Abstract
An immunochromatographic assay (ICA) based on competitive antigen-coated format using colloidal gold as the label was developed for the detection of the organophosphorus insecticide chlorpyrifos. The ICA test strip consisted of a membrane with a detection zone, a sample pad and an absorbent pad. The membrane was separately coated with chlorpyrifos hapten-OVA conjugate (test line) and anti-mouse IgG (control line). Based on the fact that the competition is between the migrating analyte in the sample and the analyte hapten immobilized on the test strip for the binding sites of the antibody-colloidal gold (Ab-CG) conjugate migrating on the test strip, this study suggests that the relative migration speed between the two migrating substances is a critically important factor for the sensitive detection by competitive ICA. This criterion was utilized for the confirmation of appropriateness of a nitrocellulose (NC) membrane for chlorpyrifos ICA. The detection limit of the ICA for chlorpyrifos standard and chlorpyrifos spiked into agricultural samples were 10 and 50 ng mL−1, respectively. The assay time for the ICA test was less than 10 min, suitable for rapid on-site testing of chlorpyrifos.
Keywords: Organophosphorus pesticide, Chlorpyrifos, Immunochromatographic assay
1. Introduction
Increasing public concern about pesticide contamination of food and the environment has increased the demand for broader pesticide monitoring. Regulatory agencies are criticized for monitoring too few samples. This stems from the high cost, sophistication, and time-consuming sample-preparation procedures involved in current chromatographic methods [1]. Immunochemical techniques began recently to gain acceptance as a fast and cost-effective tools for separating and/or detecting trace amounts of chemicals such as pesticides [1]. Among the various types of immunochemical techniques developed so far, immunoaffinity chromatography, immunoaffinity capillary electrophoresis and ICA could be attractive approaches for broader pesticide monitoring. Immunoaffinity chromatography, especially solid phase extraction, has proven to be an effective method for clean-up and concentration of pesticide-containing samples [2–4]. Immunoaffinity capillary electrophoresis in micellar electrokinetic chromatography mode can be used for the determination of small nonpolar chemicals such as organophosphorus pesticides and has several advantages such as the two dimensional functionality and determination of multiple components simultaneously [5–7]. However, the limitation of low sensitivity must be overcome in order for this technique to be utilized as a practical method for pesticide analysis [7]. The key advantageous feature of ICA using colloidal gold as the label is that it requires an assay procedure of only one step in contrast to that of three or four steps involved in the enzyme-linked immunosorbent assay (ELISA) which is currently the most prevalent type of immunoassay. Another advantageous feature of ICA is that it is easy and convenient to use because of the detection through naked eyes. If samples are screened rapidly by ICA for the presence of a pesticide, prior to chromatographic analysis, many samples could be eliminated from further inspection, which will result in a saving of the time and cost.
Most of ICAs developed so far are aimed at detection of high-molecular weight compounds with multiple binding sites for antibody and, thus, a sandwich assay format with no competition is employed. Meanwhile, ICAs for monovalent low-molecular weight compounds cannot be developed in a classical noncompetitive sandwich format and must be developed in a competitive format. Several competitive ICAs have been developed for small molecules including pesticides [8–13], pharmaceuticals [14–20], hormones [21, 22], and toxins [23–28]. However, the number of ICA developed for small molecules is much smaller than that for large molecules. The reason for the lesser achievement in the field of ICA development for small molecules might be the difficulty in establishing effective competition in a competitive ICA format. A preincubation of Ab-CG conjugate with sample before ICA described in some papers appears to have resulted from such a difficulty [29–31].
In most of the competitive ICAs developed so far, competition is between the migrating analyte and the immobilized analyte hapten (capture antigen) for the binding to the migrating Ab-CG conjugate (Fig. 1). The degree of inhibition of Ab-CG conjugate binding to the capture antigen would be proportional to the frequency of the collision between Ab-CG conjugate and analyte before Ab-CG conjugate binding to the capture antigen. Meanwhile, the collision frequency would depend on the concentration of the pesticide around the migrating Ab-CG conjugate and the time required for Ab-CG conjugate to reach the capture antigen. The concentration of the pesticide around the migrating Ab-CG conjugate would depend on the relative migration speed of the pesticide and Ab-CG conjugate on the test strip. Therefore, it is suggested in this study that the relative migration speed of the two migrating substances is critically important for sensitive detection by competitive ICA. This suggestion is adopted in this study to validate the appropriateness of the membrane selected for ICA of the organophosphorus pesticide chlorpyrifos. Using the selected membrane and a monoclonal antibody to chlorpyrifos, an ICA for this pesticide was developed and validated. This paper also describes the production of a monoclonal antibody to chlorpyrifos and the development of an ELISA for chlorpyrifos both which are prerequisites to the development of an ICA for chlorpyrifos.
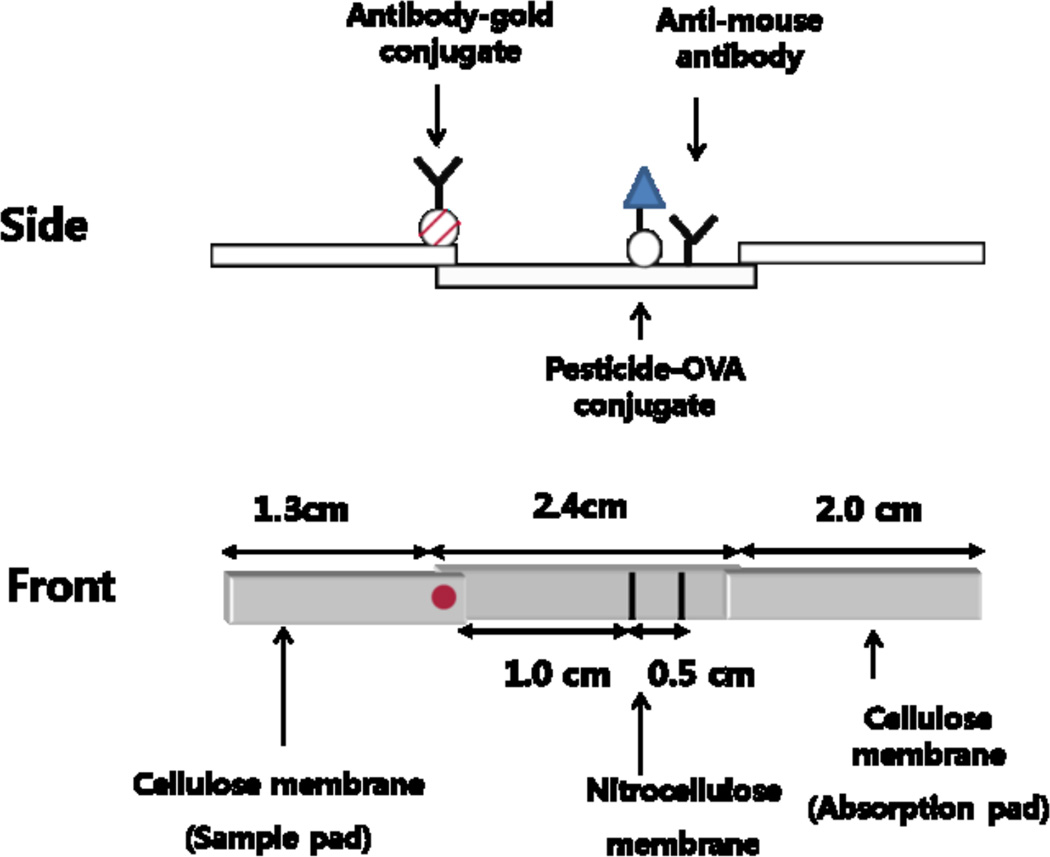
Fig. 1.
