Abstract
Docosahexaenoic acid (DHA) is important for brain function, however, the exact amount required for the brain is not agreed upon. While it is believed that the synthesis rate of DHA from α-linolenic acid (ALA) is low, how this synthesis rate compares with the amount of DHA required to maintain brain DHA levels is unknown. The objective of this work was to assess whether DHA synthesis from ALA is sufficient for the brain. To test this, rats consumed a diet low in n-3 PUFAs, or a diet containing ALA or DHA for 15 weeks. Over the 15 weeks, whole body and brain DHA accretion was measured, while at the end of the study, whole body DHA synthesis rates, brain gene expression, and DHA uptake rates were measured. Despite large differences in body DHA accretion, there was no difference in brain DHA accretion between rats fed ALA and DHA. In rats fed ALA, DHA synthesis and accretion was 100-fold higher than brain DHA accretion of rats fed DHA. Also, ALA-fed rats synthesized approximately 3-fold more DHA than the DHA uptake rate into the brain. This work indicates that DHA synthesis from ALA may be sufficient to supply the brain.
Keywords: brain, docosahexaenoic acid, kinetics, a-linolenic-acid, liver, synthesis, conversion
α-Linolenic acid (ALA) is the most accessible and sustainable source of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in the global diet (1). ALA is also a precursor to docosahexaenoic acid (DHA), an n-3 PUFA that is particularly enriched within the brain (2). While it is generally accepted that DHA is important for normal brain function, the amount of DHA required by the brain is not agreed upon (3–7). n-3 PUFAs cannot be synthesized by mammals de novo, therefore, DHA must be consumed from dietary sources or be synthesized from shorter chain n-3 PUFAs (i.e., ALA). To date, reports suggest that the synthesis rate of DHA from ALA is low and perhaps even below detection (8–17). However, plasma concentrations of DHA in vegans are only 0–40% lower than fish eaters despite having no dietary DHA (18, 19). Furthermore, vegan and vegetarian populations do not have an increased risk of neurological disorders (20, 21).
The lack of concordance between the low DHA synthesis rates and the relatively normal plasma DHA concentrations in vegans may be due to the methods used to measure DHA synthesis (22). In humans, an ALA tracer is administered orally and the appearance of labeled DHA in the plasma is measured for up to 2 weeks (8–17). From the area under the curve (AUC) of labeled DHA appearance, the fractional conversion of DHA is calculated (8–12, 15). Alternatively, plasma labeled DHA concentrations over time can be used in modeling programs to determine fractional conversion (13, 16, 17). In both cases, the calculations may preclude a quantitative measurement of the DHA synthesis rate and allow only a comparison between study groups (22). Another concern is the finding that up to 57% of the tracer remains in the adipose after oral consumption of labeled ALA (15). Because the human adipose half-life can be longer than one year, it is possible that the tracer is unavailable for DHA synthesis during the study period (23).
Recently, Rapoport and colleagues developed a new method in rats to estimate the DHA synthesis rate from ALA (24). This method requires a steady-state infusion of labeled ALA and uses nonlinear regression to determine the DHA synthesis rate. Importantly, this method estimates the DHA synthesis rate in rats to be 9.8 μmol/day (24), matching the 11 μmol/day synthesis and accretion rate of longer chain n-3 PUFAs, which we estimated from a published balance study performed in growing rats (25). The steady-state infusion method may have advantages because: i) infusing a tracer to achieve steady-state in the plasma eliminates issues around adipose tissue storage of the tracer; and ii) it allows for a quantitative determination of the DHA synthesis rate.
The goal of this study was to determine if DHA synthesis from ALA can maintain brain DHA in rats. We addressed this objective by measuring: i) brain and whole body DHA accretion; ii) DHA synthesis rates from ALA; and iii) brain DHA uptake rates in rats fed three different diets: a control diet (low n-3 PUFAs), an ALA diet (2% ALA), or a DHA diet (2% DHA). We found that rats fed ALA and DHA accreted similar amounts of brain DHA, which together with our kinetic findings suggest DHA synthesis from ALA is likely sufficient to maintain brain DHA levels. We also mimicked, in rats, the methods used in humans to determine DHA synthesis from ALA by subjecting rats to a gavage with labeled ALA. We found that the rates from this experiment, in rats, were comparable to the results of previously published human studies. Collectively, these results indicate that the rat is an appropriate model for measuring brain DHA synthesis and that brain DHA can be supplied from dietary ALA.
MATERIALS AND METHODS
Animals
All procedures were performed in accordance with the policies set out by the Canadian Council on Animal Care and were approved by the Animal Ethics Committee at the University of Toronto. Three Long Evans dams each with 18-day-old male Long Evans pups were ordered from Charles River Laboratories (St. Constant, QC, Canada). Pups were nonlittermates and each dam was housed with pups. Upon arrival, dams were allocated to one of the three diets (described below). When the pups were 21 days old, two pups from each dam were euthanized by high-energy head-focused microwave fixation (4.5 kW for 0.9 s). Brains were removed immediately and stored at −80°C until lipid analysis. Bodies were also stored at −80°C immediately after euthanization until analysis. The brains and bodies of these pups were used to determine the PUFA content of the rats at weaning (i.e., baseline PUFA content).
The remaining pups were weaned, singly housed, continued on the diet of their respective dam for 15 weeks, and were allocated to either the balance study (n = 11), the steady-state infusion study (n = 4), or the brain DHA uptake study (n = 3). For the balance study, the rats were weighed, food intake was determined, and uneaten food was replaced with fresh food on a weekly basis. Rats were euthanized by CO2 asphyxiation. Brains were removed and dissected sagittally. Carcasses and one half brain per rat were stored immediately at −80°C (to be later analyzed for lipid content). The other half brain was flash-frozen with 2-methylbutane on dry ice, and then stored at −80°C until analyzed for mRNA expression. The PUFA content in the brains and bodies of these rats after the 15 week feeding period was compared with the baseline PUFA content (described above) to determine PUFA accretion (equation 1, described below). For the steady-state infusion study and the brain DHA uptake study, a jugular vein catheter was surgically implanted into the rats. After allowing a 24 h recovery, rats were infused with 2H14-ALA or 14C-DHA, respectively (described below). The 2H14-ALA infusion was used to determine DHA synthesis rates following a previously published method (24) and the 14C-DHA infusion was utilized to measure the brain DHA uptake rate by following previously published methods (26).
For the gavage study (n = 4), a carotid artery catheter was surgically implanted in rats that had consumed a diet with ALA but no DHA for 9 weeks postweaning. After a 24 h recovery, the rats were gavaged with 10 mg of 2H5-ALA (described below).
Diets
Diets were modified from the custom low n-3 AIN-93G purified rodent diet (Dyets Inc., Bethlehem, PA) (27). The diet contained 10% lipids (by weight). The fat content of the diet was 32.8% (by weight) safflower oil, 65.2% hydrogenated coconut oil, and 2% added oil. The added oil was either DHA ethyl ester (Equateq, Callanish, Scotland), ALA ethyl ester (Equateq), or oleate ethyl ester (Nucheck Prep, Elysian, MN). Each oil was determined to be >98% pure by gas chromatography-flame ionization detection (GC-FID) and each diet contained one added oil to make the DHA, ALA, and control diets, respectively. The custom low n-3 AIN-93G diet is designed to be deficient in n-3 PUFAs, and as a result the only n-3 PUFAs in the diets were those added as ethyl ester oils (DHA or ALA) and a residual amount of ALA that made up 0.25% of the fatty acids. Oleate ethyl ester was added to the control diet to keep total fat content of the diets consistent, and to ensure a constant n-6 PUFA level across all three diets. The fatty acid composition of each diet as measured by GC-FID as shown in Table 1.
TABLE 1.
Percent composition of the three diets
| Fatty Acid | Control Diet | ALA Diet | DHA Diet | Gavage Study Diet |
| 10:0 | 3.6 | 4.0 | 3.8 | ND |
| 12:0 | 36.3 | 37.4 | 37.2 | ND |
| 14:0 | 15.1 | 14.8 | 14.8 | 0.1 |
| 16:0 | 10.0 | 9.7 | 9.9 | 14.3 |
| 16:1n-7 | 0.1 | 0.1 | 0.1 | 0.16 |
| 18:0 | 7.4 | 7.1 | 7.3 | 3.5 |
| 18:1n-9 | 7.5 | 4.6 | 4.7 | 21.8 |
| 18:1n-7 | 0.2 | 0.2 | 0.2 | 1.2 |
| 18:2n-6 | 19.5 | 19.1 | 20.1 | 52.5 |
| 18:3n-3 | 0.25 | 2.25 | 0.24 | 5.3 |
| DHA (22:6 n-3) | ND | ND | 2.00 | ND |
Data shown are means (n = 3) expressed as percent of total fatty acids. ND, fatty acid concentration below limit of detection.
The composition of the diet consumed by rats for the gavage study is shown in Table 1. This diet contained 52 and 5% of the fatty acids as linoleic acid (LNA) and ALA, respectively, with all other PUFAs <0.5%.
Balance study: whole body fatty acid extraction
Carcasses were thawed overnight in a refrigerator at 3°C, cut into pieces, and passed through a #12 hand grinder. After the whole body had passed through the grinder once, the homogenate was mixed together by hand and passed at least one more time through the grinder until the mixture was sufficiently homogeneous. Due to the small size of the pups all of the 21-day-old pup bodies were passed through the grinder together.
A portion of the whole body homogenate was weighed and further homogenized (done in duplicate for each 18-week-old rat and the baseline homogenate) using a Polytron benchtop homogenizer (Brinkman Instruments, Toronto, ON, Canada) in a mixture of chloroform:methanol:0.88%KCl (2:1:0.8 by volume) (28). The mixture was centrifuged at 500 g for 10 min, and the chloroform layer was extracted. New chloroform was added to the remaining aqueous phase, the mixture was once again centrifuged, and the chloroform layer was extracted and added to the previously extracted chloroform layer. This total lipid extract (TLE) was evaporated under N2, reconstituted in a known volume of chloroform, and stored under N2 at −80°C until further analysis.
