Abstract
The vitamin D signal transduction system involves a series of cytochrome P450-containing sterol hydroxylases to generate and degrade the active hormone, 1α,25-dihydroxyvitamin D3, which serves as a ligand for the vitamin D receptor-mediated transcriptional gene expression described in companion articles in this review series. This review updates our current knowledge of the specific anabolic cytochrome P450s involved in 25- and 1α-hydroxylation, as well as the catabolic cytochrome P450 involved in 24- and 23-hydroxylation steps, which are believed to initiate inactivation of the vitamin D molecule. We focus on the biochemical properties of these enzymes; key residues in their active sites derived from crystal structures and mutagenesis studies; the physiological roles of these enzymes as determined by animal knockout studies and human genetic diseases; and the regulation of these different cytochrome P450s by extracellular ions and peptide modulators. We highlight the importance of these cytochrome P450s in the pathogenesis of kidney disease, metabolic bone disease, and hyperproliferative diseases, such as psoriasis and cancer; as well as explore potential future developments in the field.
Keywords: 1,25-(OH)2D3; CYP2R1; CYP27A1; CYP27B1; CYP24A1; vitamin D-dependent rickets; chronic kidney disease; idiopathic infantile hypercalcemia; vitamin D analog; regioselectivity
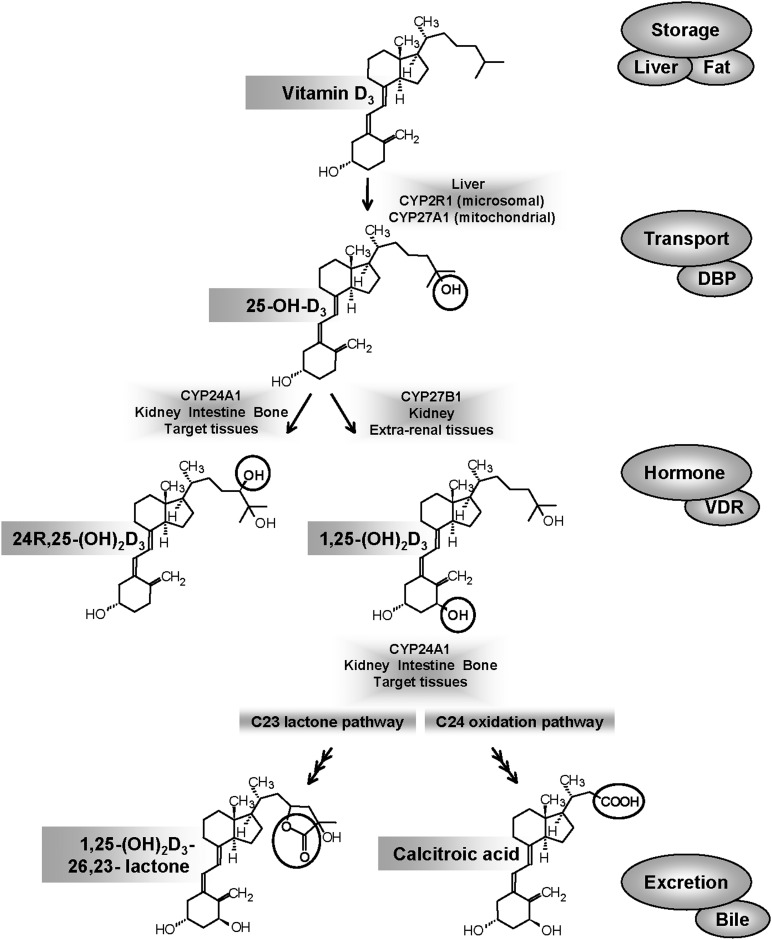
The activation of vitamin D3 is accomplished by sequential steps of 25-hydroxylation to produce the main circulating form, 25-hydroxyvitamin D (25-OH-D3), followed by 1α-hydroxylation to the hormonal form, 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3) (1) (Fig. 1). The initial step of 25-hydroxylation occurs in the liver (2), and the second step occurs both in the kidney and extrarenal sites (3, 4). The fat-soluble vitamin D and its metabolites are transported from one tissue to another on the vitamin D-binding protein (DBP), which shows different affinity for the individual metabolites (5). The cell-surface receptor megalin-cubilin is thought to facilitate the endocytosis of a DBP-bound 25-OH-D3 into a number of cell types, especially kidney cells (6). While it is widely believed that DBP is an essential component of the vitamin D signal transduction system, the DBP-knockout mouse is normocalcemic and exhibits normal tissue distribution of vitamin D metabolites and vitamin D action despite exhibiting very low blood levels of 1,25-(OH)2D3. This unexpected phenotype raised questions about DBP's exact role: essential transporter or buffer against toxicity (7, 8). Work performed on the 25-hydroxylases over the past four decades in humans and a variety of animal species has revealed that several cytochrome P450 enzymes2 (CYP), such as CYP2R1, CYP27A1, CYP3A4, CYP2D25, and perhaps others, are capable of 25-hydroxylation of vitamin D3 or related compounds. These CYPs can be referred to as vitamin D3-25-hydroxylases, and it is CYP2R1 that is emerging as the physiologically relevant enzyme (9). On the other hand, there is no ambiguity over the second step of 1α-hydroxylation or the 25-OH-D3-1α-hydroxylase enzyme responsible, which is carried by a single cytochrome P4502 named CYP27B1 (10–12).
Fig. 1.
Main cytochrome P450-mediated steps involved in vitamin D metabolism are depicted along with the main metabolites of vitamin D. Two other proteins, DBP and VDR, play essential roles in the transport of metabolites from one tissue to another and the key signal transduction events involved in target cell action, respectively.
The inactivation of vitamin D is carried out by the mitochondrial enzyme, 25-hydroxyvitamin D3-24-hydroxylase, first described in the early 1970s and initially believed to be involved solely in the renal 24-hydroxylation of 25-OH-D3 (13). Work performed over the last 35 years has shown that 24-hydroxylase enzyme activity is the result of CYP24A1 (5, 14). CYP24A1 catalyzes the conversion of both 25-OH-D3 and 1,25-(OH)2D3 into a series of 24- and 23-hydroxylated products targeted for excretion along well-established pathways culminating in the water-soluble biliary metabolite calcitroic acid or a 26,23-lactone.
This article assembles the most currently pertinent literature on the activating and inactivating enzymes of vitamin D metabolism and highlights protein structure and enzymatic properties, crystal structures, gene organization, and mutational analysis and regulation. Due to space restrictions, this overview will not cover all of the rich history that went into the early enzymology or cloning of these cytochrome P450-containing enzymes, much of which has been extensively reviewed elsewhere (5, 15–17).
General information regarding vitamin D hydroxylases
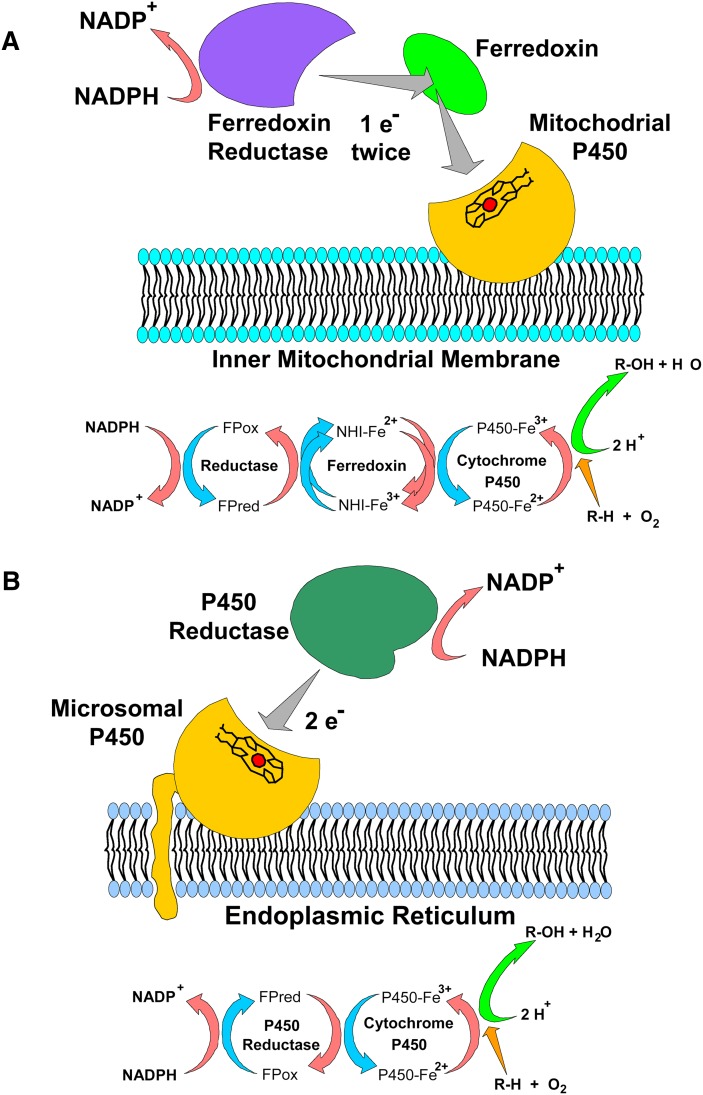
Table 1 summarizes pertinent information about all of the vitamin D-metabolizing CYPs, including both the activating and inactivating enzymes. CYPs are classified into two main subtypes based upon their subcellular location: microsomal or mitochondrial, with vitamin D metabolism featuring both subtypes (14). Both mitochondrial and microsomal CYP subtypes do not function alone but are components of electron transport chains. As with all mitochondrial CYPs, the functional enzyme activity for mitochondrial vitamin D-related CYPs (e.g., CYP27A1, CYP27B1, CYP24A1) requires the assistance of two additional electron-transporting proteins consisting of a general-purpose ferredoxin reductase, a general-purpose ferredoxin, and a highly specific CYP (Fig. 2A). In contrast, microsomal CYPs (e.g., CYP2R1) require a single general-purpose protein, NADPH-cytochrome P450 reductase (Fig. 2B). All of the vitamin D-related CYPs catalyze single or multiple hydroxylation reactions on specific carbons of the vitamin D substrate using a transient, heme-bound, oxygenated-iron (Fe-O) intermediate. The exact site of hydroxylation, termed regioselectivity, can be somewhat variable with vitamin D related-CYPs; human CYP24A1 has been documented to hydroxylate at C23, C24, or C26.
TABLE 1.
Vitamin D-metabolizing CYPs
| P450 | Species | Tissue Location | Subcellular Location | Size (aa) | Human Gene Locus | Enzyme Activity | Disease State Human or (Mouse Knockout) | Function |
| CYP2R1 | Human | Liver | Microsomes | 501 | 11p15.2 | 25-Hydroxylation of D3 | VDDR type 1B (unknown) | Physiological |
| >47 species | 25-Hydroxylation of D2 | 25-Hydroxylase | ||||||
| CYP27A1 | Human | Liver | Mitochondria | 531 | 2q33-qter | 25-Hydroxylation of D3 | CTX | Pharmacological |
| >56 species | Macrophage | 24-Hydroxylation of D2 | 25-Hydroxylase | |||||
| CYP2C11 | Rat | Liver (male) | Microsomes | 500 | 25-Hydroxylation of D3 | |||
| 25-Hydroxylation of D2 | ||||||||
| CYP2D25 | Pig | Liver | Microsomes | 500 | 25-Hydroxylation of D3 | |||
| CYP2J2 | Human | Liver | Microsomes | 502 | 1p31.3-p31.2 | 25-Hydroxylation of D2 | ||
| CYP2J3 | Rat | 25-Hydroxylation of D3 | ||||||
| CYP3A4 | Human | Liver | Microsomes | 503 | 7q22.1 | 25-Hydroxylation of D2 4α/4β-Hydroxylation of vitamin D3 | ||
| Intestine | ||||||||
| CYP27B1 | Human | Kidney | Mitochondrial | 508 | 12q13.1-q13.3 | 1α-Hydroxylation of D3 | VDDR-1A | 1α-Hydroxylase |
| >39 species | 1α-Hydroxylation of D2 | (Rickets) | ||||||
| CYP24A1 | Human | Target tissue | Mitochondrial | 514 | 20q13.2-q13.3 | 23- and 24-Hydroxylation of 25(OH)D/1,25(OH)2D | 24-Hydroxylase | |
| >51 species | ||||||||
Reproduced with permission from Ref. 15.
Fig. 2.
Electron transport chains and protein components of the vitamin D hydroxylases. (A) In mitochondria, NADPH is oxidized by the flavoprotein ferredoxin reductase, which transfers single electrons through a pool of ferredoxin iron-sulfur proteins to the mitochondrial P450s on the inner membrane. (B) In the endoplasmic reticulum, electron equivalents from NADPH are captured by the NADPH P450 reductase (also known as P450 oxidoreductase, POR). The two electrons from NADPH are transferred sequentially to the microsomal P450 (e.g., CYP2R1). (Used from Ref. 14 with permission.)
From alignments of the vitamin D-related CYPs (Fig. 3), it is immediately apparent that all CYP proteins possess around 500 amino acids and a size of 50–55 kDa, featuring abundant highly conserved residues, which suggests a common secondary structure with multiple highly conserved helices (designated A–L) connected by loops and β-sheet structures. All CYPs possess a cysteine residue and other residues that covalently bind and align the heme group, in addition to several other domains for interaction with the electron-transferring machinery, such as ferredoxin or NADPH-cytochrome P450 reductase. The N-terminus is thought to insert into the endoplasmic reticular membrane for microsomal CYPs or the inner mitochondrial membrane for mitochondrial CYPs. The substrate-binding pocket is formed by several secondary structures folded around the distal face of the heme-group so that the substrate can be brought to within 3.2 Å of the iron atom for hydroxylation. An analysis of the heme-ligand geometry of 49 substrate-bound crystal structures revealed the hydroxylation target carbons actually adopt a spatially conserved orientation to the heme iron, and this can be triangulated for use in docking studies (18).
Fig. 3.