Schematic diagram of the lateral flow ICA test strip.
Chlorpyrifos [O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate] is a broad spectrum organophosphorus insecticide that is widely used in agriculture and indoor disinfestation [32, 33]. It is moderately toxic to mammalian species, but extremely toxic to bees and a wide range of aquatic species [34]. Due to its widespread use in agriculture, a high chlorpyrifos residue occurrence in food has been reported [35–37], which poses potential health hazards [38]. Current chlorpyrifos analysis largely is carried out by multiresidue methods using gas chromatography [39].
2. Experimental
2.1. Materials and instruments
Pesticides including chlorpyrifos were purchased from Dr. Ehrenstorfer (Augsburg, Germany). Keyhole limpet hemocyanin (KLH) was obtained from Calbiochem (La Jolla, CA). Indophenyl acetate (IPA), tetrachloroauric acid, BSA, ovalbumin (OVA), horseradish peroxidase (HRP), HRP-labeled goat anti-mouse IgG, phosphate buffered saline (PBS), polyethylene sorbitan monolaurate (Tween 20), and gelatin were purchased from Sigma (St. Louis, MO). The 3,3',5,5'-tetramethylbenzidine (TMB) used was a product of Boehringer Mannheim (Mannheim, Germany). Cellulose (Millipore SA3H645H9) for absorption pad was acquired from Millipore (Billerica, MA). Cellulose for sample pad (Whatman Rapid 24) was obtained from Whatman (Maidstone, UK). Biodyne A (pore size, 1.2 µm), Biodyne B (pore size, 0.45 µm) and Immunodyne ABC membrane (pore size, 0.45µm) were acquired from Pall (Pall Filtrationstechnik GmbH, Dreieich, Germany). Ultrabind membrane (pore size, 0.8 µm) was purchased from Pall (Germany Gelman Sciences GmbH, Rossdorf, Germany). Hybond N+ membrane was obtained from Amersham Int. Plc (Buckinghamshire, UK). Immobilon membrane (pore size, 0.45 µm) was purchased from Millipore (Billerica, MA). Nitrocellulose (NC) membrane used was Immunopore RP membrane from Whatman (Maidstone, UK). Microtiter plates (Maxisorp) were purchased from Nunc (Roskilde, Denmark).
ELISA plates were washed with a Model 1575 ImmunoWash from Bio-Rad (Hercules, CA) and well absorbances were read with Vmax microplate reader from Molecular Devices (Menlo park, CA).
2.2. Synthesis of haptens
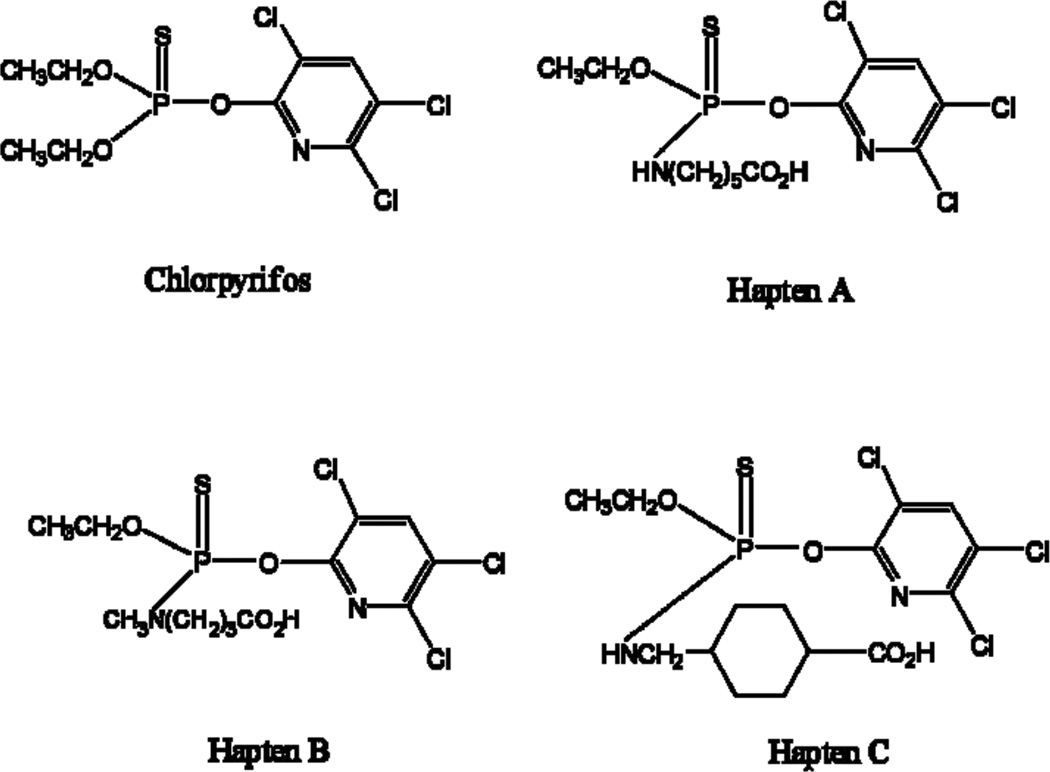
The haptens used for immunization and preparation of coating antigen are presented in Fig. 2. Haptens A and B were used for immunization and Haptens A-C were used as coating antigens for ELISA. Haptens A and B were prepared by the same procedure as described in a previous paper [40]. Hapten C was prepared similarly.