Balance study: brain fatty acid extraction
One hemisphere was thawed briefly and dissected over ice to separate the brainstem, cerebellum, cortex, hippocampus, striatum, and the rest of the brain. Total lipids were extracted from the whole brain of 21-day-old rats and each region of the dissected 15 week half brains by following the method of Folch, Lees, and Sloane Stanley (28). Briefly, whole brains or brain regions were weighed in glass mortars, homogenized with glass pestles in 0.88% KCl, and transferred into a clean test tube. The homogenizer was then washed with methanol, which was then transferred to the test tube containing the homogenized brain and KCl. The homogenizer was washed a final time with chloroform and the chloroform was transferred to the test tube containing the KCl and methanol mixture (chloroform:methanol:0.88% KCl, 2:1:0.8 by volume). The homogenate mixtures were left at 3°C overnight and were centrifuged at 500 g for 10 min the following morning. The chloroform layer was extracted and new chloroform was added to the aqueous phase. This mixture was centrifuged at 500 g for 10 min and the chloroform layer was extracted and added to the previously extracted chloroform phase.
Balance study: fecal PUFA analysis
At 8 weeks postweaning all the feces produced by a rat in 24 h were collected. Approximately 1 g of feces from 6 rats was then analyzed for PUFA content. Fatty acids were extracted with chloroform:methanol:0.88% KCl (2:1:0.8 by volume) as described above and quantified by GC-FID to determine an estimate of n-3 and n-6 PUFA fecal excretion. Mean PUFA excretion (percentage of intake) was determined and applied to all animals to determine PUFA excretion.
Transmethylation and GC-FID
A known amount of heptadecanoic acid (17:0; Nu-Check Prep, Elysian, MN) was added to the TLE. Samples were transmethylated using 14% boron trifluoride in methanol at 100°C for 1 h. Fatty acid methyl esters (FAMEs) were extracted with hexane and quantified using GC-FID. FAMEs were analyzed using a Varian-430 gas chromatograph (Varian, Lake Forest, CA) equipped with a Varian FactorFour capillary column (VF-23ms; 30 m × 0.25 mm interior diameter × 0.25 μm film thickness) and a FID. Samples were injected in splitless mode. The injector and detector ports were set at 250°C. FAMEs were eluted using a temperature program set initially at 50°C for 2 min, increasing at 20°C/min, and held at 170°C for 1 min, then increased at 3°C/min and held at 212°C for 5 min to complete the run at 32 min. The carrier gas was helium, set to a constant flow rate of 0.7 ml/min. Peaks were identified by retention times of authentic FAME standards (Nu-Chek Prep). The concentration of each fatty acid was calculated by comparison with the internal standard (17:0) (29). The concentrations were expressed on a per gram basis then multiplied by total weight of the tissue to determine the total amount of each fatty acid in the tissue.
Balance study: brain RNA extraction
RNA was extracted from the flash-frozen half brains of the 15 week postweaning rats. The brain was briefly thawed and dissected to isolate the brainstem, cerebellum, cortex, hippocampus, striatum, and the rest of the brain. RNA was extracted from the brain regions using Trizol reagent (Ambion; Life Technologies, Burlington, ON, Canada) according to the manufacturer's protocol. The tissues were placed in a volume of Trizol reagent that was 10 times greater than the volume of the tissue and homogenized using a Kontes tissue grinder with plastic pestles (Daigger, Vernon Hills, IL), or for larger regions, a TissueRuptor (Qiagen, Germantown, MD). Chloroform was added to the Trizol reagent at a ratio of 1:5 (chloroform:Trizol reagent) and the solution was mixed and incubated at room temperature. The solution was then centrifuged at 12,000 g for 15 min at 4°C. The aqueous phase was transferred into a new tube, mixed with isopropanol (1:1, isopropanol:original volume of Trizol reagent), and incubated for 10 min. The samples were then centrifuged at 12,000 g for 10 min at 4°C to precipitate the RNA pellet. Following the precipitation of the RNA pellet, isopropanol was removed and the pellet was washed with 75% ethanol (1:2, ethanol:original volume of Trizol reagent). The samples were then centrifuged at 7,500 g for 5 min at 4°C and the wash was discarded. The pellet was allowed to air dry, and was then dissolved in RNase-free water. A Nanodrop 1000 (NanoDrop Technologies Inc., Wilmington, DE) was used to determine the concentration and purity of each sample by measuring absorbance at 260 and 280 nm. Each sample was aliquoted to make 1 μg of RNA per 10 μl of sample and stored at −80°C.
Balance study: gene expression analysis
A high-capacity cDNA reverse transcription kit (Applied Biosystems, Burlington, ON, Canada) was used to reverse transcribe 1 μg of RNA according to the manufacturer's instructions. Newly synthesized cDNA was stored at −20°C until the following day when it was loaded onto TaqMan low density array (TLDA) plates.
Quantitative real-time PCR was performed using TLDA (Applied Biosystems) on the 7900HT real-time PCR system (Applied Biosystems). cDNA (50 ng) was diluted with water to make a volume of 50 μl and mixed with 50 μl of TaqMan Universal PCR Master Mix (Applied Biosystems). The mixture was then loaded onto a customized preconfigured 384-well TLDA plate according to the manufacturer's protocol. TaqMan gene expression assays were used to assess 21 genes that were previously reported to be differentially expressed with n-3 deprivation. Among these were genes involved in the arachidonic acid (ARA) cascade: group 6 iPLA2 (Rn00588064_m1) (30), group 2a sPLA2 (Rn00580999_m1) (30), group 4 cPLA2 (Rn00591916_m1) (30), and COX-2 (Rn00568225_m1) (30); genes involved in neuroplasticity: brain-derived neurotrophic factor (Rn02531967_s1) (31), transthyretin (Rn00562124_m1) (32), T-cell intracellular antigen 1 (Rn01420836_m1) (32), and α-synuclein (Rn00569821_m1) (33); genes involved in the dopaminergic system: dopamine receptor D2 (Rn00561126_m1) (34), vesicular monoamine transporter 2 (Rn00564688_m1) (34), and tyrosine hydroxylase (Rn_00562500_m1) (34); genes involved in learning and memory: retinoic acid receptor α (Rn00580551_m1) (35), retnoid X receptor α (Rn00441185_m1) (35), retnoid X receptor β (Rn01399560_m1) (35), and peroxisome proliferator-activated receptor α (Rn00440945_m1) (35); genes involved in neurodegeneration and neuroinflammation: uncoupling protein 2 (Rn01754856_m1) (32), TNF-α receptor member 1a (Rn01492348m1) (33), heme oxygenase 1 (Rn01536933_m1) (36), and 15-lipoxygenase (Rn00696151_m1) (37); as well as epidermal growth factor receptor (Rn00580398_m1) (32) and prostaglandin E synthase 3 (Rn01529546_m1) (32). Endogenous controls measured included 18S RNA, phosphoglycerate kinase 1 (Rn01474011_g1) and β-actin (Rn00667869_m1). All genes were normalized to phosphoglycerate kinase 1. Similar results were found when normalizing to β-actin or 18S rRNA.
Steady-state infusion study: surgery and 2H-ALA infusion
At 15 weeks postweaning, rats were subjected to surgery to implant a catheter into their jugular vein. The animals were anesthetized using isoflurane inhalation (5% induction, 1–3% maintenance). Before the incision was made, hair was shaved from the incision site and the site was sterilized with iodine and ethanol. A transverse incision was made anterior to the upper thorax. The jugular vein was located by blunt dissection. The vein was isolated and tied off. The vein was then nicked and a catheter (PE 50; Intramedic, Sparks, MD) with a 3.5 cm silastic tubing end (VWR, Mississauga, ON, Canada) was inserted into the vein. The catheter was secured using 3.0 silk sutures and a 16 gauge Angiocath (Becton Dickinson, Mississauga, ON, Canada) was used to guide the catheter subcutaneously to a site outside the body near the scapula. An incision was made at this site and the catheter was tucked beneath the skin at the incision site. The incision site was stapled to protect the catheter from the rats. The incision site on the chest was closed with 4.0 silk sutures and the rats were allowed to recover from the anesthetic under a heat lamp. Approximately 24 h after the surgery, the tail vein of the rats was cannulated with a 24 gauge Angiocath (Becton Dickinson). While the rats were restrained, the staple closing the incision site on the scapula was removed and the jugular vein catheter was connected to a longer polyethylene catheter. The rats were then placed in an infusion box that contained food, a chew toy, and bedding from the rat's cage. The tail vein catheter was then connected to an infusion line. Rats were able to move freely within the infusion box throughout the infusion.
Modified from the method of Rapoport, Igarashi, and Gao (38); 4.5 μmol/100 g body weight of 2H-ALA (2H14-ALA, purity >95% confirmed by GC-FID and GC-MS; Cayman Chemical, Ann Arbor, MI) was infused into the tail vein for 3 h. To prepare the infusate, a known amount of 2H-ALA was dissolved in 5 mM HEPES buffer (pH 7.4) containing 50 mg/ml fatty acid-free BSA. The infusate was mixed by sonication at 37°C. An infusion pump (Harvard Apparatus PHD 2000; Holliston, MA) was used to infuse 3.78 ml of tracer solution at a constant rate of 0.021 ml/min for 3 h. Immediately before the infusion and every 30 min during the infusion 0.2 ml of blood was drawn from the jugular vein. The jugular vein catheter was flushed with heparinized saline (5% by volume) after every blood draw to prevent coagulation in the line. After 180 min of the infusion, 1 ml of blood was drawn from the jugular vein and the animals were euthanized with a lethal injection of T-61 into the tail vein. All blood samples were centrifuged for 10 min (PC-100 microcentrifuge; Diamed, ON, Canada) and the plasma was collected and stored at −80°C.
Steady-state infusion study: determination of plasma volume
Plasma volume was determined using the method of Schreihofer, Hair, and Stepp as modified by Gao et al.(24, 39). Briefly, a known amount of Evans Blue dye was injected into the tail veins of the rats. After 15 min, 1 ml of blood was drawn from the jugular vein, twice. The plasma was collected as described above, and 0.1 ml of plasma was diluted into 1 ml of saline. Absorbance at 604 nm was measured with a Nanodrop 1000 and by comparing the absorbance to a standard curve, the concentration of the dye was determined.
