Abstract
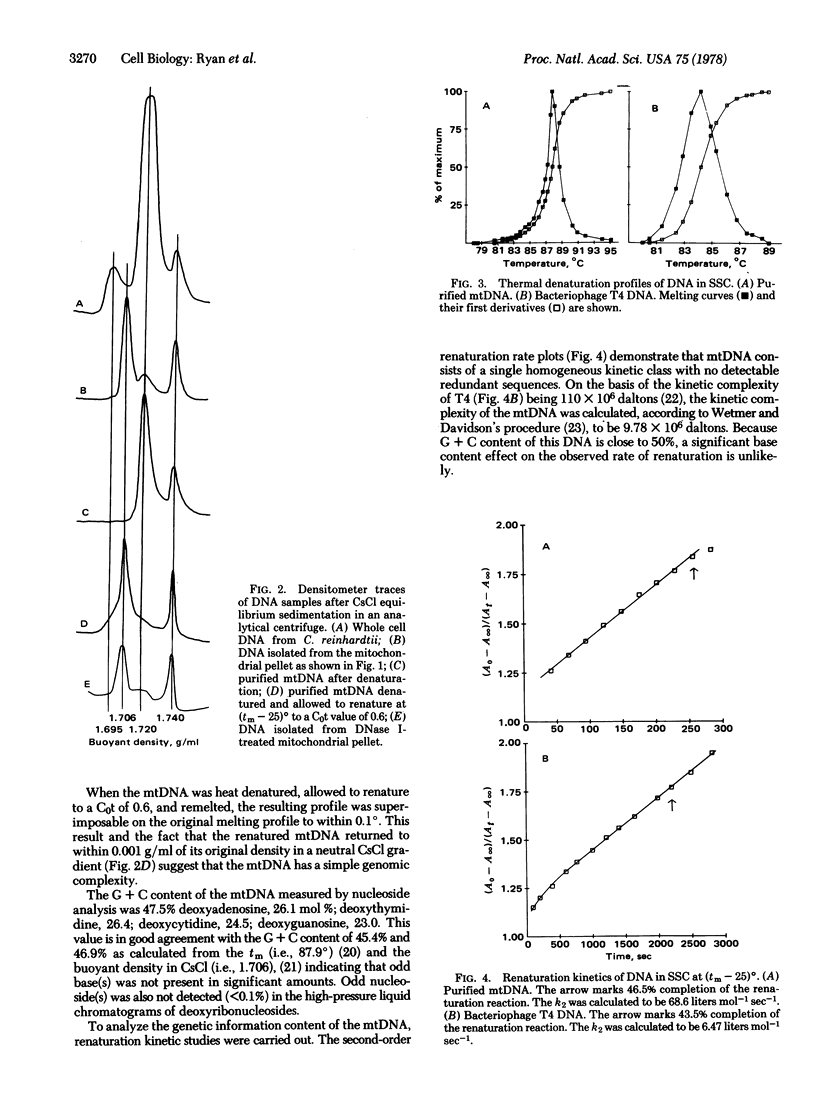
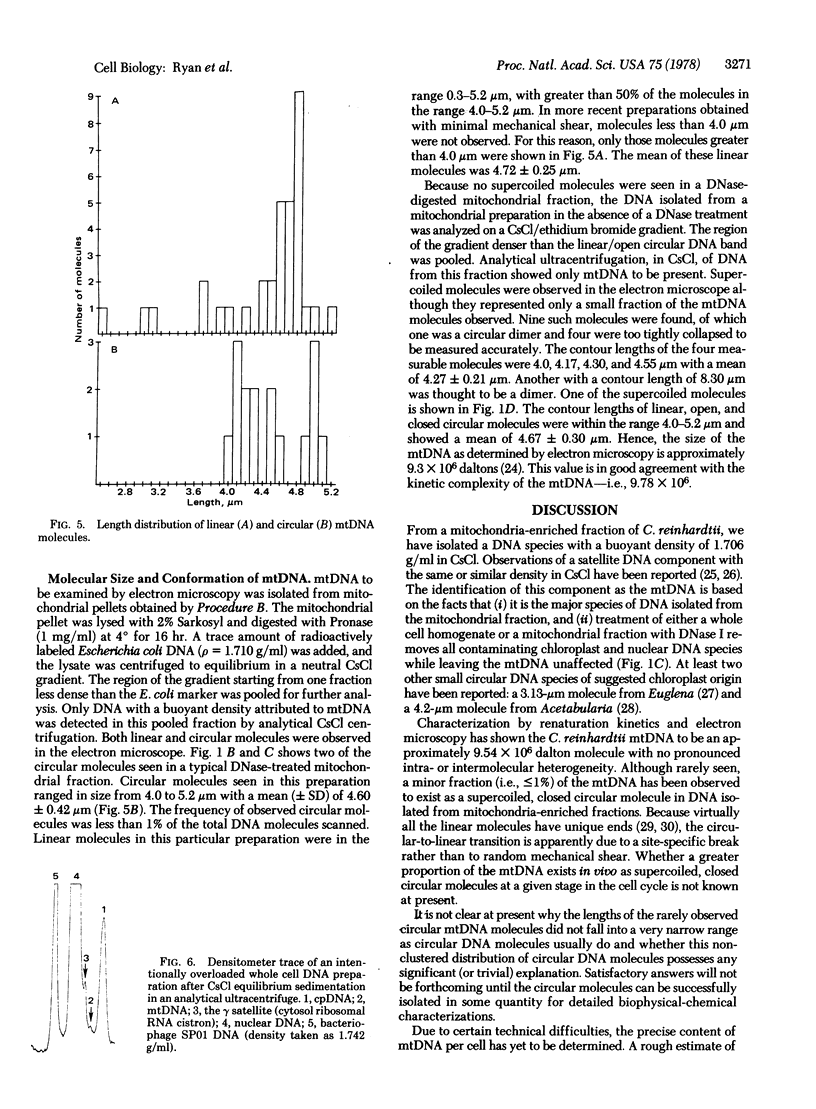
Mitochondrial DNA (mtDNA) has been isolated from a mitochondrial pellet of Chlamydomonas reinhardtii. The mtDNA has a buoyant density of 1.706 g/ml in CsCl, a melting temperature of 87.9 degrees in standard saline citrate, and a nucleoside composition of 47.5% deoxyguanidine plus deoxycytidine with no odd nucleosides. Thermal denaturation and renaturation studies have shown that (i) mtDNA contains no extensive intramolecular heterogeneity nor significant base bias between the complementary polynucleotide chains and (ii) mtDNA renatures as a single homogeneous class with a kinetic complexity of 9.78 X 10(6) daltons. Although rare (less than or equal to 1%), both open and supercoiled circular mtDNA molecules have been observed in the electron microscope. Contour lengths of linear and open and closed circular molecules are all within the range of 4.0-5.4 micron with a mean of 4.67 +/- 0.30 micron. This size is similar to that of animal mtDNA but approximately 1/8 that of the higher plant mtDNAs. The magnitude of mtDNA reiteration in C. reinhardtii is estimated to be of the same order as that of chloroplast DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastia D., Chiang K. S., Swift H., Siersma P. Heterogeneity, complexity, and repetition of the chloroplast DNA of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1157–1161. doi: 10.1073/pnas.68.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behn W., Arnold C. G. Zur Lokalisation eines nichtmendelnden Gens von Chlamydomonas reinhardii. Mol Gen Genet. 1972;114(3):266–272. doi: 10.1007/BF01788896. [DOI] [PubMed] [Google Scholar]
- Bergman K., Goodenough U. W., Goodenough D. A., Jawitz J., Martin H. Gametic differentiation in Chlamydomonas reinhardtii. II. Flagellar membranes and the agglutination reaction. J Cell Biol. 1975 Dec;67(3):606–622. doi: 10.1083/jcb.67.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamire J., Flechtner V. R., Sager R. Regulation of nuclear DNA replication by thechloroplast in Chlamydomonas. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2867–2871. doi: 10.1073/pnas.71.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert H. J. Circular mitochondrial DNA from Acanthamoeba castellanii (Neff-strain). Biochim Biophys Acta. 1973 Oct 12;324(2):199–205. doi: 10.1016/0005-2787(73)90137-8. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Burton W. G., Gillham N. W., Harris E. H. Can a non-Mendelian mutation affect both chloroplast and mithchondrial ribosomes? Proc Natl Acad Sci U S A. 1973 Dec;70(12):3463–3467. doi: 10.1073/pnas.70.12.