Abstract
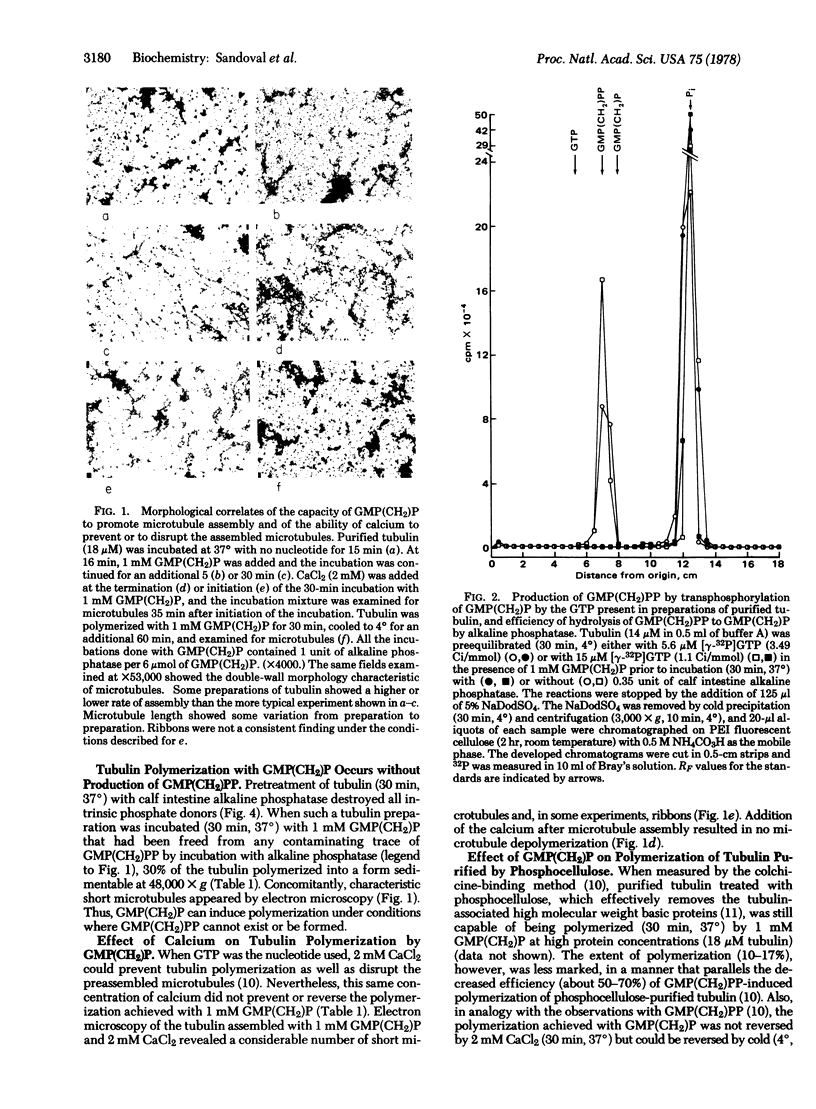
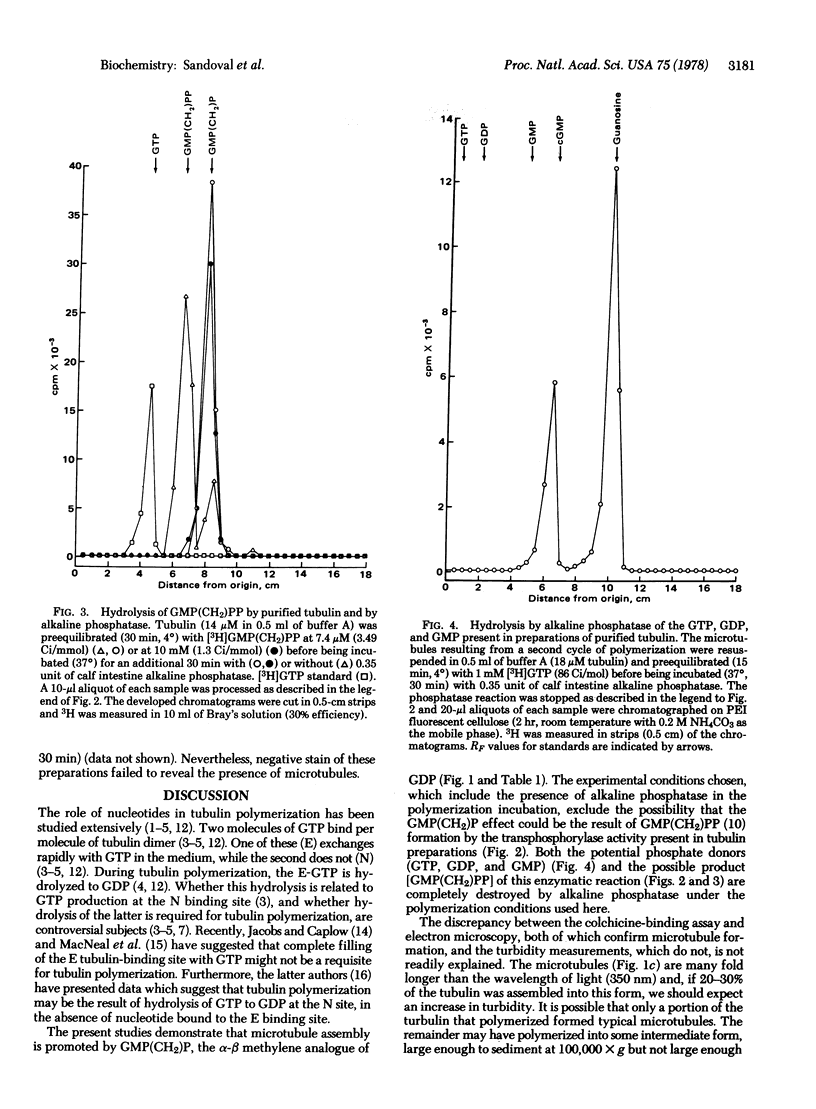
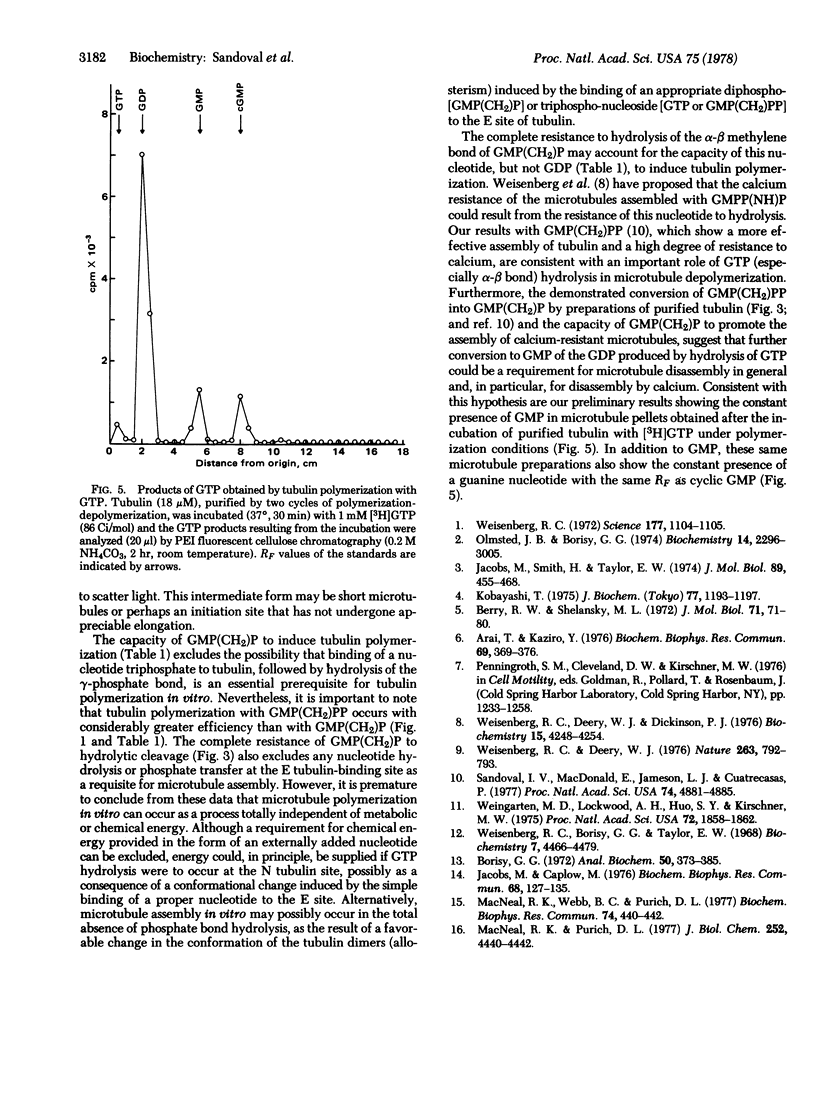
Incubation of purified rat brain tubulin with guanosine 5'-methylene diphosphonate [GMP(CH2)P] (1 mM), a GDP analog resistant to hydrolysis, results in the polymerization of 20-30% of the total tubulin present. Analogous incubations with GDP (1 mM) do not result in tubulin polymerization. Polymerization with GMP(CH2)P occurs in the presence of alkaline phosphatase (EC 3.1.3.1) under conditions that completely hydrolyze the likely phosphate donors (GTP, GDP, and GMP) as well as the potential product [GMP(CH2)PP] of the transphosphorylase activity present in purified tubulin preparations. Tubulin polymerization in vitro thus can occur in the absence of gamma-phosphate and phosphate bond hydrolysis at the exchangeable nucleotide-binding site of tubulin. Polymerization of tubulin by GMP(CH2)P is neither prevented nor reversed by concentrations of calcium (2 mM) that prevent microtubule assembly and disrupt already formed microtubules induced by GTP. However, tubulin polymerized with GMP(CH2)P is readily depolymerized by cold (4 degrees, 30 min). The possible involvement of GTP alpha-beta bond