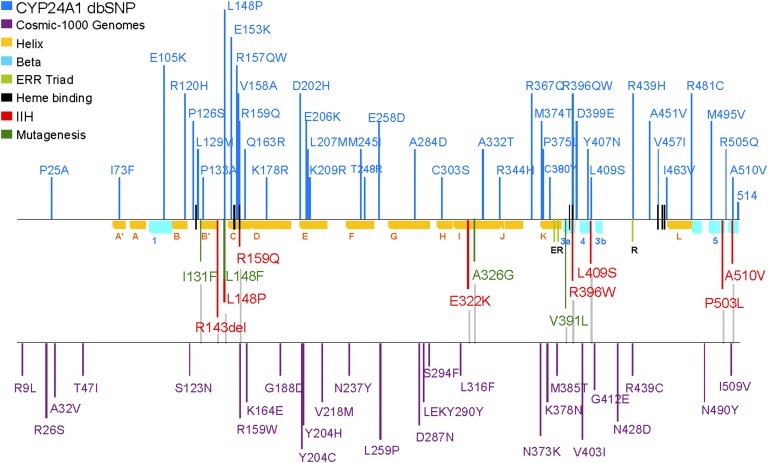
Sequence alignments of structurally determined or predicted secondary structures for vitamin D hydroxylases. Residues conserved in both mitochondrial and microsomal P450s (shaded) are structurally or functionally important, although elements of substrate recognition, binding, and specificity are inherently less conserved. Heme-binding residues and ERR-triad residues are also indicated. Locations of missense mutations leading to CYP2R1 deficiency (VDDR type 1B), CYP27A1 deficiency (CTX), CYP27B1 deficiency (VDDR type 1A), and CYP24A1 deficiency (IIH) are indicated by red shading. SNPs from dbSNP, Ensembl, Sanger Cosmic, and 1000 Genomes are shown in blue.
Attempts to identify the key substrate-binding residues were originally guided by homology models (18–22) based upon 10–20 available crystal structures from unrelated soluble prokaryotic CYPs. Recently, the study of the active site of vitamin D-related CYPs has been further advanced by the emergence of X-ray crystallography-derived models of CYP2R1 (23) and CYP24A1 (24) (Fig. 4). In addition, two bacterial vitamin D hydroxylases capable of sequentially hydroxylating vitamin D3 to 1,25-(OH)2D3 at production levels, CYP105A1 from Streptomyces griseolus (2zbz.pdb) (25) and P450 Vdh from Pseudonocardia autotrophica (3a4g.pdb) (26), have been determined. Mutational analyses to pinpoint amino-acid residues involved in contact with the main functional groups (hydroxyls) or hydrophobic cis-triene of the vitamin D substrate have been largely completed. Future work to define residues closest to the hydroxylation-sensitive 1α-position in CYP27B1 or the side-chain C-23 to C-27 carbons in the side-chain hydroxylases (CYP2R1, CYP24A1, and CYP27A1) are currently in progress.
Fig. 4.
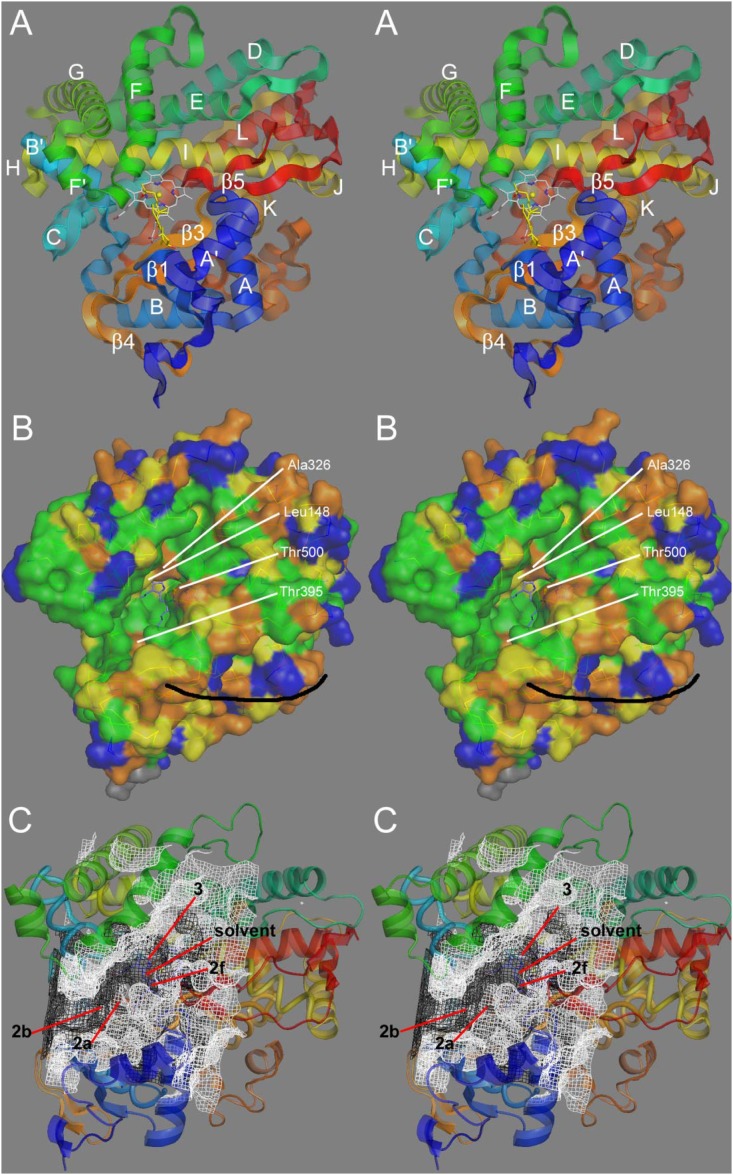
Structural analysis of CYP24A1. (A) Stereographic view of the CYP24A1 crystal structure (3k9v.pdb) with labeled secondary structures. An analysis of heme-ligand geometry in cytochrome P450s permitted docking of the substrate 1,25-(OH)2D3 (yellow) into the heme distal cavity active site. (B) Degree of amino-acid conservation in CYP24A1 observed across approximately 50 species orthologs: green (>95%), yellow (>85%), orange (>60%), and blue (<60%). The black curve indicates a possible membrane-binding surface. (C) Open active site cavity/cleft (white mesh) and an earlier model of a closed cavity (black mesh). Various access/egress channel trajectories are indicated. (Used with permission from Ref. 24).
Vitamin D3-25-hydroxylases
As outlined above, there has been no shortage of CYPs proposed as candidates for the title of physiologically relevant vitamin D3-25-hydroxylase. Early work suggested that there were both mitochondrial and microsomal 25-hydroxylase enzyme activities (27, 28), and experiments with the perfused rat liver suggested that these might be a low-affinity, high-capacity mitochondrial enzyme and a high-affinity, low-capacity microsomal enzyme (29) (reviewed in Ref. 5). More than three decades later, we can use several criteria to answer to the question, Which CYP is the physiologically important 25-hydroxylase in vivo? These criteria include i) substrate specificity toward D3 and D2 substrates; ii) Km and Vmax and enzymatic properties of the expressed enzyme; iii) tissue and subcellular location; iv) occurrence of natural mutations; and v) disease consequence of gene deletion or mutation in human and animal models.
Currently, based upon available data for these criteria, we can conclude that the answer to the above question is still not fully resolved, as there is still insufficient evidence that deletion of any single CYP results in a rickets phenotype in the mouse or vitamin D deficiency/rickets in humans. Indeed, it is possible that in vivo several CYPs could contribute to 25-hydroxylation of vitamin D and its analogs under a broad substrate concentration range. However, all available evidence suggests that CYP2R1 is probably the physiologically relevant enzyme at normal vitamin D concentrations (low nanomolar), but that it is possibly backed up by others at substrate concentrations in the pharmacological range (high nanomolar and low micromolar). Consequently, we have reviewed relevant information, first about CYP2R1 and then about the other candidate CYPs.
CYP2R1
The discovery of CYP2R1 in 2003 (30) arguably ended a three-decade search for the elusive, physiologically relevant vitamin D3-25-hydroxylase. CYP2R1 satisfies most, if not all, of the criteria listed above to describe the location and properties of the enzyme activity first defined in the early 1970s (27, 28). CYP2R1, a 501 amino-acid liver microsomal cytochrome P450, was cloned from mouse and human, and it was shown by real-time PCR to be primarily expressed in liver and testis (30). The full amino-acid sequence of hCYP2R1 is shown in Fig. 3, and alignments of all known CYP2R1 isoforms (current databases hold ∼50 species) reveal that it is highly conserved compared with other CYP2 family members, which are not highly conserved between species presumably because they are usually broad-specificity, xenobiotic-metabolizing enzymes (31). The initial studies demonstrated that CYP2R1, unlike all other putative 25-hydroxylases, would 25-hydroxylate both vitamin D2 and vitamin D3 equally well at physiologically relevant substrate concentrations (30).
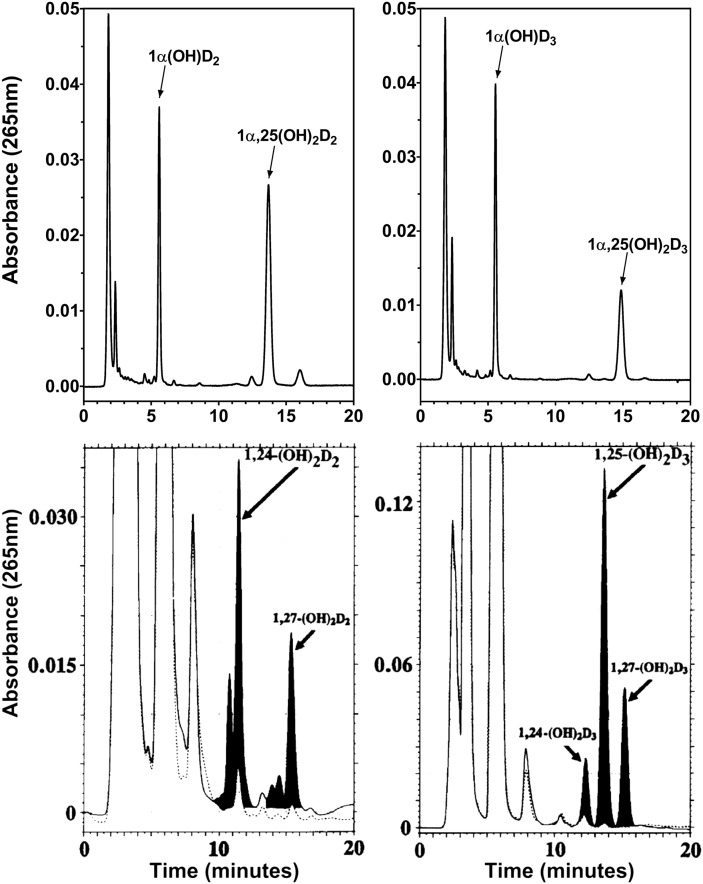
Subsequent work (32) using nanomolar substrate concentrations of [3H]1α-OH-D2, a vitamin D2 analog, has reinforced the finding that transfected mouse and human CYP2R1 enzymes are able to synthesize the predominant in vivo metabolite 1,25-(OH)2D2, and not 1,24-(OH)2D2, the minor in vivo product of 1α-OH-D2,which is also the major in vitro product of 1α-OH-D2 incubated with CYP27A1. Recent work (23) using bacterially expressed human CYP2R1 protein in a solubilized system revealed enzyme kinetic properties consistent with both of the earlier studies. hCYP2R1 showed Km values of 4.4, 11.3, and 15.8 µM for vitamin D3, 1α-OH-D2, and 1α-OH-D3 respectively, while Kcat values of 0.48, 0.45, and 1.17 mol/min/mol P450 were observed for the same three substrates. As defined in the associated LC analysis (Fig. 5A), the regioselectivity of hCYP2R1 was clearly confined to the C-25 position with no peaks corresponding to 24- or 26-hydroxylated products, which is in sharp contrast to the findings with CYP27A1 (23). Furthermore, CYP2R1 failed to metabolize 25-OH-D3, cholesterol or 7-dehydrocholesterol, thereby demonstrating a high specificity for the C-25 position on vitamin D but not for other sterol substrates. Thus, the evidence suggests that CYP2R1 has the enzymatic properties needed for a vitamin D-25-hydroxylase capable of appropriately activating known vitamin D precursors in vivo.
Fig. 5.
Comparison of the enzymatic properties of two vitamin D-25-hydroxylases: CYP2R1 and CYP27A1. The substrates used are the prodrugs 1α-OH-D2 and 1α-OH-D3 to gauge the site and efficiency of the two vitamin D 25-hydroxylases toward D2 and D3 family members. Chromatograms of metabolites from in vitro reconstituted CYP2R1 enzyme (upper panels) (23) and transiently transfected CYP27A1 in COS-1 cells (lower panels) (39). Upper left and lower left panels represent traces from incubation with 1α-OH-D2. Upper right and lower right panels represent traces from incubation with 1α-OH-D3. The lack of CYP27A1-mediated 25-hydroxylation toward 1α-OH-D2 is evident, although 1α,24(OH)2D2 metabolite is detectable in the serum of animals given large doses of vitamin D2 (41) and is a VDR agonist.
Strushkevich et al. (23) also solved the crystal structure of a functional form of CYP2R1 in complex with vitamin D3, which represented the first crystal structure of a vitamin D-related CYP. The crystal structure generally confirmed the helical nature and binding pocket of CYP2R1 predicted from other CYPs using homology modeling (19). Cocrystallized vitamin D3 in the CYP2R1 occupied a position with the side chain pointing toward the heme group, but somewhat paradoxically, it was not optimally placed for hydroxylation because the C-25 carbon was 6.5 Å from the heme iron. It is unclear at this point whether the substrate was trapped in an access/egress channel or whether there is some other explanation for the data.