Fig. 2.
Structure of haptens of chlorpyrifos
2.3. Preparation of hapten-protein conjugates for ELISA
Haptens A and B were covalently attached to KLH to be used as immunogens. Hapten A-C were attached to OVA to be used as the coating antigens for serum screening and competitive indirect ELISA. The method of conjugation used was the active ester method via succinimide ester as described in previous papers [41, 42].
2.4. Production of monoclonal antibodies
Procedures for the production of monoclonal antibodies against the hapten-KLH conjugates were similar to those previously described in an earlier paper [43].
2.5. Competitive indirect ELISA
Checkerboard assays, in which several dilutions of ascites were titrated against varying amounts of the coating antigens (Hapten A and B conjugated to OVA), were used to select the most suitable antibodies and to have a rough estimate of appropriate antigen coating and antibody concentrations for competitive assays. The procedure for the checkerboard assays was the same as that for competitive assays (see below) except that addition of pesticide standard or sample is omitted at the competition step.
From the results of the checkerboard assays, one antibody was selected as the most suitable one (4B). Then, to select the most suitable coating antigen, competitive assays were performed under various combinations of immunoreagents at several concentration levels. The concentrations of the antibodies and the chosen coating antigen were further optimized. Additionally, optimization of the concentration of methanol and phosphate ion in assay mixture was carried out as described in previous papers [42, 43].
The procedure for the competitive assay was as follows. All incubations except that for antigen coating were carried out at room temperature. Microtiter plates were coated with 100 µL/well of a coating antigen in carbonate-bicarbonate buffer (50 mM, pH 9.6) by overnight incubation at 4°C. The plates were washed five times with PBST (10 mM PBS containing 0.05% Tween 20, pH 7.4) and were blocked by incubation with 1% gelatin in PBS (200 µL/well) for 1 h. After another washing step, 50 µL/well of analyte dissolved in methanol-PBS and 50 µL/well of the antibody diluted with PBS was added. After incubation for 1 h, the plates were washed and 100 µL/well of a diluted (1/3000) goat anti-mouse IgG-HRP was added. The mixture was allowed to incubate for 1 h, and after another washing step, 100 µL/well of a TMB solution (160 µL of 0.6% TMB-DMSO and 40 µL of 1% H2O2 diluted with 10 mL of citrate-acetate buffer, pH 5.5) was added. The reaction was stopped after an appropriate time (typically 10 min) by adding 50 µL of 2 M H2SO4 and absorbance was read at 450 nm.
2.6. Determination of cross-reactivities in ELISA
Several organophosphorus pesticides and a metabolite of organophosphorus pesticides were tested for cross-reactivity using the indirect ELISA procedure described above. The cross-reactivity values were calculated as follows: (IC50 of chlorpyrifos/IC50 of compound) × 100.
2.7. Preparation of Ab-CG conjugate for ICA
Colloidal gold was prepared by Frens method [44]. The conjugation of anti-chlorpyrifos antibody (4B) to colloidal gold was carried out according to the method of Roth [45]. The optimal ratio of the antibody to colloidal gold was sought by the procedure of Beasley [46].
2.8. Experiments for examining the mobility of the pesticide and Ab-CG conjugate on the membranes
Several membranes for use as the detection zone of the test strip were tested regarding the migration speed of chlorpyrifos and Ab-CG conjugate on them. Since chlorpyrifos is colorless, a colored substitute compound that is similar in polarity to chlorpyrifos was sought and the compound chosen was IPA which is quite nonpolar and has a dark brown color. A total of 7 membranes were tested.
Each of the membranes was mounted onto a polystyrene plastic sheet using double-sided adhesive tape and cut into square pieces (2.0 × 3.0 cm). IPA dissolved in 10% methanol-PBST (10 mM) was spotted (1 µL) on the membrane at the position of 0.2 cm from the bottom and Ab-CG conjugate was spotted (1 µL) on the membrane in parallel to IPA. The membrane strip spotted with the two reagents was inserted into 10% methanol-PBST to elute the reagents and the migrating behavior of the two reagents was observed. The time required for the solvent to reach 2.2 cm height was measured and the membrane strip was taken out of the solvent, dried and the positions of the two reagents were measured. Membranes blocked with 1% BSA for 5, 15 and 60 min and, then, dried for 3 h were also tested.
2.9. Preparation of test strips and test procedure
The structure of the lateral flow strip is illustrated in Fig. 1. To be used as a test line, the capture antigen (Hapten A-OVA) in PBS was applied using a Hamilton syringe as a thin line on the membrane. Anti-mouse IgG in PBS was applied similarly above the capture antigen to be used as a control line. The membrane with test and control line was pasted at the central part of the plastic backing sheet using double-sided adhesive tape. The cellulose membrane to be used as a sample pad was pasted at the lower part of the backing sheet over-crossing previously pasted membrane with 2 mm width. The cellulose membrane to be used as an absorbent pad was pasted at the upper part of the backing sheet over-crossing the membrane with 2 mm width. The assembled plate was cut lengthwise to make square strips of 0.4 cm × 5.7 cm size. Ab-CG conjugate was applied to the upper end of the sample pad. The strip was used either without further elaboration or as in-housed format where the test strip was mounted into a cassette which had a well for sample application and a window for the detection zone. The assay was carried out with the test strip placed horizontally on a flat surface, using 80 µL of serial dilutions of chlorpyrifos standards or samples.
2.10. Optimization of ICA test strip
The test strip was optimized with regard to the concentration of Tween 20 in eluting solvent (10% methanol-PBST). The concentrations of Tween 20 tested were 0, 0.05, 0.075 and 0.1%. The position of the capture antigen (1 µL/cm, 0.5 mg mL−1) was optimized by testing the positions of 0.5, 1.0, 2.5, and 3.0 cm from the bottom of the NC membrane. The concentration of the capture antigen solution tested were 0.0625, 0.125, 0.25, and 0.5 mg mL−1.
2.11. Performance of ICA test strip verified with standard solutions of chlorpyrifos
The optimized ICA was applied to the detection of chlorpyrifos using standard solutions of 102 – 10−1 ng mL−1 and a solution with no pesticide. The minimal detection limit was defined as the lowest concentration level of the pesticide that was scored “positive” in five tests by six test persons.
