021-9150(75)90004-0. [DOI] [PubMed] [Google Scholar]
- 5.Abbott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine beta-synthase deficiency: Prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet. 1987;26:959–69. doi: 10.1002/ajmg.1320260427. [DOI] [PubMed] [Google Scholar]
- 6.Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–62. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1997;94:5923–8. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinchurkar MS, Sathye SM, Dikshit M. Retinitis pigmentosa genetics: A study in Indian population. Indian J Ophthalmol. 1996;44:77–82. [PubMed] [Google Scholar]
- 9.Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: The Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–26. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- 10.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: The Rotterdam Study. Am J Psychiatry. 2002;159:2099–101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 11.Tolmunen T, Hintikka J, Voutilainen S, Ruusunen A, Alfthan G, Nyyssonen K, et al. Association between depressive symptoms and serum concentrations of homocysteine in men: A population study. Am J Clin Nutr. 2004;80:1574–8. doi: 10.1093/ajcn/80.6.1574. [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Tsai JC, Tsang HY, Kuo YT, Lin HF, Chiang IC, et al. Homocysteine levels, MTHFR C677T genotype, and MRI Hyperintensities in late-onset major depressive disorder. Am J Geriatr Psychiatry. 2005;13:869–75. doi: 10.1176/appi.ajgp.13.10.869. [DOI] [PubMed] [Google Scholar]
- 13.Reif A, Pfuhlmann B, Lesch KP. Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1162–8. doi: 10.1016/j.pnpbp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 14.FraguasR R, Jr, Papakostas GI, Mischoulon D, Bottiglieri T, Alpert J, Fava M. Anger attacks in major depressive disorder and serum levels of homocysteine. Biol Psychiatry. 2006;60:270–4. doi: 10.1016/j.biopsych.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–74. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- 16.Muntjewerff JW, Kahn RS, Blom HJ, den Heijer M. Homocysteine, methylenetetrahydrofolate reductase and risk of schizophrenia: A meta-analysis. Mol Psychiatry. 2006;11:143–9. doi: 10.1038/sj.mp.4001746. [DOI] [PubMed] [Google Scholar]
- 17.Levine J, Stahl Z, Sela BA, Gavendo S, Ruderman V, Belmaker RH. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159:1790–2. doi: 10.1176/appi.ajp.159.10.1790. [DOI] [PubMed] [Google Scholar]
- 18.de Haan L, van Amelsvoort T, Linszen DH. Elevated homocysteine levels in schizophrenia. Am J Psychiatry. 2004;161:1131–2. doi: 10.1176/appi.ajp.161.6.1131-a. [DOI] [PubMed] [Google Scholar]
- 19.Applebaum J, Shimon H, Sela BA, Belmaker RH, Levine J. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;38:413–6. doi: 10.1016/j.jpsychires.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Goff DC, Bottiglieri T, Arning E, Shih V, Freudenreich O, Evins AE, et al. Folate, homocysteine, and negative symptoms in schizophrenia. Am J Psychiatry. 2004;161:1705–8. doi: 10.1176/appi.ajp.161.9.1705. [DOI] [PubMed] [Google Scholar]
- 21.Lee YS, Han DH, Jeon CM, Lyoo IK, Na C, Chae SL, et al. Serum homocysteine, folate level and methylenetetrahydrofolate reductase 677, 1298 gene polymorphism in Korean schizophrenic patients. Neuroreport. 2006;17:743–6. doi: 10.1097/01.wnr.0000215777.99473.52. [DOI] [PubMed] [Google Scholar]
- 22.Levine J, Stahl Z, Sela BA, Ruderman V, Shumaico O, Babushkin I, et al. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60:265–9. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Haidemenos A, Kontis D, Gazi A, Kallai E, Allin M, Lucia B. Plasma homocysteine, folate and B12 in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1289–96. doi: 10.1016/j.pnpbp.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Akanji AO, Ohaeri JU, Al-Shammri SA, Fatania HR. Associations of blood homocysteine concentrations in Arab schizophrenic patients. Clin Biochem. 2007;40:1026–31. doi: 10.1016/j.clinbiochem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Petronijevic ND, Radonjic NV, Ivkovic MD, Marinkovic D, Piperski VD, Duricic BM, et al. Plasma homocysteine levels in young male patients in the exacerbation and remission phase of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1921–6. doi: 10.1016/j.pnpbp.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Feng LG, Song ZW, Xin F, Hu J. Association of plasma homocysteine and methylenetetrahydrofolate reductase C677T gene variant with schizophrenia: A Chinese Han population-based case-control study. Psychiatry Res. 2009;168:205–8. doi: 10.1016/j.psychres.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003;72:80–7. doi: 10.1159/000068692. [DOI] [PubMed] [Google Scholar]
- 28.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–8. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 29.Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B (12) deficiency and depression in physically disabled older women: Epidemiologic evidence from the Women›s Health and Aging Study. Am J Psychiatry. 2000;157:715–21. doi: 10.1176/appi.ajp.157.5.715. [DOI] [PubMed] [Google Scholar]