Another piece of evidence that strengthens the case for CYP2R1 being the vitamin D3-25-hydroxylase is the finding of a human Leu99Pro mutation in a Nigerian family that results in vitamin D-dependent rickets (VDDR) type 1B (33). This disease was postulated four decades ago (34) following the elucidation of vitamin D metabolism. Unfortunately, the genetic nature of the Leu99Pro mutation of CYP2R1 was determined by Cheng et al. (9) a decade after the initial identification of the Nigerian rachitic patient (33), making patient and family follow-up difficult. However, subsequent genetic analysis of exon 2 of CYP2R1 (9) in 50 Nigerian individuals revealed one heterozygote with the Leu99Pro mutation, suggesting that there may be a founder gene effect in the Nigerian population in which vitamin D deficiency is quite prevalent (35). Though the Leu99 residue is not in a region of the CYP2R1 coding for substrate-binding domain, it is involved in water-mediated hydrogen bonding to the Arg445 amide nitrogen located three residues from the heme-coordinating Cys448, and thus a Leu99Pro mutation probably results in a misfolded protein with little or no enzyme activity. Numerous attempts to bacterially express hCYP2R1 with a Leu99Pro mutation at the same time as the wild-type hCYP2R1 failed, leading Strushkevich et al. (23) to conclude that CYP2R1 with Leu99Pro is misfolded or shows poor protein stability. Recently, DeLuca's group generated a CYP2R1 knockout mouse, and preliminary studies suggest that serum 25-OH-D levels are 50% reduced compared with wild-type or heterozygous littermates (36), implying that although CYP2R1 is a major physiologically relevant vitamin D3-25-hydroxylase, there is some redundancy in the vitamin D3-25-hydroxylase “family” of enzymes that can partially compensate for the deletion of CYP2R1. Furthermore, a genome-wide association study of the genetic determinants of serum 25-hydroxyvitamin D concentrations (37) concluded that variants at the chromosomal locus for CYP2R1 (11p15) was the second strongest association of only four sites, with DBP (formerly known as GC), CYP24A1, and 7-dehydrocholesterol reductase being the others. Notably, variants of the other 25-hydroxylases, such as CYP27A1, were not identified to be associated with serum 25-OH-D concentrations, again arguing that it plays no role in 25-hydroxylation of vitamin D at physiological substrate concentrations. Several polymorphisms of the CYP2R1 gene have now been identified in various single nucleotide polymorphism (SNP) databases (Fig. 3).
CYP27A1
CYP27A1 was the first cloned vitamin D-25-hydroxylase in the early 1990s, discovered by David Russell's group (38). CYP27A1, a liver mitochondrial cytochrome P450 with a homolog in more than 56 species, is 531 amino acids in size in humans. It was originally cloned from rabbit, and it was also cloned from human (38, 39). Even the earliest claims that CYP27A1 was a vitamin D-25-hydroxylase were controversial, as the purified liver enzyme seemed to be a better cholesterol-26-hydroxylase than vitamin D-25-hydroxylase; thus, it was proposed as a bifunctional enzyme involved in both bile-acid and vitamin D metabolism (40). Work with the recombinant protein demonstrated that CYP27A1 is a low-affinity, high-capacity vitamin D3-25-hydroxylase that also 25-hydroxylates 1α-OH-D3, but it seems incapable of the 25-hydroxylation of vitamin D2 or 1α-OH-D2 catalyzing 24-hydroxylation to 24-OH-D2 or 1,24S-(OH)2D2 (Fig. 5B) (39, 41). Fig. 5 shows that although CYP27A1 exhibits the ability to 24- and 26-hydroxylate 1α-OH-D3, its primary site of hydroxylation is C25, whereas with 1α-OH-D2, this switches to C24-hydroxylation with some 26-hydroxylation. The inability of CYP27A1 enzymatic properties to explain the formation of 25-OH-D2 in animals in vivo became the main impetus for the search for an alternative 25-hydroxylase that culminated in CYP2R1 (30).
Parallel enzymatic work with bile-acid substrates clearly showed that CYP27A1 could 25- and 27-hydroxylate the side chain of the cholesterol and play a role in the trimming of C-27 sterols without the secosteroid, open B-ring nucleus (42). The same workers performed mutagenesis studies that established the important residues involved in ferredoxin interaction. Although there is currently no crystal structure of CYP27A1, numerous homology models have been proposed for the enzyme predicted from other CYPs (19, 43). Until the recent emergence of crystal structures of CYP2R1 and CYP24A1, these models offered the best structural insights into general structure and substrate-binding pockets of vitamin D-related CYPs.
Several pieces of biological or clinical information argue against CYP27A1 being the physiologically relevant vitamin D-25-hydroxylase. First, the CYP27A1-null mouse phenotype does not include rickets or any other bone lesion (44). However, this animal model is complicated by the absence of any significant bile-acid defect. Second, though human CYP27A1 mutations have been documented in the literature, these result in a bile acid-related condition known as cerebrotendinous xanthomatosis (CTX) rather than rickets (45) (Fig. 3). Affected individuals usually have normal serum 25-OH-D, though some of these individuals can exhibit low serum 25-OH-D and a type of osteoporosis (46). Current opinion is that CTX is a defect in bile-acid metabolism and that the bone disease is the result of malabsorption of dietary vitamin D caused by bile-acid insufficiency rather than inadequate 25-hydroxylase enzyme activity (47). Third, the genome-wide association study of the determinants of serum 25-hydroxyvitamin D concentrations (37) concluded that variants at the locus for CYP2R1 (11p15) but not for CYP27A1 are associated with variations in serum 25-OH-D concentrations.
A more likely possibility for an in vivo role for CYP27A1 in vitamin D metabolism is as a pharmacologically relevant 25-hydroxylase for 25-hydroxylation of 1α-hydroxylated vitamin D analogs (1α-OH-D3 and 1α-OH-D2), popular prodrugs in the treatment of osteoporosis/ metabolic bone disease and the secondary hyperparathyroidism associated with chronic kidney disease-metabolic bone disease (CKD-MBD) (48). It is worth noting that in vitro CYP27A1 synthesizes 1,25-(OH)2D3 and 1,24S-(OH)2D2, metabolites from 1α-OH-D3 and 1α-OH-D2, respectively, and that 24-hydroxylated compounds, such as 1,24S-(OH)2D2, are also observed in vivo following administration of pharmacological amounts of vitamin D2 compounds (41, 49, 50). It will be interesting to assess serum 25-OH-D levels, especially after administration of graded doses of vitamin D3 in progeny of double knockouts from CYP27A-null- and CYP2R1-null mice currently being generated by DeLuca's group (36). Thus CYP27A1 may contribute to the metabolism of vitamin D compounds, including 1α-OH-D3 and 1α-OH-D2, when present at high concentrations, but it is unclear whether it is involved in vitamin D metabolism at physiologically relevant concentrations.
Other potential 25-hydroxylases
Over the past three decades, there have been numerous reports that in addition to CYP2R1 and CYP27A1, a number of other specific microsomal CYPs, partially purified from tissues or cells or studied in bacterial or mammalian expression systems, can 25-hydroxylate a spectrum of vitamin D substrates but only at micromolar substrate concentrations. These include CYP2D11, CYP2D25, CYP2J2, CYP2J3, and CYP3A4 (see Table 1). Some of these are expressed in one mammalian species (e.g., pig or rat) and have no obvious human equivalent; show gender differences not observed for human vitamin D-25-hydroxylation in vivo; or fail to 25-hydroxylate vitamin D2 or 1α-OH-D2. Again, as with CYP27A1, lack of regiospecificity for the C-25 position surfaces as an important distinguishing feature compared with CYP2R1, as many other microsomal CYPs (e.g., CYP3A4) catalyze the 24-hydroxylation of vitamin D2 and D3 compounds (51–53). Based upon the emergence of the strong case for CYP2R1 being the vitamin D-25-hydroxylase, the pursuit of these other nonspecific CYPs is becoming less urgent, but at least one of these, namely, CYP3A4, deserves special mention.
A multifunctional nonspecific enzyme such as CYP3A4, which is estimated to metabolize up to 50% of known drugs, would probably not attract special interest here were it not for the fact that recently it been shown to be selectively induced by 1,25-(OH)2D3 in the intestine (53–55). CYP3A4 has been shown to 24- and 25-hydroxylate vitamin D2 substrates more efficiently than vitamin D3 substrates (51, 52), and it also 23R- and 24S-hydroxylates the already 25-hydroxylated 1,25-(OH)2D3 (53). However, CYP3A4 is known to have Km values for vitamin D compounds in the micromolar range, a property that questions its physiological but not pharmacological relevance. Recent work (56, 57) has demonstrated that both human intestinal microsomes and recombinant CYP3A4 break down 1,25-(OH)2D2 at a significantly faster rate than 1,25-(OH)2D3, suggesting that this nonspecific cytochrome P450 might limit vitamin D2 action preferentially in selective target cells (e.g., intestine) in which it is expressed, particularly in the pharmacological dose range. Such an observation may also offer an explanation for the well-documented lower toxicity of vitamin D2 compounds compared with vitamin D3 compounds in vivo, the vitamin D2 compounds not causing such severe hypercalcemia by virtue of reduced effects on intestinal calcium absorption. The same type of mechanism involving differential induction of nonspecific CYPs, such as CYP3A4, may also underlie the occasional reports of drug-drug interactions involving vitamin D, in which coadministered drug classes (e.g., anticonvulsants) (5, 58, 59) cause accelerated degradation of vitamin D2 over vitamin D3. Thus, while CYP3A4 might be occasionally considered a vitamin D-25-hydroxylase, its main relevance to vitamin D metabolism may be in its involvement in inactivation of vitamin D compounds at high concentrations.
25-hydroxyvitamin D-1α-hydroxylase (CYP27B1)
The 25-hydroxyvitamin D-1α-hydroxylase has been investigated virtually from the day that its product 1,25-(OH)2D3 was discovered (3, 60). The need to define the enzyme in biochemical terms became urgent as soon as it became apparent that the 25-hydroxyvitamin D-1α-hydroxylase was a central regulatory axis of the calcium and phosphate homeostatic systems, subject to upregulation by parathyroid hormone (PTH), low Ca2+, and low PO43- levels (1, 61, 62). It was quickly recognized that serum 1,25-(OH)2D3 was predominantly made in the kidney (3, 63), with a PTH-regulated form located in the proximal convoluted tubule and a calcitonin-regulated form in the proximal straight tubule (64–67). Biochemical investigations showed that the enzyme involved was a mixed-function oxidase with a cytochrome P450 component (68). The exact molecular description of this enzyme took another 25 years to unravel. In the meantime, there were emerging reports of so-called “extrarenal” 25-hydroxyvitamin D-1α-hydroxylase activity in several sites, including placenta, bone, and macrophage (69–74), which evoked the question whether there was more than one cytochrome P450 with 25-hydroxyvitamin D-1α-hydroxylase activity. Unlike with the liver vitamin D-25-hydroxylase, this does not appear to be the case, and with the cloning of CYP27B1 as a single gene, the story has become much simpler.
In 1997, several groups coincidentally cloned, sequenced, and characterized CYP27B1 from rat, mouse, and human species (10, 11, 75). Although many of these groups used kidney libraries as the source of the enzyme, interestingly, other groups reported finding the same CYP27B1mRNA in keratinocyte (76) and human colonic cell HT-29 (77) libraries, suggesting that the enzyme was identical in all locations. Subsequently, it has been confirmed that the CYP27B1 protein is identical in all locations (4, 78), whether renal or extrarenal, although the regulation in these different tissue locations must involve different hormones and effectors.
hCYP27B1 is a 507 amino-acid protein with a molecular mass of ∼55 kDa. The best available information suggests that the enzyme 1α-hydroxylates 25-OH-D2 and 25-OH-D3 equally efficiently to give the active metabolite of each form of the vitamin. The genetic rachitic condition termed vitamin D-dependency rickets (VDDR) type 1A, in which the 1α-hydroxylase enzyme is absent or defective presumably due to mutation of CYP27B1, was recognized in the early 1970s by Fraser and colleagues (34, 79), who showed that patients had low or absent serum 1,25-(OH)2D and could be successfully treated using small amounts of synthetic 1,25-(OH)2D3. VDDR type 1A involves a resistant-rickets phenotype, characterized by hypocalcemia, hypophosphatemia, secondary hyperparathyroidism, and undermineralized bone. It is cured by physiological (microgram) amounts of 1,25-(OH)2D3 or pharmacological (milligram) amounts of 25-OH-D3 or vitamin D, which is consistent with a block in 1α-hydroxylation activity (34). Subsequent work mapped the CYP27B1 gene to 12q13.1-q13.3, which is the same location established for the VDDR type 1A disease (10). Human CYP27B1 mutations occur throughout the gene (Fig. 3), resulting in defective and misfolded proteins with little or no activity (80–84).
At least two groups have created CYP27B1-null mice (85, 86) that exhibit a lack of 1α-hydroxylated metabolites in the blood and tissues, revealing that CYP27B1 is the sole source of 1,25-(OH)2D in the body. The mouse phenotype mirrors human VDDR type 1 in terms of resistant rickets. The animals also show a reduction in CD4- and CD8-positive peripheral lymphocytes, and female mice are infertile (85). Detailed bone histomorphometric analyses of the CYP27B1 and CYP27B1/PTH double-knockout mice established that 1,25-(OH)2D3 deficiency resulted in epiphyseal dysgenesis and only minor changes in trabecular bone volume (87). Bikle and colleagues showed that CYP27B1 is also required for optimal epidermal differentiation and permeability barrier homeostasis in the skin of mice (88). Administration of a normal diet supplemented with either small amounts of 1,25-(OH)2D3 or use of a high-calcium “rescue diet” largely corrects the mineral metabolism and bone defects seen in the CYP27B1-null mouse (89–92). Global CYP27B1-null animals given high-calcium intakes for several months show growth plate abnormalities, probably exacerbated by secondary hyperparathyroidism and hypophosphatemia (85, 87). However, tissue-specific knockout of the mouse CYP27B1 gene in chondrocytes suggests that growth plate abnormalities are not merely the result of blood mineral ion defects and that local production of 1,25-(OH)2D3 plays a role in growth plate development (93, 94).