2.12. Performance of ICA test strip in the detection of chlorpyrifos spiked into agricultural samples
Solutions of chlorpyrifos in methanol for the spiking of rice and lettuce samples were prepared at various concentrations (1000 – 1 ng mL−1). One mL of a spiking solution was added to 1 gram of pesticide-free lettuce leaves or rice grains that had been chopped or ground in fine pieces, respectively. After setting this aside for 24 h, the leaves or grains were incubated in 10 mL of methanol for 10 min with vigorous shaking and then filtered through a Whatman No. 1 filter paper. The container and the residues were rinsed with 5 mL of methanol, the rinsate was filtered, and the filtrate was combined with the previous filtrate. The methanol was evaporated to dryness under reduced pressure, and the residue was extracted with 10 mL of a 10% methanol-PBS. The extract was analyzed with the ICA strip.
2.13. Determination of cross-reactivity in ICA
Several organophosphorus pesticides, a metabolite of organophosphorus pesticides and a carbamate pesticide were tested for cross-reactivity using the ICA developed. The cross-reactivity was estimated visually on a positive/negative basis.
3. Results and discussion
3.1. Production of monoclonal antibodies
All of the twelve mice injected with each of the two immunogens produced sera exhibiting high titer values after the third injection (second boost). Among the twelve antisera, antisera A-5 (from Hapten A-KLH) and B-5 (from Hapten B-KLH) with the highest inhibition by chlorpyrifos were selected for cell fusion. Cloning of the cells in the most inhibited well and screening of the hybridoma clones by indirect ELISA, using several hapten-OVA conjugates as coating antigens and chlorpyrifos as analyte, allowed selection of the most suitable antibody for ELISA. The isotype of the antibody was IgG and the type of light chain was κ.
3.2. Competitive indirect ELISA
The results of the experiments carried out to select the most suitable immunoreagents and their concentrations using various combinations of antibody dilutions and amounts of the coating antigens allowed selection of the optimum condition as the combination of antiserum 4B from Hapten B–KLH (second boost) diluted 1:2700 and the coating antigen Hapten A–OVA at 25 ng/well.
Table 1 presents the effect of the concentration of methanol and phosphate ion on ELISA characteristics at the competition step. The optimum conditions selected were 20% methanol and 100 mM phosphate based on lower IC50 value and/or faster color development.
Table 1.
Influence of concentrations of methanol and phosphate buffer of the assay solution on indirect competitive ELISAa
| Variables | A | B | C (ng mL−1) |
D | |
|---|---|---|---|---|---|
| Methanol (%) | 5 | 1.044 | 0.843 | 322 | 0.030 |
| 10 | 1.117 | 0.952 | 43 | 0.053 | |
| 20 | 1.131 | 0.954 | 27 | 0.052 | |
| 30 | 0.954 | 0.825 | 30 | 0.041 | |
| bPBS (mM) | 10 | 1.197 | 1.093 | 38 | 0.006 |
| 50 | 0.989 | 1.072 | 29 | 0.002 | |
| 100 | 0.814 | 1.120 | 23 | 0.002 | |
| 200 | 0.611 | 1.075 | 23 | 0.002 | |
| 400 | 0.491 | 1.064 | 16 | 0.006 | |
Assay conditions: antibody to Hapten B-KLH, diluted 1/2700 with 10 mM PBS; coating antigen, Hapten A-OVA, 25 ng/well; goat anti-mouse IgG-HRP diluted 1/3000. Maximal absorbance (A), slope (B), IC50 (C, ng/mL), and minimal absorbance (D) are values from the four-parameter sigmoidal fitting. The data are the means of triplicates.
Final concentration of phosphate ion of the competition buffer containing 138 mM NaCl and 2.7 mM KCl.
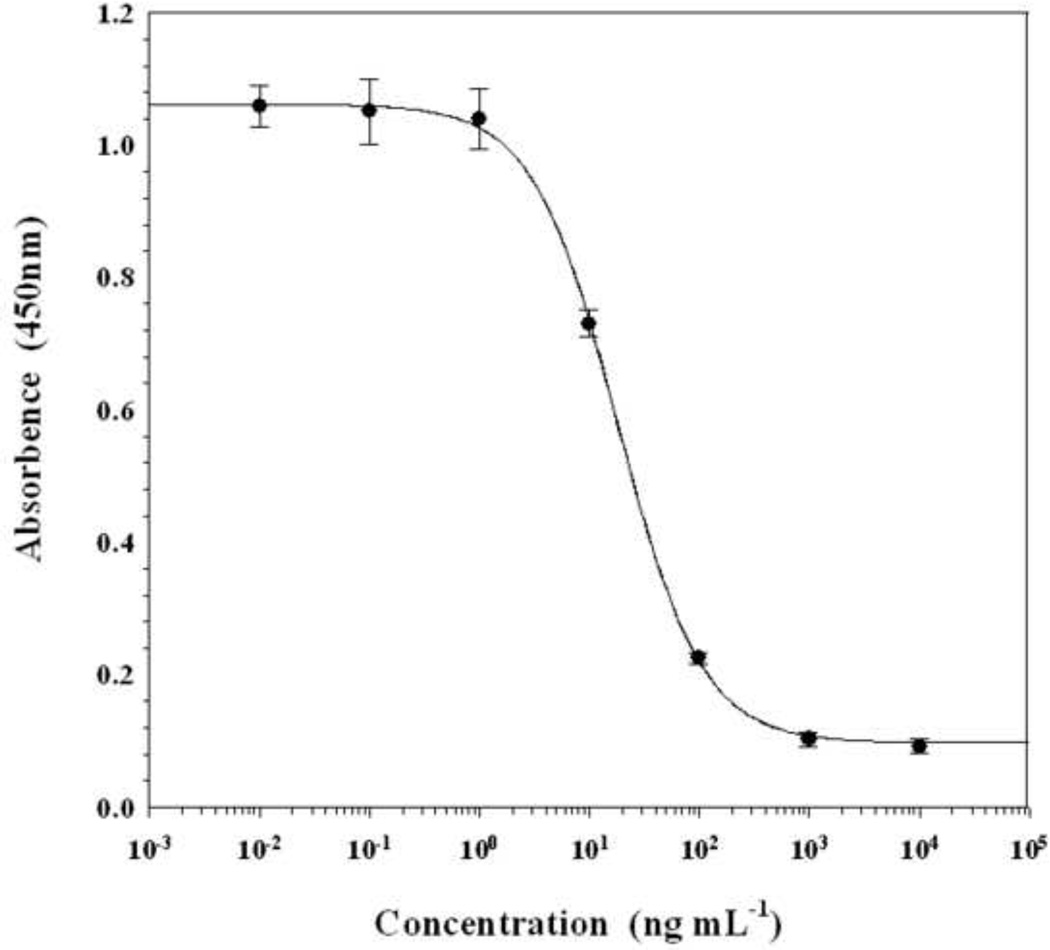
Fig. 3 shows a typical inhibition curve obtained under these optimized conditions. The IC50 value of the assay was 18 ng mL−1 with a detection limit of 2.5 ng mL−1 (10% inhibition).