Steady-state infusion study: plasma lipid extraction
Fifty microliters of plasma were added to a test tube which contained known amounts of unesterified 17:0 standard and di-17:0 phosphatidylcholine standard. Lipids were extracted from the plasma using the method of Folch, Lees, and Sloane Stanley (28), as described above.
Steady-state infusion study: thin layer chromatography
Thin layer chromatography (TLC) was used to separate esterified and unesterified lipids. The TLE was dried under N2 and reconstituted in 250 μl chloroform. TLC plates (TLC silica gel 60, EMD) that were washed in chloroform and methanol (2:1) were activated by heat at 100°C for 1 h. TLE was loaded onto the TLC plates and the plates were run in heptane-diethyl ether-glacial acetic acid (60:40:2 v/v/v) alongside authentic standards (Nu-Check Prep). The plates were sprayed with 0.1% (w/v) 8-anilino-1-naphthalenesulfonic acid. Total phospholipid, unesterified fatty acid, triglyceride, and cholesteryl ester bands were identified under UV light by comparison to standards. Esterified lipid (phospholipid, triglyceride, and cholesteryl ester) bands were collected and transferred to a glass test tube. The unesterified fatty acid band was scraped off and transferred to a separate glass test tube.
Steady-state infusion study: plasma lipid hydroxylation and esterification
Plasma samples taken at time 0 min of the infusion were transmethylated as described above and run on GC-FID. Unesterified fatty acids were extracted from the silica using chloroform:methanol:0.88% KCl (2:1:0.8) as described above. Esterified lipids were hydrolyzed with 1 ml of 10% methanoic KOH at 70°C for 1 h (24). After the reaction, 1 ml of concentrated HCl was added to the sample followed by 1 ml of dH2O. The fatty acids were extracted twice with 3 ml of hexane. The unesterified lipids (free fatty acids and hydrolyzed esterified fatty acids) were converted to fatty acid-pentafluorobenzyl (PFB) esters by following the method of Strife and Murphy (40). One hundred microliters of a mixture of pentafluorobenzylbromide-diisopropylamine-acetonitrile (10:100:1,000 by volume) was added to the samples, which were then shaken for 15 min. The PFB mixture was then evaporated under N2 gas and the fatty acid-PFB esters were reconstituted in 50 μl of hexane and run on the GC mass spectrometer.
GC-MS
Fatty acid-PFB esters were analyzed using an Agilent 6890 series gas chromatograph (Agilent Technologies, Wilmington, DE) equipped with a DB-FFAP capillary column (30 m × 0.25 mm interior diameter, 0.25 mm film thickness; J and W Scientific, Folsom, CA) according to the method of Pawlosky, Sprecher, and Salem (41). Fatty acid-PFB esters dissolved in hexane were injected with a splitless injection technique. The GC oven temperature was programmed from 80°C to 185°C at 20°C/min then to 240°C at 10°C/min and held for 30 min. The injector and transfer line were maintained at 250°C and 280°C, respectively. The negative chemical ionization source temperature was 150°C. Methane was the ionization gas. Selected ion mode was used to analyze fatty acids using the mass-PFB ion for PFB derivatives. The m/z values for 2H-ALA and 2H-DHA were 291 and 337, respectively. Concentrations were determined by using calibration equations that relate the fatty acid peak area:standard peak area ratio to a fatty acid concentration.
Brain DHA uptake study: 14C-DHA infusion
At 15 weeks postweaning, a surgery was performed to implant a catheter into the jugular vein (described above). Approximately 24 h after the surgery, the tail vein of the rats was cannulated and 14C-DHA (76 μCi per rat) was infused into the tail vein at a rate of 0.223(1 + e−19.2t) ml/min (t is infusion time in minutes) for 5 min. At approximately 0, 15, 30, 45, 90, 180, 240, and 300 s during the infusion, 200 μl of blood was drawn from the jugular vein and the plasma was extracted and stored at −80°C as described above. After 5 min of 14C-DHA infusion rats were euthanized by high-energy head-focused microwave fixation (13.5 kW for 1.6 s). The brains were then removed, dissected sagittally and stored at −80°C until analysis.
Brain DHA uptake study: plasma radioactivity analysis
Lipids were extracted from 50 μl of plasma using the method of Folch, Lees, and Sloane Stanley (28), as described above. A known portion of the plasma TLE was then added to a scintillation vial with 5 ml of scintillation cocktail (GE Healthcare Life Sciences, Baie d'Urfe, QC, Canada). Radioactivity was measured using liquid scintillation counting to determine the plasma AUC for radioactivity. For plasma samples drawn at time 0 s of the infusion, the unesterified lipids were isolated by TLC and quantified using GC-FID (described above).
Brain DHA uptake study: liquid scintillation counting
Radioactivity was quantified by a Packard TRI-CARB2900TR liquid scintillation analyzer (Packard, Meriden, CT) with a detector efficiency of 48.8%. Radioactivity was expressed in disintegrations per minute; then converted to nCi.
Brain DHA uptake study: brain radioactivity analysis
Brain hemispheres were homogenized and lipids extracted as described above. A known portion of the TLE was then added to a scintillation tube with 5 ml of scintillation cocktail. Radioactivity was quantified using liquid scintillation counting.
Gavage study: surgery and blood sampling
At 10 weeks postweaning rats were subjected to a surgery to implant a catheter into their carotid artery. The animals were anesthetized using isoflurane inhalation (5% induction, 1–3% maintenance). Before the incision was made, hair was shaved from the incision site and the site was sterilized with iodine and ethanol. A transverse incision was made anterior to the upper thorax. The carotid artery was located by blunt dissection. The artery was isolated and tied off. The artery was then nicked and a catheter (PE 50, Intramedic, USA) was inserted into the vessel. The catheter was secured using 3.0 silk sutures and a 16 gauge Angiocath (Becton Dickinson) was used to guide the catheter subcutaneously to a site outside the body near the scapula. The incision site on the chest of rats was closed with 4.0 silk sutures and the rat was then injected with 1ml of saline solution subcutaneously and allowed to recover from the anesthetic under a heat lamp. The day after the surgery, 200 μl of blood was drawn from the carotid artery catheter; the rat was then gavaged with 10 mg of 2H5-ALA that was dissolved in 1 ml of olive oil (Sigma-Aldrich Corporation, St. Louis, MO). To prepare the gavage solution, 2H5-ALA ethyl ester (Cambridge Isotope Laboratories Inc., Andover, MA), which was generously donated by Dr. Stephen Cunnane, was hydrolyzed with 10% methanolic KOH at 70°C for 1 h. Unesterified fatty acids were formed by adding 1 ml of HCl followed by 1 ml of dH2O. Lipids were extracted twice with 3 ml of hexane. The unesterified 2H5-ALA was then dried down and mixed with olive oil to make a solution that was 10 mg 2H5-ALA per ml of olive oil. Blood samples (200 μl) were drawn at 30, 60, 90, 120, 180, 240, 300, and 360 min after the gavage. Plasma was separated and stored in −80°C as described above. Lipids were extracted from 50 μl of plasma by the Folch method, and the TLE was hydrolyzed to create unesterified fatty acids (described above). Hydrolyzed TLE was stored in −80°C until analyzed with liquid chromatography-tandem mass spectrometry (LC-MS/MS)
LC-MS/MS
Half of the TLE was evaporated under N2 gas and reconstituted in 100 μl of water/acetonitrile (80:20 v/v). Fatty acids were detected using an Agilent HPLC 1290 (Agilent Technologies, Santa Clara, CA) equipped with an Agilent Zorbax SB-phenyl column (3 × 50 mm, 3.5 μm; AgilentTechnologies). The initial HPLC conditions of elution were set at 500 μl/min gradient system consisting of (A) 50% water and (B) 50% acetonitrile. The gradient started with 50% (A) and 50% (B) and maintained for 1.5 min, increased to 100% (B) from 1.5 to 6 min, and maintained at 100% B for 4 min to complete the total run of 10 min. Mass spectrometry analyses were carried out on QTRAP 5500 triple quadruple mass spectrometer (AB SCIEX, Framingham, MA) in electrospray ionization negative ion mode. The source temperature was 600°C and the ion spray voltage was −4,500 eV. The optimized parameters were as follows: declustering potential, −40; entrance potential, −10; collision energy, −20; and collision cell exit potential, −11. Mass transitions for 2H5-ALA, 2H5-eicosapentaenoic acid (EPA), 2H5-docosapentaenoic acid (DPA)n-3, and 2H5-DHA were: m/z 282.2 to 59.0, m/z 306.2 to 262.2, m/z 334.2 to 290.2, and m/z 332.2 to 288.2, respectively. Concentration was quantified by comparing the peak area ratios (peak of interest:internal standard) and correcting for a response factor that was determined for each fatty acid of interest. Response factors were determined by analyzing a standard mixture of 100 ng/ml each of DHA, EPA, ALA, DPA, and 2H8-ARA by LC/MS/MS and comparing peak areas for each of the four fatty acids in relation to the peak area for 2H8-AA to generate response factors. The response factors were 10, 0.75, 0.25, and 0.75 for ALA, EPA, DPAn-3, and DHA, respectively.
Balance study: equation
In the balance study, PUFA content in the whole body of animals at time 0 (21 days of age) and after 15 weeks (126 days of age) consuming the diets was determined. Because PUFAs cannot be synthesized de novo, it is possible to determine the accretion of a specific PUFA by subtracting the baseline amount from the amount of that PUFA in rats at 15 weeks postweaning. Using equation 1, it is then possible to determine the metabolic consumption of the dietary PUFAs (25).
| (Eq. 1) |
where i refers to a PUFA consumed in the diet of the animals and x refers to a longer chain PUFA synthesized from i.