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K. S., Sueoka N. Replication of chloroplast DNA in Chlamydomonas reinhardi during vegetative cell cycle: its mode and regulation. Proc Natl Acad Sci U S A. 1967 May;57(5):1506–1513. doi: 10.1073/pnas.57.5.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde M. F., Boynton J. E., Gillham N. W., Harris E. H., Tingle C. L., Wang W. L. Chloroplast genes in Chlamydomonas affecting organelle ribosomes. Genetic and biochemical analysis of analysis of antibiotic-resistant mutants at several gene loci. Mol Gen Genet. 1975 Oct 3;140(3):183–220. doi: 10.1007/BF00334266. [DOI] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Structure and replication of mitochondrial DNA from Paramecium aurelia. J Mol Biol. 1975 Oct 5;97(4):593–609. doi: 10.1016/s0022-2836(75)80061-1. [DOI] [PubMed] [Google Scholar]
- Hollenberg C. P., Borst P., van Bruggen E. F. Mitochondrial DNA. V. A 25 micron closed circular duplex DNA molecule in wild-type yeast mitochondria. Stucture and genetic complexity. Biochim Biophys Acta. 1970 May 21;209(1):1–15. [PubMed] [Google Scholar]
- Howell S. H., Walker L. L. Synthesis of DNA in toluene-treated Chlamydomonas reinhardi (DNA replication-chloroplast DNA-cell cycle-electron microscopy). Proc Natl Acad Sci U S A. 1972 Feb;69(2):490–494. doi: 10.1073/pnas.69.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H. Estimation of the molecular length of petite negative yeast mitochondrial DNA. FEBS Lett. 1976 Aug 15;67(2):134–136. doi: 10.1016/0014-5793(76)80350-x. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Physicochemical characterization of mitochondrial DNA from pea leaves. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1830–1834. doi: 10.1073/pnas.69.7.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- Mets L., Bogorad L. Altered chlorplast ribosomal proteins associated with erythromycin-resistant mutants in two genetic systems of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3779–3783. doi: 10.1073/pnas.69.12.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass M. M., Ben-Shaul Y. A novel closed circular duplex DNA in bleached mutant and green strains of Euglena gracilis. Biochim Biophys Acta. 1972 Jun 22;272(1):130–136. doi: 10.1016/0005-2787(72)90041-x. [DOI] [PubMed] [Google Scholar]