Fig. 3.
ELISA standard curve for chlorpyrifos by indirect competitive assay. Assay conditions: monoclonal antibody (4B) against Hapten B-KLH, diluted 1/2700; coating antigen, Hapten A-OVA, 25 ng/well; goat anti-mouse IgG-HRP, 1/3000; organic cosolvent, 20% methanol; phosphate buffer, 100 mM; pH 7.4. Each point represents the mean of 16 determinations. Vertical bars indicate ± standard deviation about the mean.
Table 2 shows the cross-reactivities of several organophosphorus pesticides as well as a chlorpyrifos metabolite that was found by the indirect ELISA. The interference to the assays was negligible except with chlorpyrifos-methyl, which showed a cross-reactivity of 13%.
Table 2.
Cross-reactivity of organophosphorus pesticides determined by indirect competitive ELISAa
| Indirect ELISA |
|||
|---|---|---|---|
| Compound | Structure | IC50 (ng mL−1)b |
Cross-reactivity (%)c |
| Chlorpyrifos | 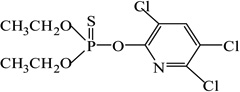 |
24 | 100 |
| Chlorpyrifos-methyl | 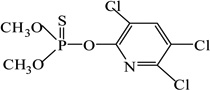 |
190 | 13 |
| Diazinon | 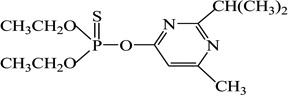 |
ni | - |
| Bromophos-ethyl | 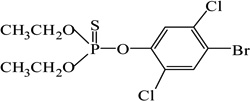 |
ni | - |
| Bromophos-methyl | 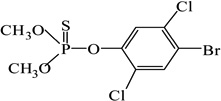 |
ni | - |
| Parathion | 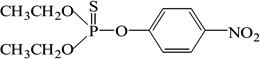 |
ni | - |
| Parathion-methyl | 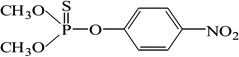 |
ni | - |
| Fenitrothion | 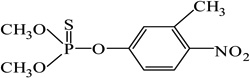 |
ni | - |
| EPN | 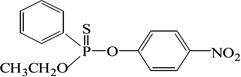 |
ni | - |
| O,O-diethylthiophosphate | 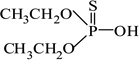 |
ni | - |
Assay conditions were the same as those described in the legend of Fig. 3.
“ni” means no inhibition (cross-reactivity lower than 0.5%).
Cross-reactivity (%) = (IC50 of chlorpyrifos/IC50 of other compound) × 100.
3.3. Mobility of the pesticide and Ab-CG conjugate on the membranes
ICA for the detection of low-molecular weight compounds cannot be developed readily in sandwich format because of their monovalency, and, thus, competition must be introduced between analyte and its competitor. All of the ICAs developed so far for small molecules are based on such competition [8–28]. In all of the ICAs except one developed so far, competition is between the migrating analyte and immobilized analyte hapten for binding to migrating Ab-CG conjugate. In one exceptional case [10], competition is between the analyte and the analyte hapten-protein-CG conjugate for binding to immobilized antibody. In currently prevailing ICAs where competition is between the analyte and immobilized analyte hapten, the intensity of the red color of Ab-CG conjugate coated at the test line is inversely proportional to the degree of inhibition of Ab-CG conjugate binding to the immobilized antigen by analyte. The degree of inhibition of Ab-CG conjugate binding, in turn, would be proportional to the collision frequency between Ab-CG conjugate and the analyte before Ab-CG conjugate binding to the capture antigen. Theoretically, the collision frequency would depend on the concentration of the pesticide around the migrating Ab-CG conjugate and the time required for Ab-CG conjugate to reach the capture antigen.
Longer migration time leads to a higher degree of inhibition. However, since slow migration speed of Ab-CG conjugate requires a long assay performance time which is undesirable, compromise is needed to select an appropriate migration speed of Ab-CG conjugate.
Higher concentration of pesticides around the Ab-CG conjugate leads to a higher degree of inhibition. Meanwhile, the concentration of pesticide around the Ab-CG conjugate would depend on the relative migration speed of the pesticide and Ab-CG conjugate on the test strip. In lateral flow ICA where the sample is applied once only, the analyte must go ahead of the Ab-CG conjugate, but not too far from the conjugate for the maximum overlap of the two substances. To achieve such a condition, the migration speed of the analyte must be slightly higher than that of the conjugate.
NC membranes have been commonly used in most of ICA tests because lateral flow on them is fast enough due to their large pore sizes [47]. We decided to test if NC membrane provides an appropriate relative migration speed for the development of chlorpyrifos ICA. We also tested other kinds of membranes for two reasons. First reason is that it was not certain whether NC membrane is better than others in respect to the relative migration speed of the two substances. The other reason is that we thought other membranes with too slow flow rate due to small pore sizes may be made suitable for ICA through a certain means such as blocking of membranes. We decided to test seven different types of membranes which are currently used as solid supports for adsorption or filtration of biomolecules. Experiments for investigating the migration speed of the pesticide and Ab-CG conjugate on each of these membranes was carried out. Since chlorpyrifos is not visible on the test strip due to its being colorless, IPA with a dark brown color and a polarity similar to chlorpyrifos was used as a substitute for chlorpyrifos. The results of the experiment for estimating the mobility of IPA and Ab-CG conjugate are presented in Table 3. The two reagents moved with tailing, thus, most of the Rf values presented are the range of the spots, i. e., Rf values of the front and tail end. Both reagents were immobile on Immobilon (PVDF) membrane, both in an unblocked and blocked state. Both reagents were mobile on the NC membrane and Ultrabind membrane both in an unblocked and blocked state. In the case of other membranes, IPA was mobile, but Ab-CG conjugate was immobile. Blocking of the membranes with 1% BSA made the Ab-CG conjugate to move on Immunodyne ABC and Biodyne A membrane. Therefore, Biodyne B, Hybond N+ and Immobilon membranes were eliminated from the list of choices due to immobility of the conjugate on them. Immunodyne ABC membrane was eliminated because the two substances moved too slowly. Ultrabind membrane was also eliminated, because the band of the Ab-CG conjugate moved irregularly with too much spreading. NC and Biodyne A membrane blocked with 1% BSA for 15 min were the only ones that satisfied the criteria of the selection. However, Biodyne A membrane was also eliminated because the two substances moved too slowly on the membrane when the membrane was assembled into a strip containing a sample and an absorbent pad. NC membrane was the only one left showing no serious problem. The results of the experiment carried out to compare the degree of inhibition by chlorpyrifos demonstrated that unblocked NC membrane was better than blocked ones.