Steady-state infusion study: equations
To determine the DHA synthesis rate (steady-state infusion study), appearance of 2H-DHA in the plasma-esterified pool was measured and fit to a Boltzmann sigmoidal curve ([2H-DHA] × plasma volume vs. time) using nonlinear curve (24) (Graphpad Prism Version 4.0, La Jolla, CA). At any point on this curve, the slope (S) will be determined by the ability of the body to synthesize 2H-DHA from 2H-ALA and the ability of the periphery to uptake 2H-DHA (equation 2).
| (Eq. 2) |
where k1,DHA is the steady-state synthesis-secretion coefficient for DHA, [2H-ALA]unesterified is the plasma concentration of the infusate, k−1,DHA is the disappearance coefficient for DHA, and [2H-DHA]esterified is the concentration of DHA in the plasma that has been synthesized from the infusate, packaged into a lipoprotein and secreted into the plasma.
The maximum first derivative (Smax) of this curve is assumed to be the time point when the uptake of esterified DHA from the periphery is negligible, i.e., 0 (equation 3).
| (Eq. 3) |
Therefore, the derivative at this point is equal to the rate of 2H-DHA synthesis. By correcting the Smax by the tracee:tracer ratio, the rats actual DHA synthesis is determined, Jsyn,DHA (nmol/min) (24).
| (Eq. 4) |
Brain DHA uptake study: equations
To determine the brain DHA uptake rate, the incorporation coefficient for DHA from the unesterified plasma pool into the brain total lipid pool is (26):
| (Eq. 5) |
where is the total brain radioactivity at the end of the infusion and is the plasma radioactivity AUC.
Multiplying the incorporation coefficient by the concentration of plasma unesterified DHA (cpl) allows for the determination of the brain DHA uptake rate:
| (Eq. 6) |
Statistics
Mean lipid concentrations and accretions, as well as mean relative quantitation for genes and brain DHA uptake rates, were compared by one-way ANOVA using Tukey's test for multiple comparison (Graphpad Prism version 4.0). If variances were determined to be unequal, by Bartlett's test for equality of variances, then the Kruskal-Wallis test was used to compare the means followed by Dunn's test for multiple comparisons.
Mean plasma lipid concentrations were compared using the Kruskal-Wallis test followed by Dunn's test for multiple comparisons.
DHA synthesis rates between rats consuming the ALA and control diet was compared using Student's t-test due to the fact that DHA synthesis rates were not quantifiable in rats consuming the DHA diet.
RESULTS
Body weight and food intake: balance study
There were no statistical differences in body weight and food intake between rats consuming different diets throughout the 15 week feeding period. At 15 weeks, mean weights were 713 ± 9 g, 673 ± 25 g, and 724 ± 18 g in rats fed the control, ALA, and DHA diet, respectively. Average weekly food intake throughout the study was 190 ± 5 g, 175 ± 5 g, and 189 ± 7 g for the control, ALA, and DHA diet, respectively.
Fecal excretion of PUFAs: balance study
On average, 0.27 ± 0.002%, 0.07 ± 0.02%, and 0.5 ± 0.05% of dietary ALA, DHA, and LNA, respectively, were excreted in the feces.
Baseline PUFA concentrations: balance study
Total n-6 and n-3 PUFAs in 21-day-old rats were 1505 ± 35 μmol and 148 ± 4 μmol, respectively (supplementary Table I and Table 2). The majority of PUFAs were in the carcasses of the rats. LNA was the main n-6 PUFA at baseline 1,279 ± 30 μmol (data not shown). DHA and ALA were the most abundant n-3 PUFAs at baseline (55 ± 1 μmol and 41 ± 0.9 μmol, respectively, data not shown).
TABLE 2.
Summary of n-3 PUFA Balance
| Dietary Group | Control | ALA | DHA |
| Intake of n-3 PUFAs (μmol) | 2560 ± 69* | 21273 ± 661* | 17301 ± 627** |
| Fecal excretion (μmol) | 7 ± 0.2 | 57 ± 2 | 10 ± 0.4** |
| Body content of n-3 PUFAs (μmol) | |||
| Day 0 | 139 ± 3 | 139 ± 3 | 139 ± 3 |
| Day 105 | 380 ± 23a | 2352 ± 166b | 1607 ± 106b |
| Brain content of n-3 PUFAs (μmol) | |||
| Day 0 | 10 ± 0.6 | 10 ± 0.6 | 10 ± 0.6 |
| Day 105 | 9.7 ± 1a | 14 ± 2b | 15 ± 3b |
| Total accretion (μmol) | |||
| ALA | 47 ± 6a | 1500 ± 100b | 51 ± 7a |
| 20:3n-3 | 84 ± 7a | 110 ± 11ab | 164 ± 21b |
| EPA | 45 ± 3a | 70 ± 10b | 71 ± 8b |
| DPAn-3 | 5 ± 2a | 90 ± 7b | 66 ± 7b |
| DHA | 60 ± 6a | 440 ± 39b | 1119 ± 62c |
| Total n-3 PUFA | 240 ± 17a | 2209 ± 107b | 1472 ± 99b |
| Metabolic consumption of dietary PUFAs (μmol) | 2317 ± 61a | 19037 ± 617b | 15783 ± 548b,** |
Data are mean ± SEM. Different letters signify means are significantly different (P < 0.05) measured by one-way ANOVA followed by Tukey's test for multiple comparisons. n = 2 for day 0 measurements and n = 11 for day 105 measurements.
*Consumption of ALA.
**Refers to DHA.
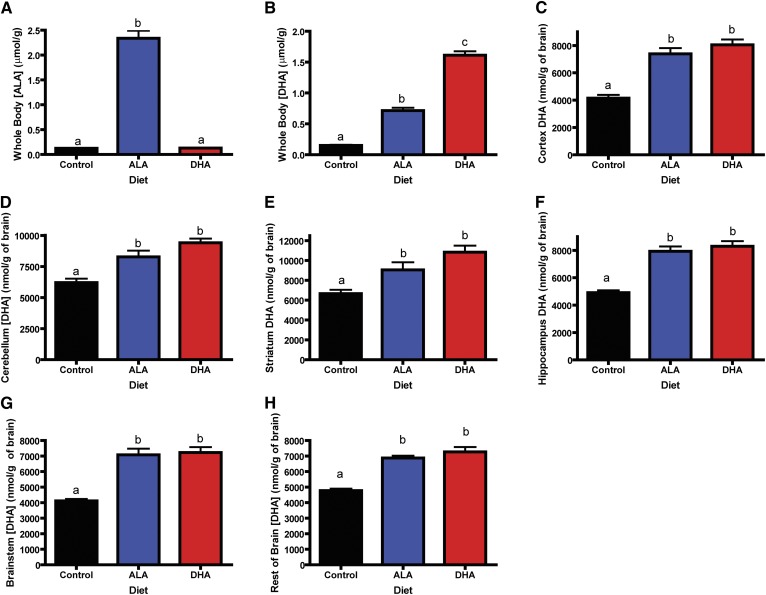
Final PUFA concentrations: balance study
Concentrations of major n-3 PUFAs in the brain and whole body are shown in Fig. 1. Whole body ALA concentrations (Fig. 1A) were 30-fold greater in rats consuming the ALA diet compared with rats consuming the DHA and control diets (ALA diet > DHA diet = control diet, P < 0.05). Whole body DHA concentrations (Fig. 1B) were highest in rats consuming the DHA diet, followed by the rats consuming the ALA diet, and lowest in rats consuming the control diet (P < 0.05). Regional brain DHA concentrations did not differ significantly between rats consuming the DHA and ALA diets (Fig. 1C–H). However, DHA concentrations were significantly lower in rats consuming the control diet compared with rats consuming the ALA and DHA diets in all brain regions (P < 0.05). Total (body + brain) n-6 PUFA concentrations did not differ between rats consuming different diets (supplementary Table I). However, DPAn-6 in both the brain and body were highest in the rats consuming the control diet, followed by the rats consuming the ALA diet, and lowest in rats consuming the DHA diet (supplementary Tables I, II; P < 0.05). Whole body ARA concentrations only differed between rats consuming the control and DHA diets (2280 ± 98 μmol vs. 1688 ± 44 μmol respectively, P < 0.05). Brain ARA concentrations only differed in the brainstem of rats fed the control and DHA diets, while brainstem ARA concentrations for rats fed the ALA diet were similar to those of rats fed both diets (supplementary Tables III–VIII, P < 0.05).
Fig. 1.
Whole body ALA and DHA concentrations and brain DHA concentrations in rats consuming the control, ALA, or DHA diet. A: Whole body ALA concentration (μmol/g) is highest in rats consuming ALA and is not different in animals consuming DHA or the control diet. B: Whole body DHA concentration (μmol/g) is highest in rats consuming DHA diet > ALA diet > control diet. Brain DHA concentrations (nmol/g) are not different in rats consuming the ALA and DHA diets but are significantly lower in rats consuming the control diet in the cortex (C), cerebellum (D), striatum (E), hippocampus (F), brainstem (G), and the rest of the brain (H). All data are mean ± SEM. Different letters signify the means are significantly different (P < 0.05) measured by one-way ANOVA followed by Tukey's multiple comparison test or Kruskal-Wallis test followed by Dunn's multiple comparison test (if variances were significantly different) (n = 11).
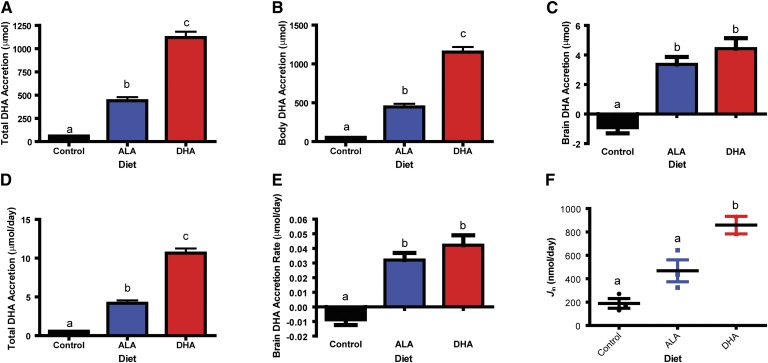
PUFA accretion: balance study
Table 2 and supplementary Table I summarize the n-3 and n-6 PUFA balance. Total and body DHA accretion (Fig. 2A, B) was highest in rats consuming the DHA diet, followed by rats consuming the ALA diet, followed by rats consuming the control diet (P < 0.05). Brain DHA accretion (Fig. 2C), however, did not differ in rats consuming the ALA and DHA diets but was significantly lower in rats consuming the control diet (P < 0.05). Rats consuming the ALA diet synthesized and accreted 4.2 ± 0.4 μmol/day of total DHA (Fig. 2D). The DHA uptake and accretion rate into the brain for rats consuming the DHA diet was 42 ± 7 nmol/day (Fig. 2E).
