Abstract
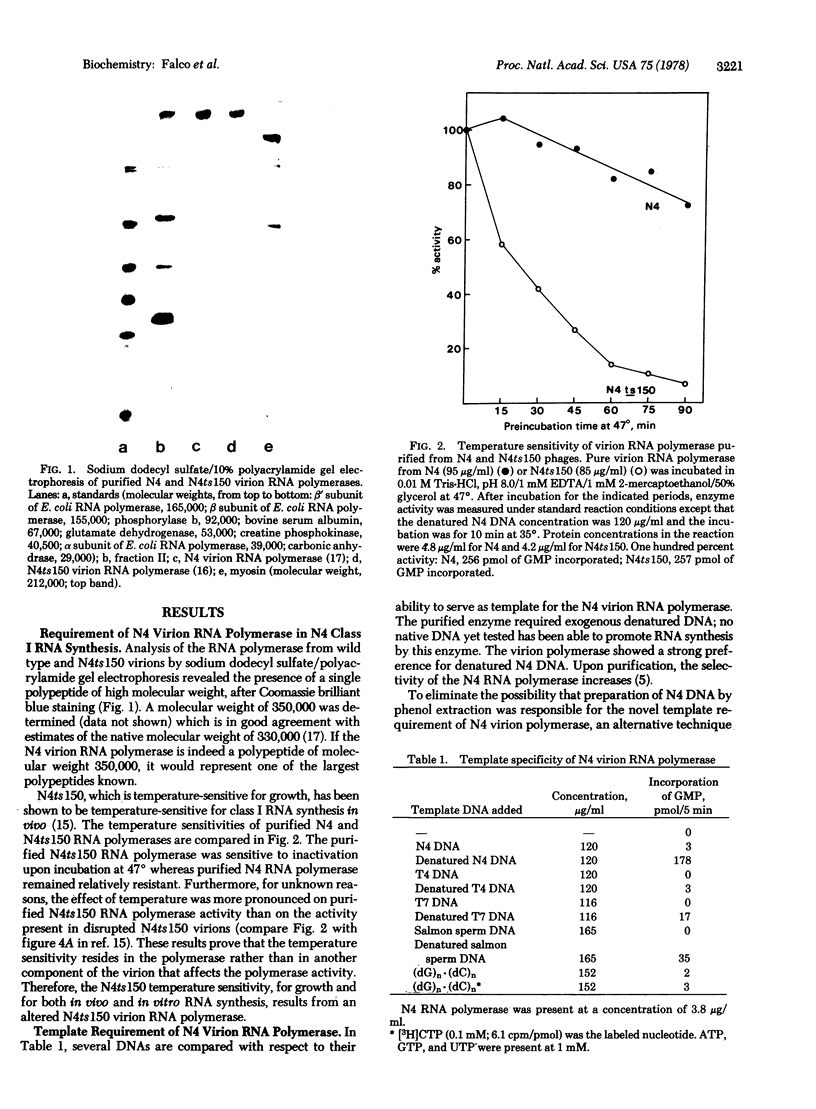
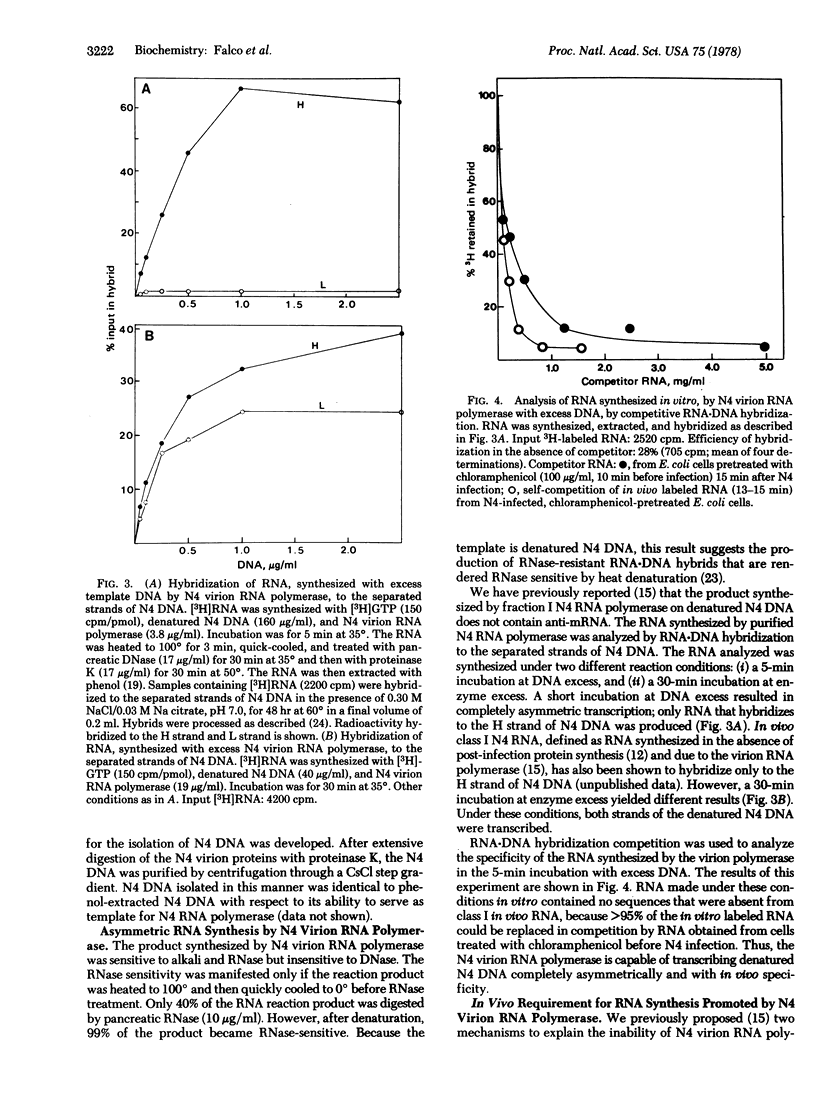
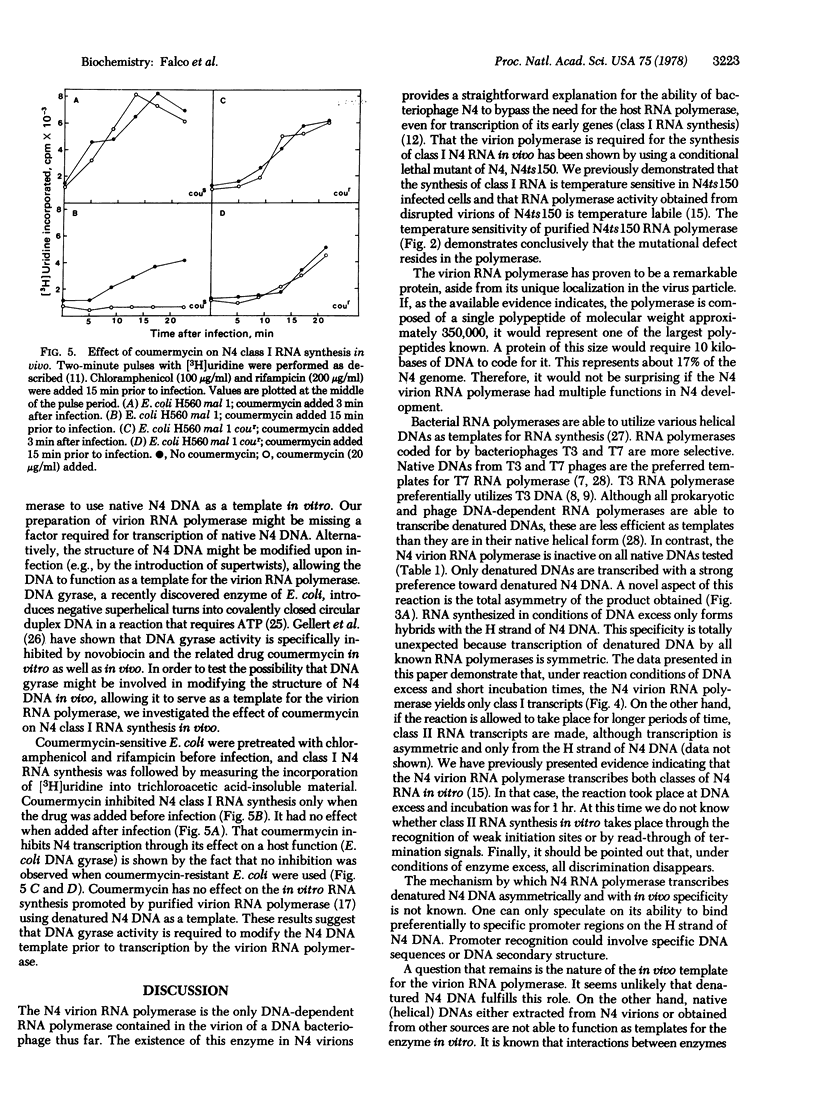
A DNA-dependent RNA polymerase has been purified from disrupted virions of the Escherichia coli bacteriophage N4. The RNA polymerase is phage-coded and is required for class I N4 RNA synthesis, which is defined as RNA synthesized in vivo in the absence of post-infection protein synthesis. A polypeptide of molecular weight 350,000 is detected when the purified enzyme is analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. N4 RNA polymerase requires denatured DNA as a template in vitro and shows a strong preference for denatured N4 DNA. With this template, transcription is asymmetric. The RNA product is complementary to only the H strand of N4 DNA. Furthermore, only class I N4 RNA is synthesized. In vivo transcription by the N4 virion RNA polymerase is inhibited by coumermycin. This result suggests that the activity of E. coli DNA gyrase, an enzyme that introduces negative supertwists into DNA, is required for N4 transcription.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: synthesis and relative stability of early and late RNA. J Mol Biol. 1968 Feb 14;31(3):325–348. doi: 10.1016/0022-2836(68)90413-0. [DOI] [PubMed] [Google Scholar]
- Botchan P., Wang J. C., Echols H. Effect of circularity and superhelicity on transcription from bacteriophagelambda DNA. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3077–3081. doi: 10.1073/pnas.70.11.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., Ring J. Characterization of T7-specific ribonucleic acid polymerase. 1. General properties of the enzymatic reaction and the template specificity of the enzyme. J Biol Chem. 1973 Mar 25;248(6):2235–2244. [PubMed] [Google Scholar]