Table 3.
The mobility of IPA and CG-Ab on seven types of membranesa
| 1% BSA Blockingb | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No blocking | ||||||||||||
| 5min | 15min | 1hr | ||||||||||
| Rf | Flow time (s) of solvent |
Rf | Flow time (s) of solvent |
Rf | Flow time (s) of solvent |
Rf | Flow time (s) of solvent |
|||||
| IPA | CG-Ab | IPA | CG-Ab | IPA | CG-Ab | IPA | CG-Ab | |||||
| 1 Whatman NC (RP) | 0~0.65 | 0.15 | 25 | 0~0.60 | 0~0.30 | 110 | 0~0.50 | 0~0.30 | 120 | 0~0.40 | 0.5~0.70 | 116 |
| 2 UltraBind | 0~0.75 | 0~0.20 | 45 | 0~0.80 | 0~1.0 | 90 | 0~0.90 | 0~1.0 | 131 | 0~0.90 | 0.5~1.0 | 98 |
| 3 Immunodyne ABC | 0~0.25 | nm | 253 | 0~0.30 | 0~0.40 | 229 | 0~0.30 | 0~0.40 | 233 | 0~0.30 | 0~0.45 | 239 |
| 4 Biodyne A | 0~0.28 | nm | 110 | 0~0.27 | 0.02 | 92 | 0~0.40 | 0~0.20 | 120 | 0.20~0.30 | 0~0.70 | 117 |
| 5 Biodyne B | 0~0.25 | nm | 122 | 0~0.25 | nm | 238 | 0.25~0.35 | nm | 256 | 0.25~0.35 | nm | 229 |
| 6 Hybond N+ | 0~0.10 | nm | 67 | 0~0.25 | nm | 347 | 0~0.10 | nm | 321 | 0~0.20 | nm | 319 |
| 7 Immobilon | Nm | nm | nm | nm | nm | nm | nm | nm | nm | nm | nm | nm |
Each membrane strip was a regular square of 3.0 × 2.0 cm. One microliter of IPA and CG-Ab was pipeted at 0.2 cm from the starting point. The reagents were developed by soaking the membranes with 10% methanol-PBST The Rf and flow time were measured when the solvent reached 2.2 cm from the starting point. “nm” means no migration.
Membranes were blocked by incubation with 1% BSA for indicated time.
3.4. Optimization of the ICA format
The test strip made with unblocked NC membrane was further optimized regarding the concentration of Tween 20 in the elution solvent, and the position and concentration of the capture antigen. While the assay performed using the eluting solvent containing 0.05% Tween 20 gave a clear color development at the test line, those with 0.075 and 0.125% Tween 20 gave a faint color development at the test line. This can be attributed to faster migration of Ab-CG conjugate at higher concentrations of Tween 20 which would lead to the shorter time of contact between the Ab-CG conjugate and the capture antigen. The Tween 20 concentration of 0.05% was selected as the most suitable. Among the positions of the capture antigen tested, higher positions made the color development at the test line weaker, and the position of 1 cm from the bottom of the NC membrane was estimated to be appropriate. Among the concentrations of the capture antigen tested, 0.0625 mg per mL gave too faint color development at the test line with negative control. Among the remaining concentrations of 0.125 and 0.25 mg mL−1, the lower concentration was chosen as more appropriate because lesser amount of competitor would give higher sensitivity.
3.5. Performance of ICA test strip
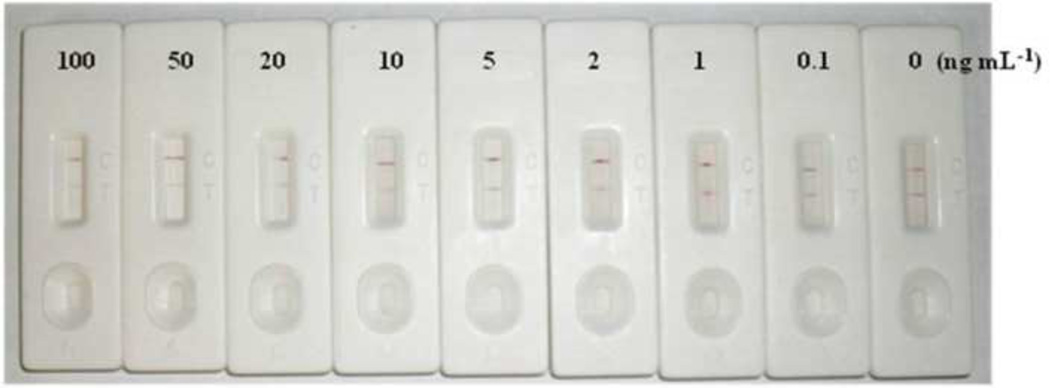
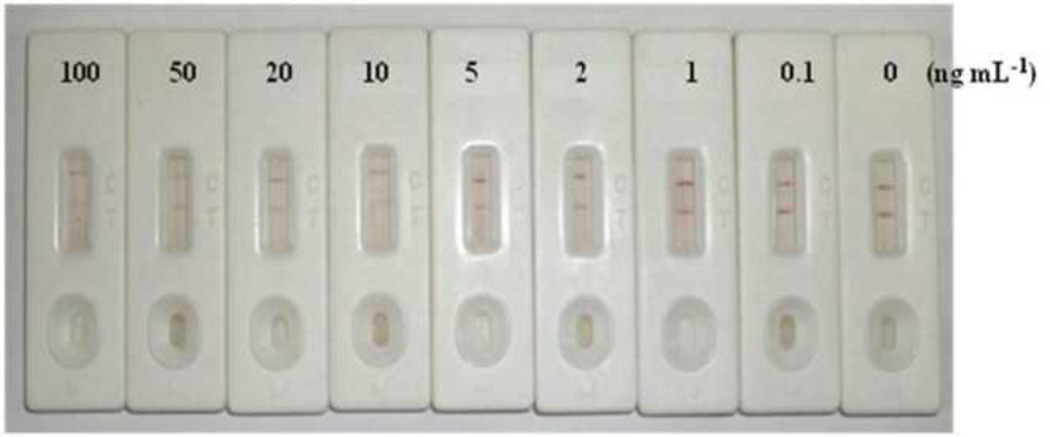
The results of the ICA using the optimized test strip and chlorpyrifos standard solutions are presented in Fig. 4. A negative control without chlorpyrifos gave a clear red color both at the test line and control line. Chlorpyrifos concentrations above 102 ng mL−1 caused no color development at the test line, but gave a red color at the control line. A chlorpyrifos concentration of 101 ng mL−1 caused a distinguishable difference compared to the negative control. Therefore, the visual detection limit for chlorpyrifos was considered to be 101 ng mL−1.