The availability of specific CYP27B1mRNA and anti-CYP27B1 protein antibodies has allowed a more rigorous exploration of the extrarenal expression of the enzyme. Diaz et al. (95) used Northern analysis and RT-PCR to examine mRNA expression in human synctiotrophoblasts and concluded that there was CYP27B1 expression in human placenta. Using similar techniques, several groups reported low but detectable expression of CYP27B1 in a variety of cultured cell lines (e.g., prostate and colonic cells) (96–98). Immunohistochemistry data from analysis of animal and human tissues has revealed the presence of the CYP27B1 protein in several tissues purported to express 1α-hydroxylase activity (e.g., skin, colon, macrophage, prostate, breast) (4, 78). Not all studies have supported the conclusion that CYP27B1 is expressed outside of the kidney in normal, nonpregnant animals. Using a β-galactosidase reporter system, Vanhooke et al. (92) found no evidence for expression of CYP27B1 in murine skin or primary keratinocytes, although there was expression in kidney and placenta. It is possible that the lack of detection of low-abundance, extrarenal CYP27B1 transcripts is due to some inherent insensitivity of the β-galactosidase reporter system, whereas it is sufficiently sensitive to detect abundant renal CYP27B1 transcripts.
Despite the fact that the existence of the extrarenal 1α-hydroxylase remains tentative, there has been much speculation about its possible role of this enzyme in health and disease (99–101). It is now widely believed the enzyme exists in nonrenal tissues to boost local production of cellular 1,25-(OH)2D3 in a paracrine/autocrine system. Such a role would suggest that cellular 1,25-(OH)2D3 concentrations in extrarenal CYP27B1 tissues might be higher than in the tissues of the classical endocrine system (e.g., intestine, bone, parathyroid gland), which depend entirely on renally synthesized, blood-borne 1,25-(OH)2D3 at concentrations ∼10−10M. Cell differentiation and antiproliferative genes regulated in extrarenal tissues (e.g., macrophage, colon, prostate, skin) may require higher 1,25-(OH)2D3 concentrations. A role for the extrarenal CYP27B1 is also consistent with the epidemiological finding that serum 25-OH-D levels are associated with various health outcomes from bone health to cardiovascular health. In particular, low serum 25-OH-D levels are associated with increased mortality for colon, breast, and prostate cancer; increased autoimmune diseases and greater susceptibility to tuberculosis; and increased cardiovascular diseases and hypertension. The presence of CYP27B1 in cells of the colon, breast, prostate, monocyte/macrophage, and vasculature could explain why serum 25-OH-D levels are so critical to the normal functioning of these tissues.
CKD-MBD, with five stages defined by decreasing glomerular filtration rate (GFR), is well known to be accompanied by a gradual fall in serum 1,25-(OH)2D3 (normal range, 20–60 pg/ml), widely assumed to be due to a gradual decline in CYP27B1 activity (102). Whether this is in turn due to loss of the CYP27B1 protein caused by renal damage is debatable. It is possible that the fall in serum 1,25-(OH)2D3 to values below 20 pg/ml by the end of CKD stage 2 could be due in part to increased fibroblast growth factor-23 (FGF-23) levels, a known downregulator of CYP27B1 expression in normal kidney cells (103). Recent reports of marked increases in FGF-23 levels in CKD stage 5 dialysis patients with phosphate retention are consistent with FGF-23 playing a major role in vitamin D dysregulation and mortality in chronic kidney disease (104).
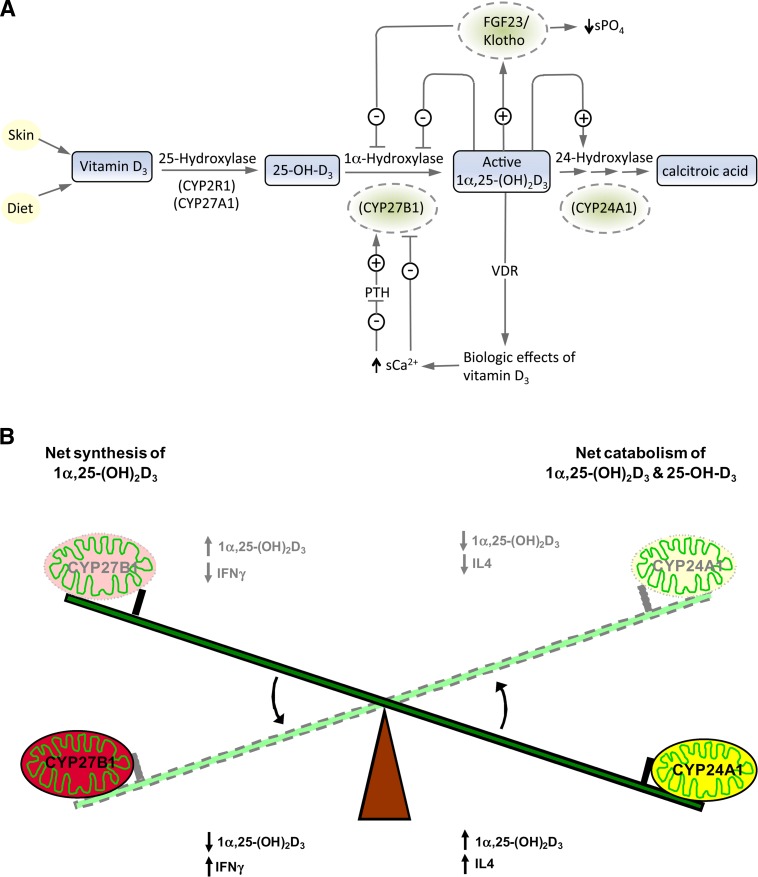
The regulation of CYP27B1 (summarized in Fig. 6A) has been a major focus since the enzyme's discovery in the early 1970s (1). Ca2+ and PO43− ions, probably through the hormones PTH, calcitonin, and FGF-23, regulate CYP27B1 expression through complex signal transduction processes (67, 105–107), whereas 1,25-(OH)2D3, the end-product of the enzyme, downregulates its own synthesis at the transcriptional level by vitamin D receptor (VDR)-mediated action at the level of the CYP27B1 gene promoter (106, 108, 109). Evidence is also accumulating to suggest that CYP27B1 expression is downregulated through DNA methylation and upregulated through DNA demethylation (109, 110). Although it is logical to isolate CYP27B1 from the rest of the calcium/phosphate homeostatic system, there is a reciprocity between CYP27B1 and CYP24A1 that suggests that the factors that upregulate one enzyme downregulate the other. This is evident in the isolated perfused kidney from the rat fed a low-calcium vitamin D-deficient diet or a low-PO4 vitamin D-deficient diet, which is in the 1α-hydroxylation mode and which over a 4 h perfusion period after being exposed to its 25-OH-D3 substrate, turns off CYP27B1 expression and 1α-hydroxylation and turns on CYP24A1 and 24-hydroxylation (111). The vitamin D metabolic system seems ideally designed to avoid synthesis of excessive amounts of the hormone and also to degrade the hormone, or even its substrate, by superinduction of catabolic processes, including CYP24A1. In the VDR-null mouse, we see a complete breakdown of this autoregulation process because CYP27B1 is not suppressed by excessive 1,25-(OH)2D3 production and CYP24A1 is not actively stimulated, both steps requiring VDR-mediated events.
Fig. 6.
(A) Physiological roles of renal CYP27B1 and CYP24A1 in calcium and phosphate homeostasis. Ca and PO4 ions through PTH, FGF-23, and the hormone 1,25-(OH)2D3 play key roles in determining the balance between the synthesis and degradation of 25-OH-D3 and 1α,25-(OH)2D3 by regulating renal CYP27B1 and CYP24A1, respectively. (B) Putative roles of extrarenal CYP27B1 and CYP24A1 in establishing the optimal target cell concentration of 1,25-(OH)2D3 for regulation of gene expression in noncalcemic functions. Cytokines are believed to regulate these extrarenal/target cell enzymes. Normal cells balance synthesis and degradation to generate optimal levels of 1,25-(OH)2D3. Cancer cells show reduced CYP27B1 and increased CYP24A1 expression. (Reproduced from Ref. 166)
The regulation of the extrarenal 1α-hydroxylase has also received attention over the last couple of decades. What is clear is that the renal and extrarenal enzymes are regulated by different factors: the kidney CYP27B1 by the calcium/phosphate homeostatic hormones described above, and the extrarenal enzyme by tissue-specific factors, including cytokines (Fig. 6B). Adams et al. (112) have shown that macrophages in the granulomatous condition sarcoidosis are driven by proinflammatory cytokines, such as γ-interferon, which also stimulate extrarenal CYP27B1 activity and can cause excessive serum 1,25-(OH)2D3, which left unchecked, results in hypercalciuria and hypercalcemia. The mechanism of γ-interferon-mediated upregulation of CYP27B1 appears to involve the Janus kinase-signal transducer and activator of transcription MAPK and nuclear factor-kappa B pathways, with a crucial role for the transcription factor CCAAT/enhancer binding protein β (113, 114). Also, the usual CYP24A1 counterregulatory mechanism seems to have been replaced in the monocyte/macrophage system by an inactive splice-variant of CYP24A1 (115). Thus, the nature of the downregulator(s) of the extrarenal CYP27B1 in these and other cells of the immune system remains largely unknown.
Recently, the normal upregulation of the monocyte/macrophage CYP27B1 system was elucidated (101, 116, 117). Toll-like receptors (TLR) on the cell surface respond to the presence of bacteria (e.g., M. tuberculosis) with a signal transduction process, which results in upregulation of VDR and CYP27B1. Uptake of 25-OH-D bound to its blood carrier DBP allows the cells to then manufacture 1,25-(OH)2D3, which in turn stimulates VDR-mediated gene transcription of cathelicidin. Cathelicidin is an antimicrobial peptide, which specifically kills M. tuberculosis. Stubbs et al. (118) have demonstrated the existence of a high VDR-high CYP27B1 subpopulation of immune cells making cathelicidin that can be selected by cell-sorting techniques in CKD stage 5 dialysis patients treated with high doses of vitamin D3 (40,000 IU two times per week). Despite the fact that these patients are virtually devoid of circulating 1,25-(OH)2D3 at baseline because of their low renal CYP27B1 activity, vitamin D3 supplementation causes a significant increase in serum 1,25-(OH)2D3, posing the question whether this metabolite is of monocyte/macrophage extrarenal origin.
24-Hydroxylase (CYP24A1)
Though, CYP24A1 was initially referred to as the 25-hydroxyvitamin D3-24-hydroxylase, work with the recombinant enzyme has shown that it is able to catalyze multiple hydroxylation reactions at carbons C-24 and C-23 of the side chain of both 25-OH-D3 and its hormonal form, 1,25-(OH)2D3 (13, 14). Indeed, our view of the role of CYP24A1 has expanded greatly to suggest that this single P450 alone is responsible for the five-step, 24-oxidation pathway from 1,25-(OH)2D3 to produce calcitroic acid, a known biliary catabolite (119, 120), as well as catalyzing a similar pathway which starts with 23-hydroxylation and culminates in the 1,25-(OH)2D3-26,23-lactone (Fig. 1) (121, 122). In addition, CYP24A1 also efficiently hydroxylates the vitamin D2 side chain of 25-OH-D2 and 1,25-(OH)2D2 to give a more limited series of polyhydroxylated products (123, 124). The 24- and 23-products of the vitamin D3 side chain appear in a specific order, reinforcing the concept of two distinct pathways initiated by a species-dependent C-24 or a C-23 hydroxylation step. Fig. 3 depicts an amino-acid sequence alignment of CYP24A1 with other vitamin D-related CYPs. In fact, an alignment of CYP24A1 from more than 50 species shows an impressive conservation of residues for at least a good part of the protein (125). Of particular note is the dichotomy that exists at residue 326 where most species of CYP24A1 have Ala326 and exhibit 24-hydroxylation to a calcitroic-acid product while more primitive organisms have Gly326 and show predominantly 23-hydroxylation to give a 26,23-lactone product. The functional significance of two distinct pathways in different species is unknown (18).
In 2010, the crystal structure of the rat CYP24A1 was elucidated in the presence of the detergents Cymal and CHAPS (24). Although the active site of rat CYP24A1 did not contain its natural substrate, for the most part, the crystal structure confirmed the predicted tertiary structure of the protein, as well as the putative active-site residues from previous homology models and mutagenesis studies (18, 20–22). The crystal structure of rat CYP24A1 reveals a canonical cytochrome P450 structure of helices and β-sheets surrounding a prosthetic heme group and a substrate-binding cavity. Virtually all of the protein is required to maintain the shape, structure, heme-binding, and function of the enzyme. The structure of the rat CYP24A1 enzyme is shown in Fig. 4A with 1,25-(OH)2D3 (sticks and spheres; yellow) minimized by triangulation into the wide-open cleft that serves as the substrate-binding cavity (24).
Even before the crystal structure of CYP24A1 was determined, mutagenesis studies were initiated based upon the remarkable conservation of structure across cytochrome P450s (Fig. 4B). Sakaki and colleagues, who had shown that rat CYP24A1 is primarily a C24-hydroxylase compared with the human enzyme, which is capable of both C24- and C23-hydroxylation, performed a follow-up study in which they mutated Thr416Met and Ile500Thr in the β3b- and β5-sheets, respectively, to try to change the properties of the rat enzyme by substituting amino acids with those found in the human enzyme (20). They postulated that these residues interact with A-ring and cis-triene moieties of the 1α,25-(OH)2D3-docked substrate. The Thr416Met and Ile500Thr substitutions caused significant changes in the C24:C23 hydroxylation ratio, from 100:1 to 12.5:1 and 6.3:1, respectively, whereas in their hands, the wild-type human enzyme had a ratio of 3.7:1 (20).
A somewhat similar approach was used to study differences between the human and opossum CYP24A1 (18), the latter representative of the orthologs that are predominantly 23-hydroxylases, with a C24:C23 hydroxylation ratio of 1:25. Using the human CYP24A1 as a starting point, they focused on mutating Ala326 to Gly326 found in the opossum and many other “primitive” CYP24A1s (see Fig. 3), because this residue occupies a critical position directly above the heme in the I-helix that abuts the side chain of 1α,25-(OH)2D3 docked within the active site of hCYP24A1 (see Fig. 4A). The single Ala326Gly substitution radically changed the metabolic pattern observed for the resultant enzyme by changing the enzyme properties from a 24-hydroxylase with a C24:C23 hydroxylation ratio of 8.1:1 to a 23-hydroxylase with a C24:C23 hydroxylation ratio of 1:8.3, a value that resembled opossum CYP24A1 (1:25). Thus, it appears that residue 326 alone was responsible for much of the regioselectivity difference observed between human and opossum CYP24A1 orthologs. Docking studies comparing the positions of 1,25-(OH)2D3 for optimal C24 versus C23 hydroxylation suggested that the loss of a methyl group from the amino acid at 326 in the I-helix by substituting Gly for Ala provides extra space for the side chain of 1,25-(OH)2D3 to slide deeper into the substrate-binding cavity to optimally place C23 (as opposed to C24) above the heme and committing catabolism through to 1,25-(OH)2D3-26,23-lactone. The striking impact of Ala326Gly on regioselectivity is logical, given its direct contact with the substrate side chain directly above the heme, compared with Ile500 and Met416, which are located in the distal substrate access channel (Fig. 4C).