- O'Connor R. M., McArthur C. R., Clark-Walker G. D. Respiratory-deficient mutants of Torulopsis glabrata, a yeast with circular mitochondrial deoxyribonucleic acid of 6 mu m. J Bacteriol. 1976 May;126(2):959–968. doi: 10.1128/jb.126.2.959-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz M., Swift H. Mitochondrial nucleic acids and their relation to the biogenesis of mitochondria. Physiol Rev. 1970 Jul;50(3):376–427. doi: 10.1152/physrev.1970.50.3.376. [DOI] [PubMed] [Google Scholar]
- Schlanger G., Sager R. Localization of five antibiotic resistances at the subunit level in chloroplast ribosomes of Chlamydomonas. Proc Natl Acad Sci U S A. 1974 May;71(5):1715–1719. doi: 10.1073/pnas.71.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H. Buoyant density of ribosomal genes in Chlamydomonas reinhardii. Exp Cell Res. 1972 Oct;74(2):568–571. doi: 10.1016/0014-4827(72)90418-1. [DOI] [PubMed] [Google Scholar]
- Singhal R. P. Ion-exlusion chromatography: analysis and isolation of nucleic acid components, and influence of separation parameters. Arch Biochem Biophys. 1972 Oct;152(2):800–810. doi: 10.1016/0003-9861(72)90276-7. [DOI] [PubMed] [Google Scholar]
- Siu C. H., Chiang K. S., Swift H. Characterization of cytoplasmic and nuclear genomes in the colorless alga Polytoma. IV. Heterogeneity and complexity of the nuclear genome. Chromosoma. 1974;48(1):19–40. doi: 10.1007/BF00284864. [DOI] [PubMed] [Google Scholar]
- Siu C. H., Chiang K. S., Swift H. Characterization of cytoplasmic and nuclear genomes in the colorless alga Polytoma. V. Molecular structure and heterogenity of leucoplast DNA. J Mol Biol. 1975 Oct 25;98(2):369–391. doi: 10.1016/s0022-2836(75)80125-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. R., Tewari K. K. Conservation of 70S ribosomal RNA genes in the chloroplast DNAs of higher plants. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3147–3151. doi: 10.1073/pnas.71.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers F., Michelson A. M., Douzou P. Conformational changes of nucleic acids in methanol-water solutions at low temperature. Biochim Biophys Acta. 1970 Sep 17;217(1):1–6. doi: 10.1016/0005-2787(70)90116-4. [DOI] [PubMed] [Google Scholar]
- Wells R., Sager R. Denaturation and the renaturation kinetics of chloroplast DNA from Chlamydomonas reinhardi. J Mol Biol. 1971 Jun 14;58(2):611–622. doi: 10.1016/0022-2836(71)90375-5. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wilson R., Chiang K. S. Temporal programming of chloroplast and cytoplasmic ribosomal RNA transcription in the synchronous cell cycle of Chlamydomonas reinhardtii. J Cell Biol. 1977 Feb;72(2):470–481. doi: 10.1083/jcb.72.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]