Fig. 4.
Results of the lateral flow ICA for standard solutions of chlorpyrifos. The structure of the ICA strip is illustrated in Fig. 1. Ab-CG prepared by conjugation of the antibody against Hapten B to CG was used. The capture antigen was Hapten A-OVA.
Fig. 5 shows the results of ICA for chlorpyrifos spiked into rice samples. The detection limit was 50 ng mL−1. The result of ICA for chlorpyrifos spiked into lettuce samples was similar. The matrix interference was not very significant. In the case of lettuce samples, adsorption of blue pigments of the lettuce on the membrane was observed, but development of red color at the test line was not significantly masked by the blue pigment.
Fig. 5.
Results of the lateral flow ICA for chlorpyrifos spiked into rice samples. Assay conditions were the same as those described in the legend of Fig. 4.
Table 4 shows the cross-reactivities of several organophosphorus pesticides as well as a chlorpyrifos metabolite that was found by ICA. The interference to the assays was negligible except with chlorpyrifos-methyl, which showed a cross-reactivity of 13% in ELISA.
Table 4.
| Compound | Concentration (µg mL−1) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 102 | 101 | 100 | 10−1 | 10−2 | 10−3 | 0 | ||
| Chlorpyrifos | Control line | a + | + | + | + | + | + | + |
| Test line | - | - | - | ± | + | + | + | |
| Chlorpyrifos-methyl | Control line | + | + | + | + | + | + | + |
| Test line | - | ± | + | + | + | + | + | |
| Diazinon | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Bromophos-ethyl | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Bromophos-methyl | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Parathion | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Parathion-methyl | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Fenitrothion | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| EPN | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| O,O-diethylthiophosphate | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
| Carbaryl | Control line | + | + | + | + | + | + | + |
| Test line | + | + | + | + | + | + | + | |
+: Dark red band. ±: Faint red band. −: No red band.
The assay conditions were the same as those described in the legend of Fig. 4.
4. Conclusion
An ICA based on competitive antigen-coated format using colloidal gold as the label was developed for the detection of the organophosphorus insecticide chlorpyrifos. Since competition is between the migrating analyte in the sample and the analyte hapten immobilized on the test strip for the binding sites of the migrating Ab-CG conjugate, this study illustrated that the relative migration speed on the membrane between the analyte and the Ab-CG conjugate is critically important for the sensitive detection by competitive ICA. This proposal was utilized for the selection of a membrane for chlorpyrifos ICA. Of the seven membranes tested, NC membrane was found to be the most suitable. The detection limit of the ICA for chlorpyrifos standard and chlorpyrifos spiked into agricultural samples, based on the detection by naked eyes, were 10 and 50 ng mL−1, respectively. The assay time for the ICA test was less than 10 min. ICA has an advantage of its being rapid due to the assay procedure of only one step. ICA also has a disadvantage of its being semi-quantitative. Therefore, the developed ICA is suitable for rapid and sensitive on-site testing of chlorpyrifos.
Acknowledgement
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-531-F00013). Partial support was provided by NIEHS P42 ES004699 and PHS OH07550.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammock BD, Gee SJ, Harrison RO, Jung F, Goodrow MH, Li QX, Lucas A, Szekacs A, Sundaram KMS. Immunochemical Methods for Environmental Analysis. In: Van Emon J, Mumma R, editors. ACS Symposium Series 442, American Chemical Society. Washington, DC: 1990. pp. 112–139. [Google Scholar]
- 2.Chuang JC, Van Emon JM, Jones R, Dunford J, Lordo RA. Anal. Chim. Acta. 2007;583:32–39. doi: 10.1016/j.aca.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 3.Liang Y, Zhou S, Hu L, Li L, Zhao M, Liu H. J. Chromatogr. B. 2010;878:278–282. doi: 10.1016/j.jchromb.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Yang M, Huai LF. Advanced Materials Research. 2010;113–114:947–951. [Google Scholar]
- 5.Amundsen LA, Sirén H. Electrophoresis. 2007;28:99–123. doi: 10.1002/elps.200500962. [DOI] [PubMed] [Google Scholar]
- 6.Guzman NA, Blanc T, Phillips TM. Electrophoresis. 2008;29:3259–3278. doi: 10.1002/elps.200800058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suntornsuk L. Anal. Bioanal. Chem. 2010;398:29–52. doi: 10.1007/s00216-010-3741-5. [DOI] [PubMed] [Google Scholar]
- 8.Lyubavina IA, Zinchenko AA, Salomatina IS, Zherdev AV, Dzantiev BB. Russian J. Bioorg. Chem. 2004;30:178–183. [PubMed] [Google Scholar]
- 9.Shim W-B, Yang Z-Y, Kim J-Y, Choi J-G, Je J-H, Kang S-J, Kolosova AY, Eremin SA, Chung D-H. J. Agric. Food Chem. 2006;54:9728–9734. doi: 10.1021/jf0620057. [DOI] [PubMed] [Google Scholar]