Mutations at other sites in human CYP24A1 that have been shown to modulate the regioselectivity of the enzyme include Ile131, Leu148, Met246, and Val391 (21). In mutagenesis studies of residues over a single turn of the F-helix forming the top of the substrate binding-cavity of rat CYP24A1, it was shown that mutations at sites facing away from the cavity (Met245, Ser247, Thr248) retained 1,25-(OH)2D3 binding affinity similar to the wild-type, whereas mutations at sites Phe249 and Met 246 directly protruding into the cavity impaired substrate binding to different degrees. Based upon this work (22, 126), CYP24A1 is a 1,25-(OH)2D3-binding protein first and a catabolic enzyme second. All of these residues, including Ala326 and Ile500, originally selected on the basis of homology modeling (18, 20–22, 127) as putative substrate contact points, have been implicated in forming the CHAPS-containing substrate-binding site in the crystal structure of rat CYP24A1 (24). A recent report (128) suggests that a Val391Leu mutation in human CYP24A1 also changes enzymatic properties by introducing 1α-OH-D3-25-hydroxylase activity absent in the wild-type enzyme and ascribes this to a combination of a change in the position of substrate within the active site and altered substrate-binding affinity (18, 126). From homology models and mutagenesis studies of CYP24A1, we now have an unprecedented understanding of the amino-acid architecture of the substrate-binding pocket, details which have been confirmed by the availability of the crystal structure of rat CYP24A1. Our current view of the substrate-binding site is depicted in Fig. 4A, in which many of the residues that we have discussed are highlighted.
During the same period of time in which the role of CYP24A1 in multistep hydroxylation of the side chain of vitamin D was being elucidated, it was shown that the enzyme is expressed in many, if not all, target cells containing the VDR, including kidney, bone, and intestine, and that it is strongly inducible by VDR agonists in such tissues (125). This led some to propose that the role of CYP24A1 is primarily to limit or attenuate the action of 1,25-(OH)2D3 on target cells after an initial round of transcriptional activation in a negative-feedback loop (129) (Fig. 6A). The cloning of CYP24A1 in the early 1990s (130) confirmed both the target cell pattern of CYP24A1 expression and its inducibility by its substrate 1,25-(OH)2D3. Moreover, analysis of the CYP24A1 gene revealed the presence of a strong, positive vitamin D response element (VDRE) in the upstream promoter that mediates this induction at the transcriptional level (131). This concept has been extended using CHIP technology, which shows that there are multiple VDR-binding response elements in the CYP24A1 gene and that regulation involves downstream elements as well (132). This suggests that raising 1,25-(OH)2D3 in target cells could trigger CYP24A1-mediated catabolism and thus protect cells from excess VDR pathway activation. Work with the CYP24A1-null mouse also added support to a catabolic role for CYP24A1, as the clearance of 1,25-(OH)2D3 is dramatically reduced and the plasma half-life of the hormone increases 10-fold from ∼6–60 h when CYP24A1 is absent (133, 134). Thus, there is abundant evidence that CYP24A1 exists in normal physiology to catabolize 25-OH-D3 to prevent its eventual activation to 1,25-(OH)2D3 and/or to degrade the hormone 1,25-(OH)2D3 within its target cells to terminate its biological activity.
Recent work by St-Arnaud et al. (135) has challenged this solely catabolic role for CYP24A1 by noting the accelerated healing of bone fractures in laboratory animals after the administration of 24-hydroxylated metabolites of vitamin D. The CYP24A1-null mouse exhibits an “intramembranous bone” lesion originally thought to be due to the absence of a bone-specific 24-hydroxylated metabolite. However, it was later noted that the bone lesion was similar to that observed after excessive 1,25-(OH)2D3 administration and that the lesion is absent when a double-CYP24A1/VDR-null mouse is engineered, implying that it is caused by excessive VDR-mediated gene expression (136). However, in more recent studies of bone fracture healing in the CYP24A1-null mouse, 24-hydroxylated metabolites appear to accelerate the rate of repair (135). Acceptance of a unique anabolic role for 24,25-(OH)2D3 in bone healing would seem to depend heavily on the demonstration of a 24,25-(OH)2D3 receptor and elucidation of the signal transduction pathway mediating the effect. Reports indicate that a putative 24,25-(OH)2D3 receptor has been cloned, and attempts are being made to engineer a receptor-knockout mouse (137). It will be also important to show that this 24,25-(OH)2D3 receptor-knockout mouse has a defective bone fracture-healing phenotype.
Although CYP24A1 has been clearly established as the key enzyme responsible for vitamin D catabolism, it has become evident that CYP24A1 works in balance with CYP27B1, which is the cytochrome P450 enzyme responsible for converting 25-OH-D3 to 1,25-(OH)2D3 both in the kidney where its role in vitamin D hormone activation was first established, as well as in extrarenal tissues where its specific purpose remains to be elucidated. The emergence of the extrarenal 1α-hydroxylase (CYP27B1) as a mechanism for raising the cellular concentration of 1,25-(OH)2D3 (99, 100) has refocused our attention on the crucial role of target-cell CYP24A1 as a fine-tuning mechanism to attenuate and eventually reduce its level after gene expression has been modulated. While the renal CYP24A1 enzyme may function to balance systemic 25-OH-D3 and 1,25-(OH)2D3 levels, the target-cell extrarenal enzyme probably acts in conjunction with CYP27B1 to “fine tune” target tissue exposure to 1,25-(OH)2D3 hormone (138) (Fig. 6B).
Vitamin D signaling plays a critical role in regulating bone and mineral homeostasis, and consequently, enzymes such as CYP24A1 that control vitamin D levels are regulated by hormones that are integral to mineral metabolism (5) (see Fig. 6A). In addition to the self-induced regulation of CYP24A1 by 1,25-(OH)2D3 itself, the enzyme is regulated by key factors such as PTH and FGF-23.1,25-(OH)2D3-mediated induction of CYP24A1 expression is significantly attenuated by PTH (139–141) due to destabilization and increased degradation of CYP24A1 mRNA (142). As with PTH, FGF-23 also plays a central role in the regulation of mineral homeostasis, affecting both expression of genes regulating serum phosphate and those controlling vitamin D metabolism (143–145). Induction of FGF-23 expression in osteocytes and osteoblasts follows rising serum phosphate levels; subsequently, FGF-23 reduces renal phosphate reabsorption by inhibiting sodium-phosphate cotransporter activity (146, 147) and indirectly suppresses intestinal phosphate absorption by suppressing renal expression of CYP27B1, thus lowering blood 1,25-(OH)2D3 (147–149). FGF-23 also controls 1,25-(OH)2D3 levels by inducing expression of CYP24A1 mRNA in the kidney (103, 148–151).
The initial demonstration that 1,25-(OH)2D3 is an antiproliferative, prodifferentiating agent for certain cell types in vivo and many cell lines in vitro (152), coupled with the fact that cancer cell studies have showed decreased CYP27B1 and increased CYP24A1 expression in prostatic, colonic, and breast cell lines as they progress toward a more tumorigenic phenotype (97, 153–156), has caused some researchers to speculate that cancer progression involves dysfunctional vitamin D metabolism (Fig. 6B) (156). But the hypothesis that vitamin D deficiency contributes to cancer incidence or that supplemental vitamin D3 might prevent cancer is difficult to test because of the duration of clinical trials or the multiple confounding factors that accompany vitamin D deficiency. The VDR-knockout mouse, which lacks vitamin D-mediated signaling altogether, is more susceptible to chemically induced cancers, arguing that vitamin D plays a role in cancer prevention (157). Although elevated CYP24A1 expression and reduced CYP24A1 gene silencing has been reported in specific tumors (158–160), proof that it is a causative agent in cancer development is still lacking. Nevertheless, there are many claims that CYP24A1 is a candidate oncogene (161–163).
Mining of several genomic databases reveals that a number of polymorphisms of CYP24A1 have been identified in recent years, and the list is growing rapidly (Fig. 7). Although little is known of the effects of these polymorphisms on CYP24A1 enzyme activity, inactivating mutations would be expected to give rise to a hypercalcemic phenotype. Hypercalcemic conditions are not uncommon in the pediatric literature, but they appear to be a heterogeneous group of diseases, including Williams-Beuren syndrome and idiopathic infantile hypercalcemia (IIH) that are characterized by transient hypercalcemia and other features. Of these, only IIH has unknown etiology and, until recently, had no gene locus assigned to it (164, 165). In 2011, Schlingmann et al. (166) demonstrated that loss-of-function mutations of CYP24A1 were an underlying cause of IIH in nine families of German, Turkish, and Russian origin. This work, which recently confirmed by other US-based groups (167–169), reinforced the important conclusion drawn from the CYP24A1-null mouse studies that CYP24A1 is primarily a catabolic enzyme.
Fig. 7.
Location of CYP24A1 polymorphisms (in SNP databases) and CYP24A1 missense mutations identified in patients with IIH. The relative positions of the conserved α-helices and β-strands in the CYP24A1 protein are indicated in yellow and blue, respectively. Secondary structures positioning substrate contact residues are located in the β-1, A-helix, B′-helix, B′/C-loop, F/G-loop, I-helix, β-3a, β-3b, and β-5 structures; heme-binding and ERR-triad residues stabilize protein structure and are involved in ferredoxin binding and electron transfer to the heme iron.
Dysfunctional CYP24A1 activity has been implicated in a number of acquired diseases, including CKD-MBD and several types of cancer; as well as involved in genetically linked hypophosphatemia due underlying defects in FGF-23 signaling (170, 171). A detailed description of the connection between CYP24A1 and these diseases is beyond the scope of this review (see Refs. 170, 171).
Future perspectives
The study of the cytochrome P450s involved in vitamin D metabolism has come of age with the cloning and structural elucidation of several of the family members. Just as the crystal structure of the VDR has opened the door to new families of vitamin D analogs that more precisely position the vitamin D ligand in the ligand-binding pocket, the substrate-binding pockets of the vitamin D-related CYPs, especially CYP24A1, will allow us to design “metabolism-resistant” or “metabolism-sensitive” vitamin D analogs as well as a second generation of CYP24A1 or CYP27B1 inhibitors using rational drug design (172). From a biochemical perspective such information will also allow us to better understand the mechanism of multiple hydroxylation reactions executed by these enzymes
As was pointed out throughout this review, the number of CYP2R1, CYP27A1, CYP27B1, and CYP24A1 polymorphisms in the genomic databases is expanding at an exponential pace. Undoubtedly, the recent discovery of inactivating CYP24A1 mutations in IIH patients (166) will also drive clinical interest in CYP24A1 research. One would expect that more of these polymorphisms may be loss-of-function mutations associated with mild and more severe diseases in the hypercalcemic constellation, including IIH, but it remains to be seen whether CYP24A1 dysregulation can be connected with other disease states (e.g., nephrolithiasis). There is no doubt that the CYP24A1-knockout mouse (133–137) still has much more to reveal about the roles of CYP24A1 in vivo. Likewise, the development of the CYP2R1-null mouse (36) and its crossing with the CYP27A1-null mouse should lead to a much better understanding of vitamin D-25-hydroxylase. Lastly, and perhaps most importantly, the exact role of the extrarenal CYP27B1 should also be clarified over the next few years. This is an exciting time to be involved in the study of vitamin D-related cytochromes P450 and vitamin D metabolomics.