Fig. 2.
DHA kinetics. Total DHA accretion (body + brain DHA accretion) (A) and body DHA accretion (μmol) (B) was highest in rats consuming DHA > ALA > control diet (n = 11). C: Brain DHA accretion (μmol) was significantly lower in rats consuming the control diet compared with the rats consuming the ALA and DHA diets. There was no difference in brain DHA accretion between rats consuming the ALA and DHA diets (n = 11). D: Total DHA accretion rate (μmol/day) in rats consuming the three diets. For rats consuming the ALA diet, this is the synthesis-accretion rate (4.2 μmol/day) (n = 11). E: Brain DHA accretion rate (μmol/day) of rats consuming the three diets. Rats consuming the DHA diet accreted 0.042 μmol DHA/day in their brains (n = 11). F: Brain DHA uptake rate (nmol/day) (n = 3 for the ALA and control groups and n = 2 for the DHA group). All data are mean ± SEM. Different letters signify the means are significantly different (P < 0.05) measured by one-way ANOVA followed by Tukey's multiple comparison test or Kruskal-Wallis test followed by Dunn's multiple comparison test (if variances were significantly different).
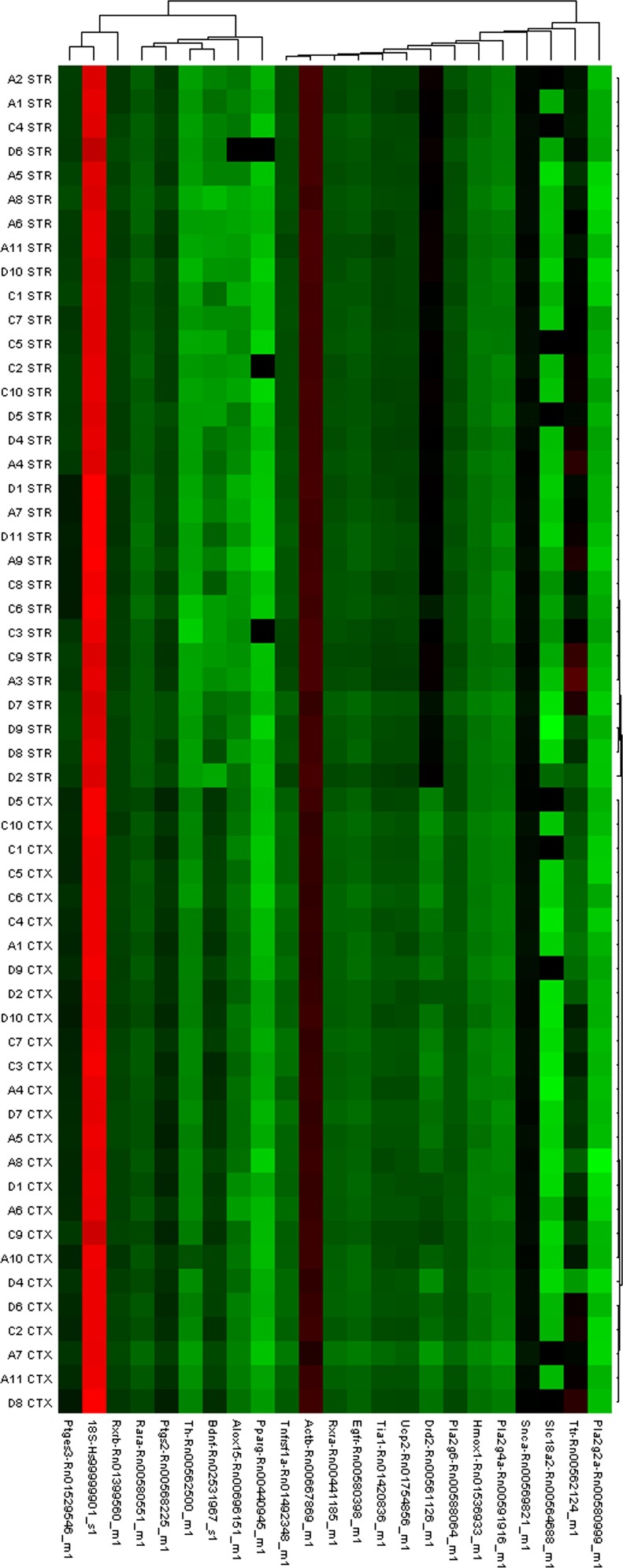
Gene expression: balance study
Relative gene expression of 21 genes was measured in all six brain regions. A heat map representing gene expression in the cortex and striatum illustrates regional differences in gene expression (Fig. 3). While there were strong effects of brain region, diet did not have an effect on gene expression for most of the measured genes. There were only 11 differences in gene expression out of 378 comparisons. A full list of relative gene expression of all the genes measured is available in supplementary Tables IX–XIV. Due to the high number of comparisons, it is not possible to rule out these differences as a chance finding.
Fig. 3.
Heat map depicting gene expression in cortex and striatum brain samples. The figure illustrates that there are no differences within brain regions for any of the measured genes (average linkage and Pearson's distance metric). The clustering of all the cortex (CTX) samples and all the striatum (STR) samples together indicates that samples of the same brain region are highly correlated. There is also a clear difference in dopamine receptor D2 indicating brain regions were properly dissected as there was less expression of this receptor in the cortex relative to the striatum. Expression profiles are illustrated as ΔCt values with red indicating higher expression and green indicating lower expression compared with a striatal sample from a rat fed the ALA diet. Dendrograms indicate the correlation between groups of samples and genes. Samples are in columns and transcripts in rows. Gene names are available in the Materials and Methods section (n = 10).
Plasma volume: steady-state infusion study
Mean plasma volume (Vplasma) was measured to be 37 ± 1.6 ml/kg which agrees with a previous report that measured plasma volume in Long Evans rats (42).
Plasma concentrations: steady-state infusion study
Unesterified ALA in the plasma was higher in rats consuming the ALA diet compared with rats consuming the control diet (supplementary Table XV, P < 0.05) but did not differ from rats consuming the DHA diet (4 ± 0.9 nmol/ml, P > 0.05). Rats consuming the DHA diet had significantly higher plasma unesterified DHA levels than rats consuming the control diet (65 ± 21 nmol/ml vs. 1 ± 0.3 nmol/ml, P < 0.05). Rats consuming the ALA diet did not have statistically different concentrations of plasma unesterified DHA compared with rats consuming the DHA or control diets (4 ± 0.9 nmol/ml, P > 0.05). Plasma esterified ALA and DHA followed the same pattern as plasma unesterified ALA and DHA (supplementary Table XVI).
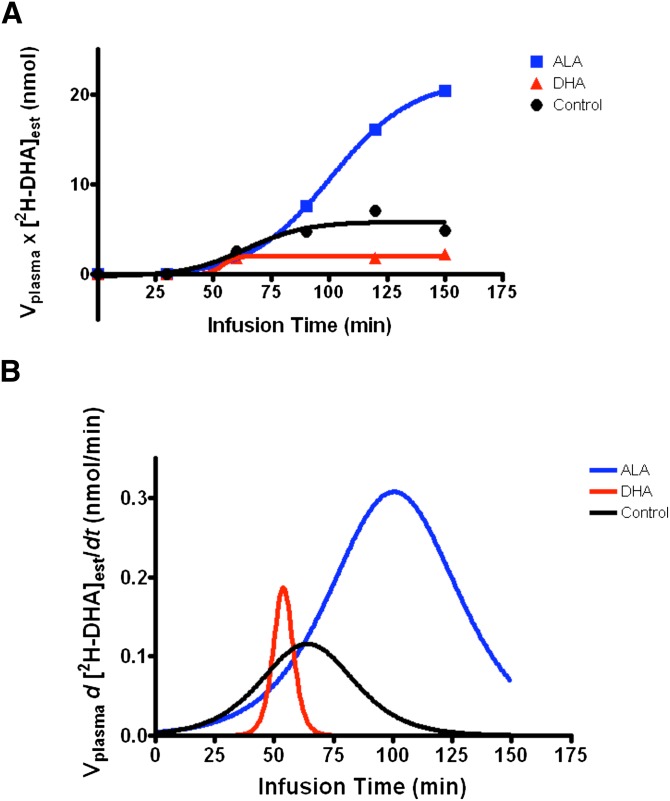
DHA synthesis: steady-state infusion study
Curves in Fig. 4A show Vplasma × [2H-DHA]esterified plotted versus time for three rats (one per dietary group). Due to the fact that [2H-DHA]esterified did not increase throughout the infusion in rats consuming the DHA diet, and thus, the data did not fit to a sigmoidal curve; a DHA synthesis rate was not calculated in these animals. Mean infusion parameters are presented in Table 3. Mean [2H-ALA]unesterified was 2.5 ± 0.5 nmol/ml and 10.5 ± 4.5 nmol/ml in the ALA diet and control diet groups, respectively. Mean Smax for rats consuming the ALA diet was 0.339 ± 0.135 nmol/min and 0.089 ± 0.023 nmol/min in rats consuming the control diet (P = 0.12). Corresponding daily DHA synthesis rates were 1452 ± 395 nmol/day and 45 ± 19 nmol/day in rats consuming the ALA and control diets, respectively (P < 0.05, Table 3).
Fig. 4.
Example of infusion curves for a rat from each diet group. A: Plasma volume (ml) (Vplasma) × concentration of esterified 2H-DHA (nmol) ([2H-DHA]est) plotted against time (min) and fit to a sigmoidal curve. This figure shows the resulting infusion curve for one rat from each dietary group that was infused with unesterified 2H-ALA for 3 h. B: First derivatives of the curves from (A). The maximum first derivative is used to determine the DHA synthesis rate. For the rat consuming DHA there was no increase in esterified 2H-DHA throughout the infusion resulting in an artificially high maximum first derivative, therefore, the DHA synthesis rate in this rat was deemed unquantifiable.