- Dunn J. J., Bautz F. A., Bautz E. K. Different template specificities of phage T3 and T7 RNA polymerases. Nat New Biol. 1971 Mar 17;230(11):94–96. doi: 10.1038/newbio230094a0. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Laan K. V., Rothman-Denes L. B. Virion-associated RNA polymerase required for bacteriophage N4 development. Proc Natl Acad Sci U S A. 1977 Feb;74(2):520–523. doi: 10.1073/pnas.74.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Sklar J. Continual requirement for a host RNA polymerase component in a bacteriophage development. Nature. 1969 Mar 1;221(5183):833–836. doi: 10.1038/221833a0. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M. Template activities of the phi X-174 replicative allomorphic deoxyribonucleic acids. Biochemistry. 1971 Nov;10(23):4212–4218. doi: 10.1021/bi00799a009. [DOI] [PubMed] [Google Scholar]
- Holland M., Whiteley H. R. RNA polymerase from Bacillus amyloliquefaciens infected with phi29 bacteriophage. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2234–2237. doi: 10.1073/pnas.70.8.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra U. Induction of a new RNA polymerase in Escherichia coli infected with bacteriophage T3. Biochem Biophys Res Commun. 1971 Apr 16;43(2):443–450. doi: 10.1016/0006-291x(71)90773-x. [DOI] [PubMed] [Google Scholar]
- Marians K. J., Ikeda J. E., Schlagman S., Hurwitz J. Role of DNA gyrase in phiX replicative-form replication in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1965–1968. doi: 10.1073/pnas.74.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Nash H. A. Restriction assay for integrative recombination of bacteriophage lambda DNA in vitro: requirement for closed circular DNA substrate. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3524–3528. doi: 10.1073/pnas.73.10.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Pesce A., Casoli C., Schito G. C. Rifampicin-resistant RNA polymerase and NAD transferase activities in coliphage N4 virions. Nature. 1976 Jul 29;262(5567):412–414. doi: 10.1038/262412a0. [DOI] [PubMed] [Google Scholar]