Footnotes
Abbreviations:
- 25-OH-D3
- 25-hydroxyvitamin D3
- 1
- 25-(OH)2D3, 1α,25-dihydroxyvitamin D3
- CKD-MBD
- chronic kidney disease-metabolic bone disease
- CYP
- cytochrome P450
- CTX
- cerebrotendinous xanthomatosis
- DBP
- vitamin D-binding protein
- FGF-23
- fibroblast growth factor-23
- GFR
- glomerular filtration rate
- IIH
- idiopathic infantile hypercalcemia
- PTH
- parathyroid hormone
- SNP
- single nucleotide polymorphism
- TLR
- toll-like receptor
- VDDR
- vitamin D-dependent rickets
- VDR
- vitamin D receptor
- VDRE
- vitamin D responsive element
REFERENCES
- 1.DeLuca H. F. 1974. Vitamin D: the vitamin and the hormone. Fed. Proc. 33: 2211–2219 [PubMed] [Google Scholar]
- 2.Ponchon G., DeLuca H. F. 1969. The role of the liver in the metabolism of vitamin D. J. Clin. Invest. 48: 1273–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser D. R., Kodicek E. 1970. Unique biosynthesis by kidney of a biologically active vitamin D metabolite. Nature. 228: 764–766 [DOI] [PubMed] [Google Scholar]
- 4.Hewison M., Adams J. S. 2011. Extrarenal 1α-hydroxylase. In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. 777–806. [Google Scholar]
- 5.Jones G., Strugnell S., DeLuca H. F. 1998. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 78: 1193–1231 [DOI] [PubMed] [Google Scholar]
- 6.Nykjaer A., Dragun D., Walther D., Vorum H., Jacobsen C., Herz J., Melsen F., Christensen E. I., Willnow T. E. 1999. An endocytic pathway essential for renal uptake and activation of the steroid 25(OH) vitamin D3. Cell. 96: 507–515 [DOI] [PubMed] [Google Scholar]
- 7.Safadi F. F., Thornton P., Magiera H., Hollis B. W., Gentile M., Haddad J. G., Liebhaber S. A., Cooke N. E. 1999. Osteopathy and resitance to vitamin D toxicity in mice null for vitamin D binding protein. J. Clin. Invest. 103: 239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zella L. A., Shevde N. K., Hollis B. W., Cooke N. E., Pike J. W. 2008. Vitamin D-binding protein influences total circulating levels of 1,25-dihydroxyvitamin D3 but does not directly modulate the bioactive levels of the hormone in vivo. Endocrinology. 149: 3656–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J. B., Levine M. A., Bell N. H., Mangelsdorf D. J., Russell D. W. 2004. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc. Natl. Acad. Sci. USA. 101: 7711–7715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St-Arnaud R., Messerlian S., Moir J. M., Omdahl J. L., Glorieux F. H. 1997. The 25-hydroxyvitamin D 1-α-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J. Bone Miner. Res. 12: 1552–1559 [DOI] [PubMed] [Google Scholar]
- 11.Takeyama K., Kitanaka S., Sato T., Kobori M., Yanagisawa J., Kato S. 1997. 25-Hydroxyvitamin D3 1α-hydroxylase and vitamin D synthesis. Science. 277: 1827–1830 [DOI] [PubMed] [Google Scholar]
- 12.Nelson D. R. 2009. The cytochrome P450 homepage. Hum. Genomics. 4: 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutson J. C., DeLuca H. F. 1974. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 13: 1543–1548 [DOI] [PubMed] [Google Scholar]
- 14.Prosser D. E., Jones G. 2004. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 29: 664–673 [DOI] [PubMed] [Google Scholar]
- 15.Jones G., Prosser D. E. 2011. The activating enzymes of vitamin D metabolism (25- and 1α-hydroxylases). In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. 23–42. [Google Scholar]
- 16.St-Arnaud R. 2011. CYP24A1: Structure, function and physiological role. In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. 43–56. [Google Scholar]
- 17.Haussler M. R., Whitfield G. K., Haussler C., Hsieh J-C., Jurutka P. W. 2011. Nuclear vitamin D receptor: natural ligands, molecular structure-function and transcriptional control of vital genes. In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. 137–170. [Google Scholar]
- 18.Prosser D. E., Kaufmann M., O'Leary B., Byford V., Jones G. 2007. Single A326G mutation converts hCYP24A1 from a 25-OH-D3-24-hydroxylase into -23-hydroxylase generating 1α,25-(OH)2D3-26,23-lactone. Proc. Natl. Acad. Sci. USA. 104: 12673–12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prosser D. E., Guo Y-D., Geh K. R., Jia Z., Jones G. 2006. Structural motif-based homology modeling of CYP27A1 and site-directed mutational analyses affecting vitamin D hydroxylation. Biophys. J. 90: 3389–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamamoto H., Kusudo T., Urushino N., Masuno H., Yamamoto K., Yamada S., Kamakura M., Ohta M., Inouye K., Sakaki T. 2006. Structure-function analysis of vitamin D 24-hydroxylase (CYP24A1) by site-directed mutagenesis: amino acid residues responsible for species-based difference of CYP24A1 between humans and rats. Mol. Pharmacol. 70: 120–128 [DOI] [PubMed] [Google Scholar]
- 21.Masuda S., Prosser D. E., Guo Y-D., Kaufmann M., Jones G. 2007. Generation of a homology model for the human cytochrome P450, CYP24A1, and the testing of putative substrate binding residues by site-directed mutagenesis and enzyme activity studies. Arch. Biochem. Biophys. 460: 177–191 [DOI] [PubMed] [Google Scholar]
- 22.Annalora A. J., Bobrovnikov-Marjon E., Serda R., Pastuszyn A., Graham S. E., Marcus C. B., Omdahl J. L. 2007. Hybrid homology modeling and mutational analysis of vitamin D-24-hydroxylase (CYP24A1) of the vitamin D pathway: insights into substrate specificity and membrane-bound structure-function. Arch. Biochem. Biophys. 460: 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strushkevich N., Usanov S. A., Plotnikov A. N., Jones G., Park H-W. 2008. Structural analysis of CYP2R1 in complex with vitamin D3. J. Mol. Biol. 380: 95–106 [DOI] [PubMed] [Google Scholar]
- 24.Annalora A. J., Goodin D. B., Hong W. X., Zhang Q., Johnson E. F., Stout C. D. 2010. Crystal structure of CYP24A1, a mitochondrial cytochrome P450 involved in vitamin D metabolism. J. Mol. Biol. 396: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto H., Shinkyo R., Hayashi K., Yoneda S., Yamada M., Kamakura M., Ikushiro S., Shiro Y., Sakaki T. 2008. Crystal structure of CYP105A1 (P450SU-1) in complex with 1α,25-dihydroxyvitamin D3. Biochemistry. 47: 4017–4027 [DOI] [PubMed] [Google Scholar]
- 26.Yasutake Y., Fujii Y., Cheon W. K., Arisawa A., Tamura T. 2009. Crystallization and preliminary X-ray diffraction studies of vitamin D3 hydroxylase, a novel cytochrome P450 isolated from Pseudonocardia autotrophica. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65: 372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhattacharyya M. H., DeLuca H. F. 1973. The regulation of rat liver calciferol-25-hydroxylase. J. Biol. Chem. 248: 2969–2973 [PubMed] [Google Scholar]
- 28.Bhattacharyya M. H., DeLuca H. F. 1974. Subcellular location of rat liver calciferol-25-hydroxylase. Arch. Biochem. Biophys. 160: 58–62 [DOI] [PubMed] [Google Scholar]
- 29.Fukushima M., Nishii Y., Suzuki M., Suda T. 1978. Comparative studies on the 25-hydroxylations of cholecalciferol and 1α-hydroxycholecalfierol in perfused rat liver. Biochem. J. 170: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng J. B., Motola D. L., Mangelsdorf D. J., Russell D. W. 2003. De-orphanization of cytochrome P450 2R1: a microsomal vitamin D 25-hydroxylase. J. Biol. Chem. 278: 38084–38093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson D. R. 2003. Comparison of P450s from human and fugu: 420 million years of vertebrate P450 evolution. Arch. Biochem. Biophys. 409: 18–24 [DOI] [PubMed] [Google Scholar]
- 32.Jones G., Byford V., West S., Masuda S., Ibrahim G., Kaufmann M., Knutson J. C., Strugnell S., Mehta R. 2006. Hepatic activation and inactivation of clinically relevant vitamin D analogs and prodrugs. Anticancer Res. 26: 2589–2595 [PubMed] [Google Scholar]
- 33.Casella S. J., Reiner B. J., Chen T. C., Holick M. F., Harrison H. E. 1994. A possible genetic defect in 25-hydroxylation as a cause of rickets. J. Pediatr. 124: 929–932 [DOI] [PubMed] [Google Scholar]
- 34.Fraser D., Kooh S. W., Kind H. P., Holick M. F., Tanaka Y., DeLuca H. F. 1973. Pathogenesis of hereditary vitamin-D-dependent rickets. An inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1α,25-dihydroxyvitamin D. N. Engl. J. Med. 289: 817–822 [DOI] [PubMed] [Google Scholar]
- 35.Thacher T. D., Fischer P. R., Pettifor J. M., Lawson J. O., Isichei C. O., Chan G. M. 2000. Case-control study of factors associated with nutritional rickets in Nigerian children. J. Pediatr. 137: 367–373 [DOI] [PubMed] [Google Scholar]
- 36.Zhu J., DeLuca H. F. 2012. Vitamin D-25-hydroxylase: four decades of searching, are we there yet? Arch. Biochem. Biophys. 523: 30–36 [DOI] [PubMed] [Google Scholar]
- 37.Wang T. J., Zhang F., Richards J. B., Kestenbaum B., van Meurs J. B., Kiel D. P., Streeten E. A., Ohlsson C., Koller D. L., Peltonen L., et al. 2010. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 376: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cali J. J., Russell D. W. 1991. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J. Biol. Chem. 266: 7774–7778 [PubMed] [Google Scholar]
- 39.Guo Y. D., Strugnell S., Back D. W., Jones G. 1993. Substrate specificity of the liver mitochondrial cytochrome P-450, CYP-27, towards vitamin D and its analogs. Proc. Natl. Acad. Sci. USA. 90: 8668–8672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohyama Y., Masumoto O., Usui E., Okuda K. 1991. Multi-functional property of rat liver mitochondrial cytochrome P-450. J. Biochem. 109: 389–393 [DOI] [PubMed] [Google Scholar]
- 41.Strugnell S., Byford V., Makin H. L. J., Moriarty R. M., Gilardi R., LeVan L. W., Knutson J. C., Bishop C. W., Jones G. 1995. 1α,24S-Dihydroxyvitamin D2: a biologically active product of 1α-hydroxyvitamin D2 made in the human hepatoma, Hep3B. Biochem. J. 310: 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pikuleva I. A., Björkhem I., Waterman M. R. 1997. Expression, purification, and enzymatic properties of recombinant human cytochrome P450c27 (CYP27). Arch. Biochem. Biophys. 343: 123–130 [DOI] [PubMed] [Google Scholar]
- 43.Mast N., Murtazina D., Liu H., Graham S. E., Bjorkhem I., Halpert J. R., Peterson J., Pikuleva I. A. 2006. Distinct binding of cholesterol and 5β-cholestane-3α,7α,12α-triol to cytochrome P450 27A1: evidence from modeling and site-directed mutagenesis studies. Biochemistry. 45: 4396–4404 [DOI] [PubMed] [Google Scholar]
- 44.Repa J. J., Lund E. G., Horton J. D., Leitersdorf E., Russell D. W., Dietschy J. M., Turley S. D. 2000. Disruption of the sterol 27-hydroxylase gene in mice results in hepatomegaly and hyper-triglyceridemia. Reversal by cholic acid feeding. J. Biol. Chem. 275: 39685–39692 [DOI] [PubMed] [Google Scholar]
- 45.Cali J. J., Hsieh C. L., Francke U., Russell D. W. 1991. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 266: 7779–7783 [PMC free article] [PubMed] [Google Scholar]
- 46.Berginer V. M., Shany S., Alkalay D., Berginer J., Dekel S., Salen G., Tint G. S., Gazit D. 1993. Osteoporosis and increased bone fractures in cerebrotendinous xanthomatosis. Metabolism. 42: 69–74 [DOI] [PubMed] [Google Scholar]
- 47.Björkhem I., Hansson M. 2010. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem. Biophys. Res. Commun. 396: 46–49 [DOI] [PubMed] [Google Scholar]
- 48.Jones G. 2010. Vitamin D analogs. Endocrinol. Metab. Clin. North Am. 39: 447–472 [DOI] [PubMed] [Google Scholar]
- 49.Jones G., Schnoes H. K., Levan L., DeLuca H. F. 1980. Isolation and identification of 24-hydroxyvitamin D2 and 24,25-dihydroxyvitamin D2. Arch. Biochem. Biophys. 202: 450–457 [DOI] [PubMed] [Google Scholar]
- 50.Horst R. L., Koszewski N. J., Reinhardt T. A. 1990. 1α-hydroxylation of 24-hydroxyvitamin D2 represents a minor physiological pathway for the activation of vitamin D2 in mammals. Biochemistry. 29: 578–582 [DOI] [PubMed] [Google Scholar]
- 51.Gupta R. P., Hollis B. W., Patel S. B., Patrick K. S., Bell N. H. 2004. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J. Bone Miner. Res. 19: 680–688 [DOI] [PubMed] [Google Scholar]
- 52.Gupta R. P., He Y. A., Patrick K. S., Halpert J. R., Bell N. H. 2005. CYP3A4 is a vitamin D-24- and 25-hydroxylase: analysis of structure function by site-directed mutagenesis. J. Clin. Endocrinol. Metab. 90: 1210–1219 [DOI] [PubMed] [Google Scholar]
- 53.Xu Y., Hashizume T., Shuhart M. C., Davis C. L., Nelson W. L., Sakaki T., Kalhorn T. F., Watkins P. B., Schuetz E. G., Thummel K. E. 2006. Intestinal and hepatic CYP3A4 catalyze hydroxylation of 1α,25-dihydroxyvitamin D3: implications for drug-induced osteomalacia. Mol. Pharmacol. 69: 56–65 [DOI] [PubMed] [Google Scholar]
- 54.Thummel K. E., Brimer C., Yasuda K., Thottassery J., Senn T., Ishizuka H., Khaarasch E., Schuetz J., Schuetz E. 2001. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol. Pharmacol. 60: 1399–1406 [DOI] [PubMed] [Google Scholar]
- 55.Thompson P. D., Jurutka P. W., Whitfield G. K., Myskowski S. M., Eichhorst K. R., Dominguez C. E., Haussler C. A., Haussler M. R. 2002. Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem. Biophys. Res. Commun. 299: 730–738 [DOI] [PubMed] [Google Scholar]
- 56.Helvig C., Cuerrier D., Kharebov A., Ireland B., Kim J., Ryder K., Petkovich M. 2008. Comparison of 1,25-dihydroxyvitamin D2 and calcitriol effects in an adenine-induced model of CKD reveals differential control over serum calcium and phosphate. J. Bone Min. Res. 23: S357 [Google Scholar]
- 57.Jones G., Byford V., Helvig C., Petkovich M.2009. Differential disposition of vitamin D2 does not involve CYP24A1. 14th International Vitamin D Workshop. Brugge, Belgium, October 4–8, 2009.