hydrolysis must be considered seriously as playing a role in the process of microtubule depolymerization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kaziro Y. Effect of guanine nucleotides on the assembly of brain microtubles: ability of 5'-guanylyl imidodiphosphate to replace GTB in promoting the polymerization of microtubules in vitro. Biochem Biophys Res Commun. 1976 Mar 22;69(2):369–376. doi: 10.1016/0006-291x(76)90531-3. [DOI] [PubMed] [Google Scholar]
- Berry R. W., Shelanski M. L. Interactions of tubulin with vinblastine and guanosine triphosphate. J Mol Biol. 1972 Oct 28;71(1):71–80. doi: 10.1016/0022-2836(72)90401-9. [DOI] [PubMed] [Google Scholar]
- Borisy G. G. A rapid method for quantitative determination of microtubule protein using DEAE-cellulose filters. Anal Biochem. 1972 Dec;50(2):373–385. doi: 10.1016/0003-2697(72)90046-2. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Caplow M. Microtubular protein reaction with nucleotides. Biochem Biophys Res Commun. 1976 Jan 12;68(1):127–135. doi: 10.1016/0006-291x(76)90019-x. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi T. Dephosphorylation of tubulin-bound guanosine triphosphate during microtubule assembly. J Biochem. 1975 Jun;77(6):1193–1197. [PubMed] [Google Scholar]
- MacNeal R. K., Purich D. L. On the role of the tubulin nonexchangeable GTP site in bovine neurotubule assembly. J Biol Chem. 1977 Jul 10;252(13):4440–4442. [PubMed] [Google Scholar]
- MacNeal R. K., Webb B. C., Purich D. L. Neurotubule assembly at substoichiometric nucleotide levels using a GTP regenerating system. Biochem Biophys Res Commun. 1977 Jan 24;74(2):440–447. doi: 10.1016/0006-291x(77)90323-0. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry. 1975 Jul;14(13):2996–3005. doi: 10.1021/bi00684a032. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., MacDonald E., Jameson J. L., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanylyl 5'-methylenediphosphonate. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4881–4885. doi: 10.1073/pnas.74.11.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J., Dickinson P. J. Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules. Biochemistry. 1976 Sep 21;15(19):4248–4254. doi: 10.1021/bi00664a018. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Deery W. J. Role of nucleotide hydrolysis in microtubule assembly. Nature. 1976 Oct 28;263(5580):792–793. doi: 10.1038/263792a0. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]