TABLE 3.
Parameters for synthesis-secretion of DHA in rats consuming the control, ALA, and DHA diet for 15 weeks
| Diet | Smax,i (nmol/min) | k1,DHA (ml/min) | Jsyn (nmol/min) | Daily Secretion Rate (nmol/day) | FDHA (/min) | t1/2 (days) |
| Control | 0.089 ± 0.023 | 0.012 ± 0.006 | 0.031 ± 0.013 | 45 ± 19 | 2E-05 ± 5E-06 | 57 ± 29 |
| ALA | 0.339 ± 0.135 | 0.131 ± 0.04* | 1.008 ± 0.274* | 1452 ± 395* | 2E-04 ± 4E-05* | 3 ± 1* |
| DHA | ND | ND | ND | ND | ND | ND |
Data are mean ± SEM (n = 4, independent samples per group). ND, not determinable. Smax,i, maximum first derivative; k1,DHA, synthesis-secretion coefficient for DHA synthesis from ALA; Jsyn, = synthesis rate of DHA from ALA; FDHA, turnover rate of esterified plasma DHA; t1/2, half-life of esterified plasma DHA.
*P < 0.05 versus control diet.
Brain DHA uptake rate: brain DHA uptake study
There was no significant difference in k* between rats fed the different diets (5.9 ± 0.7 × 10−4 ml/s/g, 6.5 ± 1.9 × 10−4 ml/s/g, and 6.0 ± 1.2 × 10−4 ml/s/g for control, ALA, DHA diet, respectively). Brain DHA uptake rate was significantly higher in the DHA-fed rats versus the ALA and control-fed rats (858.6 ± 74.8 nmol/day > 467.6 ± 93.7 nmol/day = 189.1 ± 41.8 nmol/day respectively, P < 0.05). There were no statistical differences in brain DHA uptake rates between the ALA and control-fed rats (Fig. 2F).
Appearance of 2H5-n-3 PUFAs: gavage study
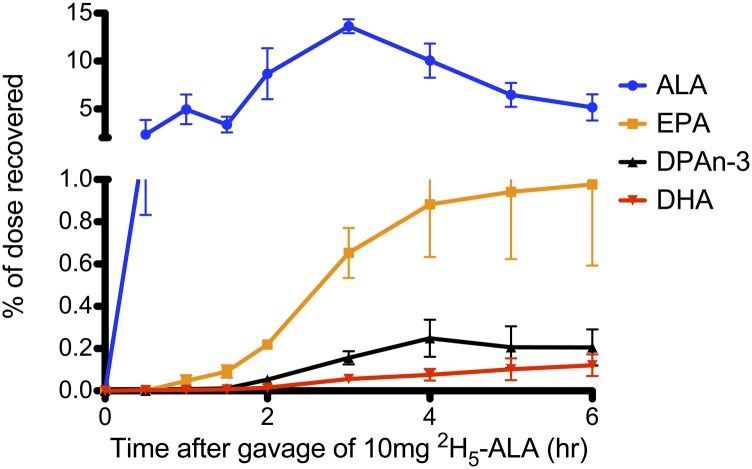
Figure 5 illustrates the appearance of 2H5-labeled n-3 PUFAs in the plasma of rats that were gavaged with 10 mg of 2H5-ALA. ALA was the first labeled n-3 PUFA to appear in the plasma and was consistently at the highest concentration. Labeled longer chain n-3 PUFAs, EPA, DPAn-3, and DHA, appeared in the plasma at later time points and concentrations of these PUFAs were lower compared with ALA.
Fig. 5.
2H5-n-3 PUFA appearance in plasma of rats gavaged with 10 mg of 2H5-ALA. Data are expressed as mean percentage of dose recovered ± SEM at time points after the gavage. Different colored lines represent different 2H5-n-3 PUFAs. Data from this graph was used to model the methods used in humans to calculate DHA synthesis (n = 4).
DISCUSSION
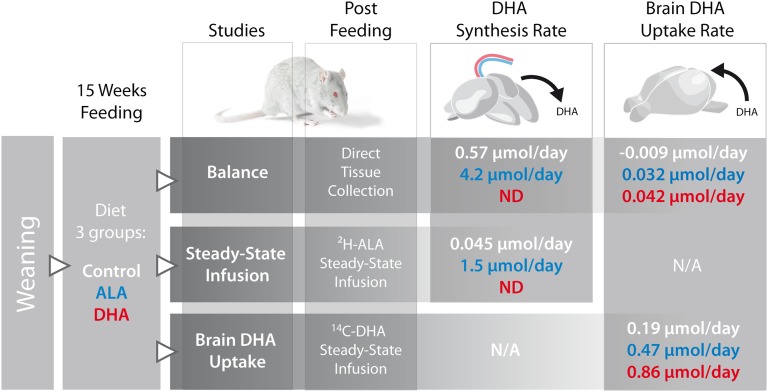
We showed that brain DHA levels in the adult rat can be maintained by dietary ALA just as well as by dietary DHA. This was supported by the finding that dietary ALA and DHA resulted in the same level and accretion of brain DHA after 15 weeks. From the 15 week balance study, the accretion of brain DHA in the ALA- and DHA-fed rats did not significantly differ, and equaled 0.032 ± 0.005 μmol per day and 0.042 ± 0.007 μmol per day, respectively (Fig. 2E). When we compare these daily brain accretion rates to the whole body DHA synthesis rate in the ALA-fed rats in the balance study, we see that DHA synthesis rates exceed brain uptake rates by 100-fold (Fig. 2D). To illustrate this, a summary of study designs and results is shown in Fig. 6. Using the steady-state infusion method, we estimated synthesis rates of DHA from ALA to be 1.5 and 0.045 μmol/day in animals consuming the ALA and control diets, respectively. These rates are lower, but in line with previously published estimates using the steady-state infusion technique (24, 43, 44). Differences could be due to the fact that we performed the steady-state infusion in rats fed a diet containing 2% of the total fatty acids as ALA, whereas Gao et al. (24) fed their animals a diet containing approximately 5% of the total fatty acids as ALA. The differences in synthesis rates could also be due to the differences in the age and strain of the rats. In particular, different rat strains are known to have different desaturase enzyme activity (45). Another important difference between our kinetic studies compared with the studies done by others is that our infusions were performed in completely free-living rats, 24 h after recovery from anesthesia (24, 46). Additionally, using a free-living infusion model, we determined the rate of DHA uptake from the unesterified plasma pool into the brain. Brain DHA uptake rates were between 189 and 618 nmol/day, which is similar to rates that have been previously published by others (46, 47).
Fig. 6.
Summary of the methods and results. Three studies were performed in rats fed the control, ALA, or DHA diet from weaning for 15 weeks. After 15 weeks the brain and body were collected to perform the balance study. For the steady-state infusion study, a jugular vein and tail vein catheter were implanted into the rats and the rats were infused with 2H-ALA to measure whole body DHA synthesis from ALA in rats fed the three diets. For the brain DHA uptake study, tail vein and jugular vein catheters were implanted into the rats and 14C-DHA was infused to determine the brain DHA uptake rate in rats fed the three diets.
Despite sizeable differences in n-3 PUFA concentrations and accretions in the bodies, we were unable to detect differences in brain DHA concentrations and accretions between rats consuming diets with DHA or ALA as the only n-3 PUFA source. In our balance study, we calculated a DHA synthesis rate of 4.2 μmol/day in animals fed the ALA diet. We also calculated, in rats consuming the DHA diet, a brain uptake and accretion rate for DHA of 42 nmol/day. Previous work in our lab found that mice fed DHA at a concentration of 2% of the fatty acids attained maximal DHA concentrations in the brain (48). Because rats fed the ALA diet were able to synthesize 100-fold more DHA than the amount of DHA accreted in the brains of rats consuming the DHA diet, and there were no significant differences in brain DHA concentrations or accretions in rats fed these two diets, it is likely that rats fed the ALA diet were able to synthesize sufficient DHA to maintain brain DHA levels. The finding that brain DHA concentrations are not different between rats fed the ALA and DHA diets is in contrast to previously published work (49). Abedin et al. (49) reported in 1999 that DHA in the brain phosphatidylethanolamine fraction was higher in Guinea pigs fed a diet containing DHA as compared with a diet containing ALA. Our study differed from this work, however, in that we measured brain total lipids, not brain phospholipid fractions, and we fed our rats pure fatty acid ethyl esters, whereas Abedin et al. (49) used oil sources to formulate their diets. As such, the high DHA diet also contained ALA in the study by Abedin et al. (49).
Furthermore, the results of our kinetic studies support the idea that rats consuming the ALA diet were able to synthesize enough DHA to supply the brain. Using the steady-state infusion method, we calculated that rats consuming the ALA diet synthesized 1.5 μmol/day of DHA, which is about 3-fold higher than the amount of DHA these rats uptake into the brain (468 nmol/day) as calculated by a 5 min infusion of radiolabeled DHA. Also, DHA synthesis rates were higher in rats fed a diet containing ALA compared with rats fed a diet with no added n-3 PUFAs. This suggests that the ALA substrate was the rate-limiting factor for DHA synthesis from ALA. Contrary to others, we were unable to measure a DHA synthesis rate in rats fed a diet containing DHA (24). Our work indicates that 2% dietary DHA reduced DHA synthesis by greater than 30-fold (24). It has been previously reported that n-3 PUFA feeding downregulates the expression of the hepatic desaturase enzymes required for DHA synthesis from ALA (50).
Despite the similar brain DHA concentrations in rats fed the ALA and DHA diets, rats consuming DHA had an almost 2-fold greater uptake rate of DHA into the brain. This means that the brains of rats fed the DHA diet took up and metabolically consumed more DHA. Despite increased metabolism of DHA in the brain, there were no differences in gene expression between rats on either diet. It is conceivable that exposing these rats to a stressor (such as brain trauma, neuroinflammation, etc.) would result in differential gene expression in the brains of these rats. Therefore, future experiments should measure the effect of diet on brain gene expression in rats that have been exposed to stress.