- 58.Tjellesen L., Gotfredsen A., Christiansen C. 1985. Different actions of vitamin D2 and D3 on bone metabolism in patients treated with phenobarbitone/phenytoin. Calcif. Tissue Int. 37: 218–222 [DOI] [PubMed] [Google Scholar]
- 59.Hosseinpour F., Ellfolk M., Norlin M., Wikvall K. 2007. Phenobarbital suppresses vitamin D3 25-hydroxylase expression: a potential new mechanism for drug-induced osteomalacia. Biochem. Biophys. Res. Commun. 357: 603–607 [DOI] [PubMed] [Google Scholar]
- 60.Holick M. F., Schnoes H. K., DeLuca H. F., Suda T., Cousins R. J. 1971. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 10: 2799–2804 [DOI] [PubMed] [Google Scholar]
- 61.Omdahl J. L., Gray R. W., Boyle I. T., Knutson J., DeLuca H. F. 1972. Regulation of metabolism of 25-hydroxycholecalciferol by kidney tissue in vitro by dietary calcium. Nat. New Biol. 237: 63–64 [DOI] [PubMed] [Google Scholar]
- 62.Tanaka Y., DeLuca H. F. 1973. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch. Biochem. Biophys. 154: 566–574 [DOI] [PubMed] [Google Scholar]
- 63.Gray R. W., Omdahl J. L., Ghazarian J. G., DeLuca H. F. 1972. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J. Biol. Chem. 247: 7528–7532 [PubMed] [Google Scholar]
- 64.Akiba T., Endou H., Koseki C., Sakai F., Horiuchi N., Suda T. 1980. Localization of 25-hydroxyvitamin D3-1α-hydroxylase activity in the mammalian kidney. Biochem. Biophys. Res. Commun. 94: 313–318 [DOI] [PubMed] [Google Scholar]
- 65.Kawashima H., Kurokawa K. 1983. Unique hormonal regulation of vitamin D metabolism in the mammalian kidney. Miner. Electrolyte Metab. 9: 227–235 [PubMed] [Google Scholar]
- 66.Kawashima H., Torikai S., Kurokawa K. 1981. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1α-hydroxylase in the proximal straight tubule of the rat kidney. Nature. 291: 327–329 [DOI] [PubMed] [Google Scholar]
- 67.Shinki T., Ueno Y., DeLuca H. F., Suda T. 1999. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1α-hydroxylase gene in normocalcemic rats. Proc. Natl. Acad. Sci. USA. 96: 8253–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghazarian J. G., Jefcoate C. R., Knutson J. C., Orme-Johnson W. H., DeLuca H. F. 1974. Mitochondrial cytochrome p450. A component of chick kidney 25-hydrocholecalciferol-1α-hydroxylase. J. Biol. Chem. 249: 3026–3033 [PubMed] [Google Scholar]
- 69.Weisman Y., Vargas A., Duckett G., Reiter E., Root A. W. 1978. Synthesis of 1,25-dihydroxyvitamin D in the nephrectomized pregnant rat. Endocrinology. 103: 1992–1996 [DOI] [PubMed] [Google Scholar]
- 70.Gray T. K., Lester G. E., Lorenc R. S. 1979. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 204: 1311–1313 [DOI] [PubMed] [Google Scholar]
- 71.Howard G. A., Turner R. T., Sherrard D. J., Baylink D. J. 1981. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J. Biol. Chem. 256: 7738–7740 [PubMed] [Google Scholar]
- 72.Somjen D., Katzburg S., Stern N., Kohen F., Sharon O., Limor R., Jaccard N., Hendel D., Weisman Y. 2007. 25-hydroxyvitamin D3-1α hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. J. Steroid Biochem. Mol. Biol. 107: 238–244 [DOI] [PubMed] [Google Scholar]
- 73.Gray T. K., Maddux F. W., Lester G. E., Williams M. E. 1982. Rodent macrophages metabolize 25-hydroxyvitamin D3 in vitro. Biochem. Biophys. Res. Commun. 109: 723–729 [DOI] [PubMed] [Google Scholar]
- 74.Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. 1983. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Invest. 72: 1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monkawa T., Yoshida T., Wakino S., Shinki T., Anazawa H., DeLuca H. F., Suda T., Hayashi M., Saruta T. 1997. Molecular cloning of cDNA and genomic DNA for human 25-hydroxyvitamin D3 1α-hydroxylase. Biochem. Biophys. Res. Commun. 239: 527–533 [DOI] [PubMed] [Google Scholar]
- 76.Fu G. K., Lin D., Zhang M. Y. H., Bikle D. D., Shackleton C. H. L., Miller W. L., Portale A. A. 1997. Cloning of human 25-hydroxyvitamin D-1α-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 11: 1961–1970 [DOI] [PubMed] [Google Scholar]
- 77.Jones G., Ramshaw H., Zhang A., Cook R., Byford V., White J., Petkovich M. 1999. Expression and activity of vitamin D-metabolizing cytochrome P450s (CYP1α and CYP24) in human non-small cell lung carcinomas. Endocrinology. 140: 3303–3310 [DOI] [PubMed] [Google Scholar]
- 78.Zehnder D., Bland R., Williams M. C., Mc R. W., Ninch A. J., Howie P. M., Stewart P. M., Hewison M. 2001. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J. Clin. Endocrinol. Metab. 86: 888–894 [DOI] [PubMed] [Google Scholar]
- 79.Scriver C. R., Reade T. M., DeLuca H. F., Hamstra A. J. 1978. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N. Engl. J. Med. 299: 976–979 [DOI] [PubMed] [Google Scholar]
- 80.Miller W. L., Portale A. A. 2000. Vitamin D 1α-hydroxylase. Trends Endocrinol. Metab. 11: 315–319 [DOI] [PubMed] [Google Scholar]
- 81.Kitanaka S., Takeyama K., Murayama A., Kato S. 2001. The molecular basis of vitamin D-dependent rickets type I. Endocr. J. 48: 427–432 [DOI] [PubMed] [Google Scholar]
- 82.Wang X., Zhang M. Y., Miller W. L., Portale A. A. 2002. Novel gene mutations in patients with 1α-hydroxylase deficiency that confer partial enzyme activity in vitro. J. Clin. Endocrinol. Metab. 87: 2424–2430 [DOI] [PubMed] [Google Scholar]
- 83.Kim C. J., Kaplan L. E., Perwad F., Huang N., Sharma A., Choi Y., Miller W. L., Portale A. A. 2007. Vitamin D 1α-hydroxylase gene mutations in patients with 1α-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92: 3177–3182 [DOI] [PubMed] [Google Scholar]
- 84.Malloy P. J., Feldman D. 2010. Genetic disorders and defects in vitamin D action. Endocrinol. Metab. Clin. North Am. 39: 333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panda D. K., Miao D., Tremblay M. L., Sirois J., Farookhi R., Hendy G. N., Goltzman D. 2001. Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA. 98: 7498–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dardenne O., Prud'homme J., Arabian A., Glorieux F. H., St-Arnaud R. 2001. Targeted inactivation of the 25-hydroxyvitamin D3-1α-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 142: 3135–3141 [DOI] [PubMed] [Google Scholar]
- 87.Xue Y., Karaplis A. C., Hendy G. N., Goltzman D., Miao D. 2005. Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum. Mol. Genet. 14: 1515–1528 [DOI] [PubMed] [Google Scholar]
- 88.Bikle D. D., Chang S., Crumrine D., Elalieh H., Man M. Q., Choi E. H., Dardenne O., Xie Z., Arnaud R. S., Feingold K., et al. 2004. 25 Hydroxyvitamin D 1α-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J. Invest. Dermatol. 122: 984–992 [DOI] [PubMed] [Google Scholar]
- 89.Hoenderop J. G., Dardenne O., Van Abel M., Van Der Kemp A. W., Van Os C. H., St-Arnaud R., Bindels R. J. 2002. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1α-hydroxylase knockout mice. FASEB J. 16: 1398–1406 [DOI] [PubMed] [Google Scholar]
- 90.Dardenne O., Prudhomme J., Hacking S. A., Glorieux F. H., St-Arnaud R. 2003. Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J. Bone Miner. Res. 18: 637–643 [DOI] [PubMed] [Google Scholar]
- 91.Dardenne O., Prud'homme J., Hacking S. A., Glorieux F. H., St-Arnaud R. 2003. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). Bone. 32: 332–340 [DOI] [PubMed] [Google Scholar]
- 92.Vanhooke J. L., Prahl J. M., Kimmel-Jehan C., Mendelsohn M., Danielson E. W., Healy K. D., DeLuca H. F. 2006. CYP27B1 null mice with LacZ reporter gene display no 25-hydroxyvitamin D3-1α-hydroxylase promoter activity in the skin. Proc. Natl. Acad. Sci. USA. 103: 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.St-Arnaud R., Dardenne O., Prud'homme J., Hacking S. A., Glorieux F. H. 2003. Conventional and tissue-specific inactivation of the 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1). J. Cell. Biochem. 88: 245–251 [DOI] [PubMed] [Google Scholar]
- 94.Naja R. P., Dardenne O., Arabian A., St-Arnaud R. 2009. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology. 150: 4024–4032 [DOI] [PubMed] [Google Scholar]
- 95.Diaz L., Sanchez I., Avila E., Halhali A., Vilchis F., Larrea F. 2000. Identification of a 25-hydroxyvitamin D3 1α-hydroxylase gene transcription product in cultures of human syncytiotrophoblast cells. J. Clin. Endocrinol. Metab. 85: 2543–2549 [DOI] [PubMed] [Google Scholar]
- 96.Whitlatch L. W., Young M. V., Schwartz G. G., Flanagan J. N., Burnstein K. L., Lokeshwar B. L., Rich E. S., Holick M. F., Chen T. C. 2002. 25-Hydroxyvitamin D-1α-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J. Steroid Biochem. Mol. Biol. 81: 135–140 [DOI] [PubMed] [Google Scholar]
- 97.Tangpricha V., Flanagan J. N., Whitlatch L. W., Tseng C. C., Chen T. C., Holt P. R., Lipkin M. S., Holick M. F. 2001. 25-hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 357: 1673–1674 [DOI] [PubMed] [Google Scholar]
- 98.Bareis P., Bises G., Bischof M. G., Cross H. S., Peterlik M. 2001. 25-hydroxy-vitamin D metabolism in human colon cancer cells during tumor progression. Biochem. Biophys. Res. Commun. 285: 1012–1017 [DOI] [PubMed] [Google Scholar]
- 99.Holick M. F. 2007. Vitamin D deficiency. N. Engl. J. Med. 357: 266–281 [DOI] [PubMed] [Google Scholar]
- 100.Jones G. 2007. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin. Dial. 20: 316–324 [DOI] [PubMed] [Google Scholar]
- 101.Adams J. S., Hewison M. 2010. Update in vitamin D. J. Clin. Endocrinol. Metab. 95: 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez I., Saracho R., Montenegro J., Llach F. 1996. A deficit of calcitriol synthesis may not be the initial factor in the pathogenesis of secondary hyperparathyroidism. Nephrol. Dial. Transplant. 11(Suppl. 3): 22–28 [DOI] [PubMed] [Google Scholar]
- 103.Inoue Y., Segawa H., Kaneko I., Yamanaka S., Kusano K., Kawakami E., Furutani J., Ito M., Kuwahata M., Saito H., et al. 2005. Role of the vitamin D receptor in FGF23 action on phosphate metabolism. Biochem. J. 390: 325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olauson H., Qureshi A. R., Miyamoto T., Barany P., Heimburger O., Lindholm B., Stenvinkel P., Larsson T. E. 2010. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: are gender and cardiovascular disease confounding the relationship? Nephrol. Dial. Transplant. 25: 3033–3038 [DOI] [PubMed] [Google Scholar]
- 105.Brenza H. L., Kimmel-Jehan C., Jehan F., Shinki T., Wakino S., Anazawa H., Suda T., DeLuca H. F. 1998. Parathyroid hormone activation of the 25-hydroxyvitamin D3-1α-hydroxylase gene promoter. Proc. Natl. Acad. Sci. USA. 95: 1387–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murayama A., Takeyama K., Kitanaka S., Kodera Y., Hosoya T., Kato S. 1998. The promoter of the human 25-hydroxyvitamin D3 1α-hydroxylase gene confers positive and negative responsiveness to PTH, calcitonin, and 1α,25(OH)2D3. Biochem. Biophys. Res. Commun. 249: 11–16 [DOI] [PubMed] [Google Scholar]
- 107.Liu S., Quarles L. D. 2007. How fibroblast growth factor 23 works. J. Am. Soc. Nephrol. 18: 1637–1647 [DOI] [PubMed] [Google Scholar]
- 108.Kong X. F., Zhu X. H., Pei Y. L., Jackson D. M., Holick M. F. 1999. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D3-1α-hydroxylase gene. Proc. Natl. Acad. Sci. USA. 96: 6988–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim M-S., Fujiki R., Kitagawa H., Kato S. 2007. 1α,25(OH)2D3-induced DNA methylation suppresses the human CYP27B1 gene. Mol. Cell. Endocrinol. 265–266: 168–173 [DOI] [PubMed] [Google Scholar]
- 110.Kouzmenko A, Ohtake F., Fujiki R., Kato S. 2011. Epigenetic modifications in receptor-mediated transrepression. In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. Chapter 12, 227–234 [Google Scholar]
- 111.Rosenthal A. M., Jones G., Kooh S. W., Fraser D. 1980. 25-hydroxyvitamin D3 metabolism by isolated perfused rat kidney. Am. J. Physiol. 239: E12–E20 [DOI] [PubMed] [Google Scholar]
- 112.Adams J. S., Singer F. R., Gacad M. A., Sharma O. P., Hayes M. J., Vouros P., Holick M. F. 1985. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J. Clin. Endocrinol. Metab. 60: 960–966 [DOI] [PubMed] [Google Scholar]
- 113.Esteban L., Vidal M., Dusso A. 2004. 1α-Hydroxylase transactivation by gamma-interferon in murine macrophages requires enhanced C/EBPbeta expression and activation. J. Steroid Biochem. Mol. Biol. 89–90: 131–137 [DOI] [PubMed] [Google Scholar]
- 114.Overbergh L., Stoffels K., Waer M., Verstuyf A., Bouillon R., Mathieu C. 2006. Immune regulation of 25-hydroxyvitamin D-1α-hydroxylase in human monocytic THP1 cells: mechanisms of interferon-γ-mediated induction. J. Clin. Endocrinol. Metab. 91: 3566–3574 [DOI] [PubMed] [Google Scholar]
- 115.Wu S., Ren S., Nguyen L., Adams J. S., Hewison M. 2007. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology. 148: 3410–3418 [DOI] [PubMed] [Google Scholar]
- 116.Liu P. T., Stenger S., Li H., Wenzel L., Tan B. H., Krutzik S. R., Ochoa M. T., Schauber J., Wu K., Meinken C., et al. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 311: 1770–1773 [DOI] [PubMed] [Google Scholar]