- Price A. R., Frabotta M. Resistance of bacteriophage PBS2 infection to rifampicin, an inhibitor of Bacillus subtilis RNA synthesis. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1578–1585. doi: 10.1016/0006-291x(72)90894-7. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Schito G. C. Novel transcribing activities in N4-infected Escherichia coli. Virology. 1974 Jul;60(1):65–72. doi: 10.1016/0042-6822(74)90366-3. [DOI] [PubMed] [Google Scholar]
- Ryan M. J. Coumermycin A1: A preferential inhibitor of replicative DNA synthesis in Escherichia coli. I. In vivo characterization. Biochemistry. 1976 Aug 24;15(17):3769–3777. doi: 10.1021/bi00662a020. [DOI] [PubMed] [Google Scholar]
- Sinha R. K., Misra D. N., Das Gupta N. N. Electron microscopy of DNA from coliphage N4. Virology. 1973 Feb;51(2):493–498. doi: 10.1016/0042-6822(73)90449-2. [DOI] [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. Purification of ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1476–1482. [PubMed] [Google Scholar]
- Summers W. C., Siegel R. B. Control of template specificity of E. coli RNA polymerase by a phage-coded protein. Nature. 1969 Sep 13;223(5211):1111–1113. doi: 10.1038/2231111a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Oyama Y., Nakajima K., Yura T. Role of host RNA polymerase for lambda phage development. Biochem Biophys Res Commun. 1969 Aug 15;36(4):533–538. doi: 10.1016/0006-291x(69)90337-4. [DOI] [PubMed] [Google Scholar]
- Towle H. C., Jolly J. F., Boezi J. A. Purification and characterization of bacteriophage gh-I-induced deoxyribonucleic acid-dependent ribonucleic acid polymerase from Pseudomonas putida. J Biol Chem. 1975 Mar 10;250(5):1723–1733. [PubMed] [Google Scholar]
- Wang J. C. Interactions between twisted DNAs and enzymes: the effects of superhelical turns. J Mol Biol. 1974 Aug 25;87(4):797–816. doi: 10.1016/0022-2836(74)90085-0. [DOI] [PubMed] [Google Scholar]
- vander Laan K., Falco S. C., Rothman-Denes L. B. The program of RNA synthesis in N4-infected Escherichia coli. Virology. 1977 Feb;76(2):596–601. doi: 10.1016/0042-6822(77)90241-0. [DOI] [PubMed] [Google Scholar]