The steady-state infusion method developed by Rapoport and Igarashi (51) in 2009 is an in vivo kinetic approach to measure DHA synthesis rates at a given time, whereas the balance method measures an average synthesis rate over the balance period. Using the steady-state infusion method, we calculated that rats consuming the ALA diet were synthesizing 1.5 μmol of DHA per day at 15 weeks postweaning; whereas using the balance method, we calculated that over the 15 weeks these rats synthesized, on average, 4.2 μmol of DHA per day. One explanation as to why the balance method gives a higher DHA synthesis rate than the steady-state infusion method is because the balance study measures an average DHA synthesis rate over the 15 weeks. Therefore, included in the average rate is the growth and development period of the rat, when DHA synthesis rates are likely to be highest. In contrast, the steady-state infusion method measured DHA synthesis rates at the end of the 15 week feeding period. This rate is only a measure of the synthesis rate in these rats at this time, when the rats are older and when the DHA synthesis rate is also likely low. It has been shown previously that the DHA synthesis rate decreases with age in rats (44).
Using the balance method to calculate DHA synthesis rates is advantageous because it measures DHA in all body pools to determine how much DHA was synthesized and accreted. This method is limited, however, because it cannot determine how much DHA was synthesized and then metabolically consumed. Therefore this method likely underestimates the actual DHA synthesis rate. While the steady-state infusion method only measures the DHA in the plasma pool, this is unlikely to be a limitation because the method utilizes kinetic modeling to calculate the actual DHA synthesis rate. The steady-state infusion method is advantageous because it measures a DHA synthesis rate in conditions where the substrate may be rate limiting, which can be different from in vitro kinetic models where the substrate is assumed to not be rate limiting. More importantly, the steady-state infusion method may be advantageous to the oral administration method that is performed in humans to measure DHA synthesis. When ALA is consumed orally, the majority will be β-oxidized or stored in the adipose. Our study, as well as other balance studies, found that the majority of ALA intake is metabolically consumed and not accumulated in the tissues (25, 52). In our study approximately 90% of dietary ALA was metabolically consumed. While we did not measure adipose composition, other balance studies have found the majority of PUFAs that are not metabolically consumed are stored in the adipose (25). These findings are in agreement with oral tracer administration studies performed in humans (15). This is problematic because tracer that is stored in the adipose would not be available for DHA synthesis in the liver over the duration of the study. The steady-state infusion method eliminates this problem because the tracer is infused in a way that it achieves a steady-state level in the plasma despite uptake into the adipose.
It is generally accepted that the rat can synthesize more DHA than the human; however, the methods used to measure DHA synthesis in the human have not been validated in the rat. In humans, DHA synthesis is measured by administering an oral bolus of labeled ALA and measuring the appearance of labeled DHA in plasma. These calculations can be applied to the data from our gavage study to mimic the “human” method for determining DHA synthesis. When we applied three of the calculations previously performed in humans (12, 15, 53) to our data in rats, we obtained three different values for DHA synthesis. When using the calculation of McCloy et al. (15), mean AUC for 2H5-DHA in our rats was 0.31% of dose, which was in line with the value previously published in humans (0.99% of dose, corrected for plasma volume assuming plasma volume equals 4.5% of body weight). When we applied the calculation of Emken, Adlof, and Gulley (12) to our data in rats, the rats had lower average percent conversion to DHA compared with previously published values in humans (0.64 vs. 3.8%, respectively). Finally, when we used the calculation of Gillingham et al. (53), we found that the value of apparent conversion to DHA in the rat was comparable to that found in humans (0.12% of dose recovered as DHA in rat vs. 0.19% of dose recovered as DHA in human). Therefore, the results from the oral gavage study are inconsistent with the belief that the rat is more efficient at synthesizing DHA than the human. Also, the fact that applying different kinetic calculations to the same data gave markedly different results for DHA synthesis indicates that the calculations used to measure DHA synthesis are inconsistent and should be used largely to compare relative DHA synthesis between experimental groups within a study (22). While pilot data indicated that labeled DHA peaked within 6 h of an oral gavage in a rat, if this study was extended beyond 6 h, the DHA synthesis rates measured could be higher. However, we do not expect that extending this study beyond 6 h would increase synthesis rates enough to change our conclusions.
This study had several limitations. First, with respect to the gene expression data, we analyzed different sub-regions of the brain than others. For example, many studies analyzing expression of genes involved in the ARA cascade focused on the frontal cortex, whereas our study only investigated gene expression in the cortex, which could partly explain why we were unable to reproduce previously published findings (30, 31). This study was also limited by the 15 week feeding period. As all experiments were conducted on the rats at 15 weeks postweaning, we cannot draw conclusions about outcomes during the growth and development phase of the rat's life, when brain accretion peaks. It is possible that differences in brain DHA concentrations were more pronounced during adolescence and started to equilibrate in adulthood. In fact, it has been reported that infants who were breastfed had higher brain DHA concentrations than those fed formula, therefore, the results from our study do not apply to infants (54). Also, heparin was used as an anti-coagulant for our infusion studies. Heparin is known to activate lipoprotein lipase and may have contributed to the large variability in plasma unesterified fatty acid concentrations (55, 56). However, the effect of heparin is likely an overestimation of the rate of DHA uptake into the brain. Another limitation to this study was the use of pure fatty acid ethyl esters. Pure oil sources were used so that the effect of n-3 PUFAs could be compared directly (ALA vs. DHA). However, the use of pure oil sources limited the applicability of the study to free-living situations because n-3 PUFAs are consumed from food or oil sources, not as pure fatty acids, and most diets that contain DHA also contain ALA.
This study showed that despite large differences in fatty acid accumulation in the body, rats fed a diet containing DHA or ALA making up 2% of the fatty acids did not have differences in brain DHA accumulation. Using in vivo kinetic approaches, we were able to determine that animals consuming the ALA diet synthesized DHA at rates that exceed the rate of DHA uptake from the plasma into the brain. Importantly, rats consuming the ALA diet had a lower uptake rate of DHA into the brain than rats consuming DHA. As the uptake rate of DHA into the brain has been shown to match rates of brain DHA metabolism (27), it is likely that decreased brain DHA metabolism, in combination with an increased rate of DHA synthesis from ALA, is the reason that brain DHA accretion in rats fed the ALA diet did not differ from the rats fed the DHA diet. The overall results from this study indicate that DHA synthesis from ALA in the rat may be sufficient to maintain brain DHA concentrations in the absence of dietary DHA consumption. Importantly, the steady-state infusion method can be used in humans to calculate an actual DHA synthesis rate that can be compared with brain DHA uptake rates measured in humans with positron emission tomography scanning (57).
Supplementary Material
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- ARA
- arachidonic acid
- AUC
- area under the curve
- DPA
- docosapentaenoic acid
- FAME
- fatty acid methyl ester
- GC-FID
- gas chromatography-flame ionization detection
- LNA
- linoleic acid
- PFB
- pentafluorobenzyl
- TLDA
- TaqMan low density assay
- TLE
- total lipid extract
This study was funded by Bunge Ltd. R.P.B. holds a Canada Research Chair in Brain Lipid Metabolism. LC/MS/MS analyses were performed by the Analytical Facility for Bioactive Molecules in the Centre for the Study of Complex Childhood Diseases at the Hospital for Sick Children and is supported by the Canadian Foundation for Innovation.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of 16 tables.