- 117.Schauber J., Dorschner R. A., Coda A. B., Buchau A. S., Liu P. T., Kiken D., Helfrich Y. R., Kang S., Elalieh H. Z., Steinmeyer A., et al. 2007. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 117: 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stubbs J. R., Idiculla A., Slusser J., Menard R., Quarles L. D. 2010. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J. Am. Soc. Nephrol. 21: 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Makin G., Lohnes D., Byford V., Ray R., Jones G. 1989. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem. J. 262: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reddy G. S., Tserng K. Y. 1989. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 28: 1763–1769 [DOI] [PubMed] [Google Scholar]
- 121.Yamada S., Nakayama K., Takayama H., Shinki T., Takasaki Y., Suda T. 1984. Isolation, identification, and metabolism of (23S,25R)-25-hydroxyvitamin D3 26,23-lactol. A biosynthetic precursor of (23S,25R)-25-hydroxyvitamin D3 26,23-lactone. J. Biol. Chem. 259: 884–889 [PubMed] [Google Scholar]
- 122.Sakaki T., Sawada N., Komai K., Yamada S., Yamamoto K., Ohyama Y., Inouye K. 2000. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur. J. Biochem. 267: 6158–6165 [DOI] [PubMed] [Google Scholar]
- 123.Masuda S., Strugnell S. A., Knutson J. C., St-Arnaud R., Jones G. 2006. Evidence for the activation of 1α-hydroxyvitamin D2 by 25-hydroxyvitamin D-24-hydroxylase: delineation of pathways involving 1α,24-dihydroxyvitamin D2 and 1α,25-dihydroxyvitamin D2. Biochim. Biophys. Acta. 1761: 221–234 [DOI] [PubMed] [Google Scholar]
- 124.Urushino N., Yasuda K., Ikushiro S., Kamakura M., Ohta M., Sakaki T. 2009. Metabolism of 1α,25-dihydroxyvitamin D2 by human CYP24A1. Biochem. Biophys. Res. Commun. 384: 144–148 [DOI] [PubMed] [Google Scholar]
- 125.Jones G., Kaufmann M., Prosser D. E. 2012. 25-hydroxyvitamin D3-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 523: 9–18 [DOI] [PubMed] [Google Scholar]
- 126.Annalora A. J., Bobrovnikov-Marjon E., Serda R., Lansing L., Chiu M. L., Pastuszyn A., Iyer S., Marcus C. B., Omdahl J. L. 2004. Rat cytochrome P450C24 (CYP24A1) and the role of F249 in substrate binding and catalytic activity. Arch. Biochem. Biophys. 425: 133–146 [DOI] [PubMed] [Google Scholar]
- 127.Gomaa M. S., Simons C., Brancale A. 2007. Homology model of 1α,25-dihydroxyvitamin D3 24-hydroxylase cytochrome P450 24A1 (CYP24A1): active site architecture and ligand binding. J. Steroid Biochem. Mol. Biol. 104: 53–60 [DOI] [PubMed] [Google Scholar]
- 128.Kaufmann M., Prosser D. E., Jones G. 2011. Bioengineering anabolic vitamin D-25-hydroxylase activity into the human vitamin D catabolic enzyme, cytochrome P450 CYP24A1, by a V391L mutation. J. Biol. Chem. 286: 28729–28737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lohnes D., Jones G. 1992. Further metabolism of 1α,25-dihydroxyvitamin D3 in target cells. J. Nutr. Sci. Vitaminol. (Tokyo). Spec. No.: 75–78 [PubMed] [Google Scholar]
- 130.Ohyama Y., Noshiro M., Okuda K. 1991. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 278: 195–198 [DOI] [PubMed] [Google Scholar]
- 131.Ohyama Y., Noshiro M., Eggertsen G., Gotoh O., Kato Y., Björkhem I., Okuda K. 1993. Structural characterization of the gene encoding rat 25-hydroxyvitamin D3 24-hydroxylase. Biochemistry. 32: 76–82 [DOI] [PubMed] [Google Scholar]
- 132.Pike J. W., Meyer M. B. 2012. Regulation of mouse Cyp24A1 expression via promoter-proximal and downstream distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D3 action. Arch. Biochem. Biophys. 523: 2–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.St-Arnaud R. 1999. Targeted inactivation of vitamin D hydroxylases in mice. Bone. 25: 127–129 [DOI] [PubMed] [Google Scholar]
- 134.Masuda S., Byford V., Arabian A., Sakai Y., Demay M. B., St-Arnaud R., Jones G. 2005. Altered pharmacokinetics of 1α,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse. Endocrinology. 146: 825–834 [DOI] [PubMed] [Google Scholar]
- 135.St-Arnaud R. 2010. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J. Steroid Biochem. Mol. Biol. 121: 254–256 [DOI] [PubMed] [Google Scholar]
- 136.St-Arnaud R., Arabian A., Travers R., Barletta F., Raval-Pandya M., Chapin K., Depovere J., Mathieu C., Christakos S., Demay M. B., et al. 2000. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 141: 2658–2666 [DOI] [PubMed] [Google Scholar]
- 137.St-Arnaud R., Kupscik L., Naja R-P., Husseini A., Arabian A.2012. Novel mechanism of action for 24-hydroxylated vitamin D metabolites in fracture repair. 15th Workshop on Vitamin D. Houston, Texas, June 16–22, 2012.
- 138.Jones G., Vriezen D., Lohnes D., Palda V., Edwards N. S. 1987. Side-chain hydroxylation of vitamin D3 and its physiological implications. Steroids. 49: 29–53 [DOI] [PubMed] [Google Scholar]
- 139.Zierold C., Reinholz G. G., Mings J. A., Prahl J. M., DeLuca H. F. 2000. Regulation of the porcine 1,25-dihydroxyvitamin D3-24-hydroxylase (CYP24) by 1,25-dihydroxyvitamin D3 and parathyroid hormone in AOK-B50 cells. Arch. Biochem. Biophys. 381: 323–327 [DOI] [PubMed] [Google Scholar]
- 140.Shinki T., Jin C. H., Nishimura A., Nagai Y., Ohyama Y., Noshiro M., Okuda K., Suda T. 1992. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1α,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J. Biol. Chem. 267: 13757–13762 [PubMed] [Google Scholar]
- 141.Reinhardt T. A., Horst R. L. 1990. Parathyroid hormone down-regulates 1,25-dihydroxy vitamin D receptors (VDR) and VDR messenger ribonucleic acid in vitro and blocks homologous up-regulation of VDR in vivo. Endocrinology. 127: 942–948 [DOI] [PubMed] [Google Scholar]
- 142.Zierold C., Mings J. A., DeLuca H. F. 2001. Parathyroid hormone regulates 25-hydroxyvitamin D3-24-hydroxylase mRNA by altering its stability. Proc. Natl. Acad. Sci. USA. 98: 13572–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Razzaque M. S., Lanske B. 2007. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J. Endocrinol. 194: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fukumoto S. 2008. Physiological regulation and disorders of phosphate metabolism - pivotal role of fibroblast growth factor 23. Intern. Med. 47: 337–343 [DOI] [PubMed] [Google Scholar]
- 145.Ramon I., Kleynen P., Body J. J., Karmali R. 2010. Fibroblast growth factor 23 and its role in phosphate homeostasis. Eur. J. Endocrinol. 162: 1–10 [DOI] [PubMed] [Google Scholar]
- 146.Saito H., Kusano K., Kinosaki M., Ito H., Hirata M., Segawa H., Miyamoto K., Fukushima N. 2003. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1α,25-dihydroxyvitamin D3 production. J. Biol. Chem. 278: 2206–2211 [DOI] [PubMed] [Google Scholar]
- 147.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. 2004. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 148.Perwad F., Zhang M. Y., Tenenhouse H. S., Portale A. A. 2007. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am. J. Physiol. Renal Physiol. 293: F1577–F1583 [DOI] [PubMed] [Google Scholar]
- 149.Shimada T., Yamazaki Y., Takahashi M., Hasegawa H., Urakawa I., Oshima T., Ono K., Kakitani M., Tomizuka K., Fujita T., et al. 2005. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 289: F1088–F1095 [DOI] [PubMed] [Google Scholar]
- 150.Bai X. Y., Miao D., Goltzman D., Karaplis A. C. 2003. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 278: 9843–9849 [DOI] [PubMed] [Google Scholar]
- 151.Larsson T., Marsell R., Schipani E., Ohlsson C., Ljunggren O., Tenenhouse H. S., Jüppner H., Jonsson K. B. 2004. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 145: 3087–3094 [DOI] [PubMed] [Google Scholar]
- 152.Abe E., Miyaura C., Sakagami H., Takeda M., Konno K., Yamazaki T., Yoshiki S., Suda T. 1981. Differentiation of mouse myeloid leukemia cells induced by 1α,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. USA. 78: 4990–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen T. C., Wang L., Whitlatch L. W., Flanagan J. N., Holick M. F. 2003. Prostatic 25-hydroxyvitamin D-1α-hydroxylase and its implication in prostate cancer. J. Cell. Biochem. 88: 315–322 [DOI] [PubMed] [Google Scholar]
- 154.Bises G., Kállay E., Weiland T., Wrba F., Wenzl E., Bonner E., Kriwanek S., Obrist P., Cross H. S. 2004. 25-hydroxyvitamin D3-1α-hydroxylase expression in normal and malignant human colon. J. Histochem. Cytochem. 52: 985–989 [DOI] [PubMed] [Google Scholar]
- 155.Friedrich M., Diesing D., Cordes T., Fischer D., Becker S., Chen T. C., Flanagan J. N., Tangpricha V., Gherson I., Holick M. F., et al. 2006. Analysis of 25-hydroxyvitamin D3-1α-hydroxylase in normal and malignant breast tissue. Anticancer Res. 26: 2615–2620 [PubMed] [Google Scholar]
- 156.Cross H. S., Bises G., Lechner D., Manhardt T., Kállay E. 2005. The Vitamin D endocrine system of the gut--its possible role in colorectal cancer prevention. J. Steroid Biochem. Mol. Biol. 97: 121–128 [DOI] [PubMed] [Google Scholar]
- 157.Zinser G. M., Suckow M., Welsh J. 2005. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J. Steroid Biochem. Mol. Biol. 97: 153–164 [DOI] [PubMed] [Google Scholar]
- 158.Parise R. A., Egorin M. J., Kanterewicz B. 2006. CYP24, the enzyme that catabolizes the antiproliferative agent vitamin D, is increased in lung cancer. Int. J. Cancer. 119: 1819–1828 [DOI] [PubMed] [Google Scholar]
- 159.Chen G., Kim S. H., King A. N., Zhao L., Simpson R. U., Christensen P. J., Wang Z., Thomas D. G., Giordano T. J., Lin L., et al. 2011. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin. Cancer Res. 17: 817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Johnson C. S., Chung I., Trump D. L. 2010. Epigenetic silencing of CYP24 in the tumor micro-environment. J. Steroid Biochem. Mol. Biol. 121: 338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Albertson D. G., Ylstra B., Segraves R., Collins C., Dairkee S. H., Kowbel D., Kuo W. L., Gray J. W., Pinkel D. 2000. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat. Genet. 25: 144–146 [DOI] [PubMed] [Google Scholar]
- 162.Lassmann S., Weis R., Makowiec F., Roth J., Danciu M., Hopt U., Werner M. 2007. Array CGH identifies distinct DNA copy number profiles of oncogenes and tumor suppressor genes in chromosomal- and microsatellite-unstable sporadic colorectal carcinomas. J. Mol. Med. (Berl.). 85: 293–304 [DOI] [PubMed] [Google Scholar]
- 163.Horváth H. C., Lakatos P., Kósa J. P., Bácsi K., Borka K., Bises G., Nittke T., Hershberger P. A., Speer G., Kállay E. 2010. The candidate oncogene CYP24A1: a potential biomarker for colorectal tumorigenesis. J. Histochem. Cytochem. 58: 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Martin N. D. T., Snodgrass G. J. A., Cohen R. D. 1984. Vitamin D metabolites in idiopathic infantile hypercalcaemia. Arch. Dis. Child. 59: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Online Mendelian Inheritance in Man (OMIM) Hypercalcemia, Idiopathic, of Infancy. MIM Number: 143880. Available at: http://omim.org/entry/143880. Last updated 11/09/2011. [Google Scholar]
- 166.Schlingmann K. P., Kaufmann M., Weber S., Irwin A., Goos C., Wassmuth A., John U., Misselwitz J., Klaus G., Kuwertz-Broking E., et al. 2011. Mutations of CYP24A1 and idiopathic infantile hypercalcemia. N. Engl. J. Med. 365: 410–421 [DOI] [PubMed] [Google Scholar]
- 167.Streeten E. A., Zarbalian K., Damcott C. M. 2011. CYP24A1 mutations in idiopathic infantile hypercalcemia. N. Engl. J. Med. 365: 1741–1742 [DOI] [PubMed] [Google Scholar]
- 168.Dauber A., Nguyen T. T., Sochett E., Cole D. E., Horst R., Abrams S. A., Carpenter T. O., Hirschhorn J. N. 2012. Genetic defect in CYP24A1, the vitamin D 24-hydroxylase gene, in a patient with severe infantile hypercalcemia. J. Clin. Endocrinol. Metab. 97: E268–E274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tebben P. J., Milliner D. S., Horst R. L., Harris P. C., Singh R. J., Wu Y., Foreman J. W., Chelminski P. R., Kumar R. 2012. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1mutations: effects of ketoconazole therapy. J. Clin. Endocrinol. Metab. 97: E423–E427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Petkovich M., Helvig C., Epps T. 2011. CYP24A1 regulation in health and disease. In Vitamin D. 3rd edition. D. Feldman, J. W. Pike, and J. S. Adams, editors. Academic Press, San Diego, CA. 1525–1554. [Google Scholar]
- 171.Petkovich M. P., Jones G. 2011. CYP24A1 and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 20: 337–344 [DOI] [PubMed] [Google Scholar]
- 172.Rhieu S. Y., Annalora A. J., Gathungu R. M., Vouros P., Uskokovic M. R., Schuster I., Palmore G. T., Reddy G. S. 2011. A new insight into the role of rat cytochrome P450 24A1 in metabolism of selective analogs of 1α,25-dihydroxyvitamin D3. Arch. Biochem. Biophys. 509: 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]