- 30.Virgos C, Martorell L, Simo JM, Valero J, Figuera L, Joven J, et al. Plasma homocysteine and the methylenetetrahydrofolate reductase C677T gene variant: Lack of association with schizophrenia. Neuroreport. 1999;10:2035–8. doi: 10.1097/00001756-199907130-00008. [DOI] [PubMed] [Google Scholar]
- 31.Muntjewerff JW, van der Put N, Eskes T, Ellenbroek B, Steegers E, Blom H, et al. Homocysteine metabolism and B-vitamins in schizophrenic patients: Low plasma folate as a possible independent risk factor for schizophrenia. Psychiatry Res. 2003;121:1–9. doi: 10.1016/s0165-1781(03)00200-2. [DOI] [PubMed] [Google Scholar]
- 32.Vilella E, Virgos C, Murphy M, Martorell L, Valero J, Simo JM, et al. Further evidence that hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T and A1289C polymorphisms are not risk factors for schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1169–74. doi: 10.1016/j.pnpbp.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Bar-Sella P, Rakover Y, Ratner D. Vitamin B12 and folate levels in long-term vegans. Isr J Med Sci. 1990;26:309–12. [PubMed] [Google Scholar]
- 34.Dastur DK, Quadros EV, Wadia NH, Desai MM, Bharucha EP. Effect of vegetarianism and smoking on vitamin B12, thiocyanate, and folate levels in the blood of normal subjects. Br Med J. 1972;3:260–3. doi: 10.1136/bmj.3.5821.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasool S, Abid S, Iqbal MP, Mehboobali N, Haider G, Jafri W. Relationship between vitamin B12, folate and homocysteine levels and H. pylori infection in patients with functional dyspepsia: A cross-section study. BMC Res Notes. 2012;5:206. doi: 10.1186/1756-0500-5-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sartorius N. Classification of mental disorders according to ICD 10. Encephale. 1995;21(Spec No 5):9–13. [PubMed] [Google Scholar]
- 37.Knesevich JW, Biggs JT, Clayton PJ, Ziegler VE. Validity of the hamilton rating scale for depression. Br J Psychiatry. 1977;131:49–52. doi: 10.1192/bjp.131.1.49. [DOI] [PubMed] [Google Scholar]
- 38.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 39.Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23:99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 40.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 41.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228–32. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–38. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- 43.Hin H, Clarke R, Sherliker P, Atoyebi W, Emmens K, Birks J, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: The Banbury B12 study. Age Ageing. 2006;35:416–22. doi: 10.1093/ageing/afl033. [DOI] [PubMed] [Google Scholar]
- 44.Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–94. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- 45.Kim JM, Kim SW, Shin IS, Yang SJ, Park WY, Kim SJ, et al. Folate, vitamin B (12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig. 2008;5:36–40. doi: 10.4306/pi.2008.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adler Nevo G, Meged S, Sela BA, Hanoch-Levi A, Hershko R, Weizman A. Homocysteine levels in adolescent schizophrenia patients. Eur Neuropsychopharmacol. 2006;16:588–91. doi: 10.1016/j.euroneuro.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Kim TH, Moon SW. Serum homocysteine and folate levels in korean schizophrenic patients. Psychiatry Investig. 2011;8:134–40. doi: 10.4306/pi.2011.8.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Susser E, Brown AS, Klonowski E, Allen RH, Lindenbaum J. Schizophrenia and impaired homocysteine metabolism: A possible association. Biol Psychiatry. 1998;44:141–3. doi: 10.1016/s0006-3223(97)00427-7. [DOI] [PubMed] [Google Scholar]
- 49.Regland B. Schizophrenia and single-carbon metabolism. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1124–32. doi: 10.1016/j.pnpbp.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 50.Bottiglieri T, Hyland K. S-adenosyl methionine levels in psychiatric and neurological disorders: A review. Acta Neurol Scand Suppl. 1994;154:19–26. doi: 10.1111/j.1600-0404.1994.tb05405.x. [DOI] [PubMed] [Google Scholar]
- 51.Obeid R, McCaddon A, Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med. 2007;45:1590–606. doi: 10.1515/CCLM.2007.356. [DOI] [PubMed] [Google Scholar]
- 52.Remington G, Kapur S, Foussias G, Agid O, Mann S, Borlido C, et al. Tetrabenazine augmentation in treatment-resistant schizophrenia: A 12-week, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2012;32:95–9. doi: 10.1097/JCP.0b013e31823f913e. [DOI] [PubMed] [Google Scholar]
- 53.Hillemacher T, Frieling H, Muschler MA, Bleich S. Homocysteine and epigenetic DNA methylation: A biological model for depression? Am J Psychiatry. 2007;164:1610. doi: 10.1176/appi.ajp.2007.07060881. [DOI] [PubMed] [Google Scholar]