REFERENCES
- 1.Blasbalg T. L., Hibbeln J. R., Ramsden C. E., Majchrzak S. F., Rawlings R. R. 2011. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 93: 950–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Igarashi M., Ma K., Gao F., Kim H. W., Greenstein D., Rapoport S. I., Rao J. S. 2010. Brain lipid concentrations in bipolar disorder. J. Psychiatr. Res. 44: 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMar J. C., Jr, Ma K., Bell J. M., Igarashi M., Greenstein D., Rapoport S. I. 2006. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J. Lipid Res. 47: 172–180 [DOI] [PubMed] [Google Scholar]
- 4.Lafourcade M., Larrieu T., Mato S., Duffaud A., Sepers M., Matias I., De Smedt-Peyrusse V., Labrousse V. F., Bretillon L., Matute C., et al. 2011. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 14: 345–350 [DOI] [PubMed] [Google Scholar]
- 5.Yurko-Mauro K., McCarthy D., Rom D., Nelson E. B., Ryan A. S., Blackwell A., Salem N., Jr, Stedman M. 2010. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 6: 456–464 [DOI] [PubMed] [Google Scholar]
- 6.Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., Bennett D. A., Wilson R. S., Aggarwal N., Schneider J. 2003. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 60: 940–946 [DOI] [PubMed] [Google Scholar]
- 7.Barceló-Coblijn G., Murphy E. J. 2009. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 48: 355–374 [DOI] [PubMed] [Google Scholar]
- 8.Burdge G. C., Finnegan Y. E., Minihane A. M., Williams C. M., Wootton S. A. 2003. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br. J. Nutr. 90: 311–321 [DOI] [PubMed] [Google Scholar]
- 9.Burdge G. C., Jones A. E., Wootton S. A. 2002. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 88: 355–363 [DOI] [PubMed] [Google Scholar]
- 10.Burdge G. C., Wootton S. A. 2002. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 88: 411–420 [DOI] [PubMed] [Google Scholar]
- 11.Emken E. A., Adlof R. O., Duval S. M., Nelson G. J. 1999. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids. 34: 785–791 [DOI] [PubMed] [Google Scholar]
- 12.Emken E. A., Adlof R. O., Gulley R. M. 1994. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta. 1213: 277–288 [DOI] [PubMed] [Google Scholar]
- 13.Hussein N., Ah-Sing E., Wilkinson P., Leach C., Griffin B. A., Millward D. J. 2005. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 46: 269–280 [DOI] [PubMed] [Google Scholar]
- 14.Mayes C., Burdge G. C., Bingham A., Murphy J. L., Tubman R., Wootton S. A. 2006. Variation in [U-13C] alpha linolenic acid absorption, beta-oxidation and conversion to docosahexaenoic acid in the pre-term infant fed a DHA-enriched formula. Pediatr. Res. 59: 271–275 [DOI] [PubMed] [Google Scholar]
- 15.McCloy U., Ryan M. A., Pencharz P. B., Ross R. J., Cunnane S. C. 2004. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J. Lipid Res. 45: 474–485 [DOI] [PubMed] [Google Scholar]
- 16.Pawlosky R. J., Hibbeln J. R., Lin Y., Goodson S., Riggs P., Sebring N., Brown G. L., Salem N., Jr 2003. Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am. J. Clin. Nutr. 77: 565–572 [DOI] [PubMed] [Google Scholar]
- 17.Pawlosky R. J., Hibbeln J. R., Novotny J. A., Salem N., Jr 2001. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 42: 1257–1265 [PubMed] [Google Scholar]
- 18.Welch A. A., Shakya-Shrestha S., Lentjes M. A., Wareham N. J., Khaw K. T. 2010. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of α-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. Am. J. Clin. Nutr. 92: 1040–1051 [DOI] [PubMed] [Google Scholar]
- 19.Mann N., Pirotta Y., O'Connell S., Li D., Kelly F., Sinclair A. 2006. Fatty acid composition of habitual omnivore and vegetarian diets. Lipids. 41: 637–646 [DOI] [PubMed] [Google Scholar]
- 20.Beezhold B. L., Johnston C. S., Daigle D. R. 2010. Vegetarian diets are associated with healthy mood states: a cross-sectional study in seventh day adventist adults. Nutr. J. 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty M. F. 2001. Does a vegan diet reduce risk for Parkinson's disease? Med. Hypotheses. 57: 318–323 [DOI] [PubMed] [Google Scholar]
- 22.Demmelmair H., Iser B., Rauh-Pfeiffer A., Koletzko B. 1999. Comparison of bolus versus fractionated oral applications of [13C]-linoleic acid in humans. Eur. J. Clin. Invest. 29: 603–609 [DOI] [PubMed] [Google Scholar]
- 23.Hirsch J., Farquhar J. W., Ahrens E. H., Jr, Peterson M. L., Stoffel W. 1960. Studies of adipose tissue in man. A microtechnic for sampling and analysis. Am. J. Clin. Nutr. 8: 499–511 [DOI] [PubMed] [Google Scholar]
- 24.Gao F., Kiesewetter D., Chang L., Ma K., Bell J. M., Rapoport S. I., Igarashi M. 2009. Whole-body synthesis-secretion rates of long-chain n-3 PUFAs from circulating unesterified alpha-linolenic acid in unanesthetized rats. J. Lipid Res. 50: 749–758 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Cunnane S. C., Anderson M. J. 1997. The majority of dietary linoleate in growing rats is beta-oxidized or stored in visceral fat. J. Nutr. 127: 146–152 [DOI] [PubMed] [Google Scholar]
- 26.Robinson P. J., Noronha J., DeGeorge J. J., Freed L. M., Nariai T., Rapoport S. I. 1992. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res. Brain Res. Rev. 17: 187–214 [DOI] [PubMed] [Google Scholar]
- 27.DeMar J. C., Jr, Ma K., Bell J. M., Rapoport S. I. 2004. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J. Neurochem. 91: 1125–1137 [DOI] [PubMed] [Google Scholar]
- 28.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 29.Chen C. T., Liu Z., Ouellet M., Calon F., Bazinet R. P. 2009. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot. Essent. Fatty Acids. 80: 157–163 [DOI] [PubMed] [Google Scholar]
- 30.Rao J. S., Ertley R. N., DeMar J. C., Jr, Rapoport S. I., Bazinet R. P., Lee H. J. 2007. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol. Psychiatry. 12: 151–157 [DOI] [PubMed] [Google Scholar]
- 31.Rao J. S., Ertley R. N., Lee H. J., DeMar J. C., Jr, Arnold J. T., Rapoport S. I., Bazinet R. P. 2007. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry. 12: 36–46 [DOI] [PubMed] [Google Scholar]
- 32.Kothapalli K. S., Anthony J. C., Pan B. S., Hsieh A. T., Nathanielsz P. W., Brenna J. T. 2007. Differential cerebral cortex transcriptomes of baboon neonates consuming moderate and high docosahexaenoic acid formulas. PLoS ONE. 2: e370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitajka K., Sinclair A. J., Weisinger R. S., Weisinger H. S., Mathai M., Jayasooriya A. P., Halver J. E., Puskas L. G. 2004. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc. Natl. Acad. Sci. USA. 101: 10931–10936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuperstein F., Eilam R., Yavin E. 2008. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. J. Neurochem. 106: 662–671 [DOI] [PubMed] [Google Scholar]
- 35.Dyall S. C., Michael G. J., Michael-Titus A. T. 2010. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J. Neurosci. Res. 88: 2091–2102 [DOI] [PubMed] [Google Scholar]
- 36.Lu D. Y., Tsao Y. Y., Leung Y. M., Su K. P. 2010. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for ω-3 fatty acids. Neuropsychopharmacology. 35: 2238–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H. W., Rao J. S., Rapoport S. I., Igarashi M. 2011. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim. Biophys. Acta. 1811: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rapoport S. I., Igarashi M., Gao F. 2010. Quantitative contributions of diet and liver synthesis to docosahexaenoic acid homeostasis. Prostaglandins Leukot. Essent. Fatty Acids. 82: 273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreihofer A. M., Hair C. D., Stepp D. W. 2005. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288: R253–R261 [DOI] [PubMed] [Google Scholar]
- 40.Strife R. J., Murphy R. C. 1984. Stable isotope labelled 5-lipoxygenase metabolites of arachidonic acid: analysis by negative ion chemical ionization mass spectrometry. Prostaglandins Leukot. Med. 13: 1–8 [DOI] [PubMed] [Google Scholar]
- 41.Pawlosky R. J., Sprecher H. W., Salem N., Jr 1992. High sensitivity negative ion GC-MS method for detection of desaturated and chain-elongated products of deuterated linoleic and linolenic acids. J. Lipid Res. 33: 1711–1717 [PubMed] [Google Scholar]
- 42.Robinson D. H., Conrad K. P., Edwards B. R. 1984. Comparison of body fluid compartment sizes in Brattleboro homozygous and Long-Evans rats. Am. J. Physiol. 247: F234–F239 [DOI] [PubMed] [Google Scholar]
- 43.Gao F., Kim H. W., Igarashi M., Kiesewetter D., Chang L., Ma K., Rapoport S. I. 2011. Liver conversion of docosahexaenoic and arachidonic acids from their 18-carbon precursors in rats on a DHA-free but alpha-LNA-containing n-3 PUFA adequate diet. Biochim. Biophys. Acta. 1811: 484–489 [DOI] [PubMed] [Google Scholar]
- 44.Gao F., Taha A. Y., Ma K., Chang L., Kiesewetter D., Rapoport S. I. 2013. Aging decreases rate of docosahexaenoic acid synthesis-secretion from circulating unesterified alpha-linolenic acid by rat liver. Age (Dordr.). 35: 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.de Antueno R. J., Elliot M., Horrobin D. F. 1994. Liver delta 5 and delta 6 desaturase activity differs among laboratory rat strains. Lipids. 29: 327–331 [DOI] [PubMed] [Google Scholar]
- 46.Contreras M. A., Greiner R. S., Chang M. C., Myers C. S., Salem N., Jr, Rapoport S. I. 2000. Nutritional deprivation of alpha-linolenic acid decreases but does not abolish turnover and availability of unacylated docosahexaenoic acid and docosahexaenoyl-CoA in rat brain. J. Neurochem. 75: 2392–2400 [DOI] [PubMed] [Google Scholar]
- 47.Chang M. C., Bell J. M., Purdon A. D., Chikhale E. G., Grange E. 1999. Dynamics of docosahexaenoic acid metabolism in the central nervous system: lack of effect of chronic lithium treatment. Neurochem. Res. 24: 399–406 [DOI] [PubMed] [Google Scholar]
- 48.Orr S. K., Tong J. Y., Kang J. X., Ma D. W., Bazinet R. P. 2010. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochem. Res. 35: 811–819 [DOI] [PubMed] [Google Scholar]
- 49.Abedin L., Lien E. L., Vingrys A. J., Sinclair A. J. 1999. The effects of dietary alpha-linolenic acid compared with docosahexaenoic acid on brain, retina, liver, and heart in the guinea pig. Lipids. 34: 475–482 [DOI] [PubMed] [Google Scholar]
- 50.Igarashi M., Ma K., Chang L., Bell J. M., Rapoport S. I. 2007. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J. Lipid Res. 48: 2463–2470 [DOI] [PubMed] [Google Scholar]
- 51.Rapoport S. I., Igarashi M. 2009. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot. Essent. Fatty Acids. 81: 119–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poumès-Ballihaut C., Langelier B., Houlier F., Alessandri J. M., Durand G., Latge C., Guesnet P. 2001. Comparative bioavailability of dietary alpha-linolenic and docosahexaenoic acids in the growing rat. Lipids. 36: 793–800 [DOI] [PubMed] [Google Scholar]
- 53.Gillingham L. G., Harding S. V., Rideout T. C., Yurkova N., Cunnane S. C., Eck P. K., Jones P. J. 2013. Dietary oils and FADS1-FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am. J. Clin. Nutr. 97: 195–207 [DOI] [PubMed] [Google Scholar]
- 54.Makrides M., Neumann M. A., Byard R. W., Simmer K., Gibson R. A. 1994. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am. J. Clin. Nutr. 60: 189–194 [DOI] [PubMed] [Google Scholar]
- 55.Grossman M. I., Moeller H. C., Palm L. 1955. Effect of lipemia and heparin on free fatty acid concentration of serum in humans. Proc. Soc. Exp. Biol. Med. 90: 106–109 [DOI] [PubMed] [Google Scholar]
- 56.Grossman M. I., Palm L., Becker G. H., Moeller H. C. 1954. Effect of lipemia and heparin on free fatty acid content of rat plasma. Proc. Soc. Exp. Biol. Med. 87: 312–315 [DOI] [PubMed] [Google Scholar]
- 57.Umhau J. C., Zhou W., Carson R. E., Rapoport S. I., Polozova A., Demar J., Hussein N., Bhattacharjee A. K., Ma K., Esposito G., et al. 2009. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res. 50: 1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.