- 10.Kaur J, Vikas Singh K, Boro R, Thampi KR, Raje M, Varshney GC, Raman Suri C. Environ. Sci. Technol. 2007;41:5028–5036. doi: 10.1021/es070194j. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J, Chen W, Lu Y, Cheng G. Environ. Pollut. 2008;156:136–142. doi: 10.1016/j.envpol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y-R, Liu S-Y, Gui W-J, Zhu G-N. Anal. Biochem. 2009;389:32–39. doi: 10.1016/j.ab.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Kranthi KR, Davis M, Mayee CD, Russell DA, Shukla RM, Satija U, Kshirsagar M, Shiware D, Kranthi S. Crop Protect. 2009;28:428–434. [Google Scholar]
- 14.Verheijen R, Osswald IK, Dietrich R, Haasnoot W. Food Agric. Immunol. 2000;12:31–40. [Google Scholar]
- 15.Wang X, Li K, Shi D, Xiong N, Jin X, Yi J, Bi D. J. Agric. Food Chem. 2007;55:2072–2078. doi: 10.1021/jf062523h. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Zhang G, Liu Q, Teng M, Yang J, Wang J. J. Agric. Food Chem. 2008;56:12138–12142. doi: 10.1021/jf802648z. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Wang Z, Ferreri M, Su J, Han B. J. Agric. Food Chem. 2009;57:4674–4679. doi: 10.1021/jf900433d. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Wei S, Yang H, Li Y, Deng A. Biosen. Bioelectron. 2009;24:2277–2280. doi: 10.1016/j.bios.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Xie HL, Ma W, Liu LQ, Chen W, Peng C, Xu CL, Wang L. Anal. Chim. Acta. 2009;634:129–133. doi: 10.1016/j.aca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Khamta Y, Pattarawarapan M, Nangola S, Tayapiwatana CJ. Immunoassay Immunochem. 2009;30:441–456. doi: 10.1080/15321810903188284. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen MPA, Vuento M. Acta. Chem. Scand. 1996;50:141–145. doi: 10.3891/acta.chem.scand.50-0141. [DOI] [PubMed] [Google Scholar]
- 22.Leung W, Chan P, Bosgoed F, Lehmann K, Renneberg I, Lehmann M, Renneberg RJ. Immunolog. Mehtods. 2003;281:109–118. doi: 10.1016/j.jim.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Delmulle BS, De Saeger SMDG, Sibanda L, Barna-Vetero I, Van Peteghem CHJ. J. Agric. Food Chem. 2005;53:3364–3368. doi: 10.1021/jf0404804. [DOI] [PubMed] [Google Scholar]
- 24.Xiulan S, Xiaolian Z, Jian T, Zhou J, Chu FS. Int. J. Food Microbiol. 2005;99:185–194. doi: 10.1016/j.ijfoodmicro.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Cho Y-J, Lee D-H, Kim D-O, Min W-K, Bong K-T, Lee G-G, Seo J-H. J. Agric. Food Chem. 2005;53:8447–8451. doi: 10.1021/jf051681q. [DOI] [PubMed] [Google Scholar]
- 26.Shim W-B, Yang Z-Y, Kim J-S, Kim J-Y, Kang S-J, Woo G-J, Chung Y-C, Eremin SA, Chung D-H. J. Agric. Food Chem. 2006;54:9728–9734. doi: 10.1021/jf0620057. [DOI] [PubMed] [Google Scholar]
- 27.Liu B-H, Tsao Z-J, Wang J-J, Yu F-Y. Anal Chem. 2008;80:7029–7035. doi: 10.1021/ac800951p. [DOI] [PubMed] [Google Scholar]
- 28.Shim W-B, Kim K-Y, Chung D-H. J. Agric. Food Chem. 2009;57:4035–4041. doi: 10.1021/jf900075h. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Zhang C, Wang J, Zhang Y. Anal. Chim. Acta. 2005;546:161–166. [Google Scholar]
- 30.Zhang C, Zhang Y, Wang SJ. J. Agric. Food Chem. 2006;54:2502–2507. doi: 10.1021/jf0531407. [DOI] [PubMed] [Google Scholar]
- 31.Li K, Liu L, Chu X. Anal. Sci. 2007;23:1281–1284. doi: 10.2116/analsci.23.1281. [DOI] [PubMed] [Google Scholar]
- 32.Odenkirchen E, Eisler R. Chlorpyrifos Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review. Washington, DC: U.S. Fish and Wildlife Service; 1988. [Google Scholar]
- 33.Newman A. Environ. Sci. Technol. 1995;29:324A–326A. doi: 10.1021/es00007a748. [DOI] [PubMed] [Google Scholar]
- 34.Tomlin CDS, editor. The Pesticide Manual: A World Compendium. 11th ed. Farnham, Surrey, U.K.: The British Crop Protection Council; 1997. p. 238. [Google Scholar]
- 35.Neidert E, Trotman RB, Saschenbrecker PW. J. AOAC Int. 1994;77:18–33. [Google Scholar]
- 36.U.S. Food and Drug Administration. The Food and Drug Administration’s (FDA) pesticide residue monitoring programs 1993. J. AOAC. Int. 1994;77:161A–185A. [PubMed] [Google Scholar]
- 37.KAN-DO (Office and Pesticide Team) Accumulated pesticide and industrial chemical findings from a ten-year study of ready-to-eat foods. J. AOAC Int. 1995;78:614–631. [PubMed] [Google Scholar]
- 38.Cochran RC, Kishiyama J, Aldous C, Carr WC, Pfeifer KF. Food Chem. Toxicol. 1995;33:165–172. doi: 10.1016/0278-6915(94)00124-7. [DOI] [PubMed] [Google Scholar]
- 39.Association of Official Analytical Chemists. Official Methods of Analysis. Arlington, VA: AOAC; 1995. pp. 331–343. [Google Scholar]
- 40.Cho YA, Lee H-S, Park EY, Lee YT, Hammock BD, Ahn KC, Lee JK. Bull. Korean Chem. Soc. 2003;23:481–487. [Google Scholar]
- 41.Kim YJ, Cho YA, Lee HS, Lee YT, Gee SJ, Hammock BD. Anal. Chim. Acta. 2003;475:85–96. [Google Scholar]
- 42.Kim MJ, Lee HS, Chung DH, Lee YT. Anal. Chim. Acta. 2003;493:47–62. [Google Scholar]
- 43.Shim JY, Kim YA, Lee YT, Hammock BD, Lee H-S. J. Agric. Food Chem. 2010;58:5241–5247. doi: 10.1021/jf904528y. [DOI] [PubMed] [Google Scholar]
- 44.Frens G. Nat. Phys. Sci. 1973;241:20–22. [Google Scholar]
- 45.Roth J. Histochem. J. 1982;14:791–801. doi: 10.1007/BF01033628. [DOI] [PubMed] [Google Scholar]
- 46.Beasley JE. Colloidal gold: A new perspective for cytochemical marking. Kent, U.K.: Oxford University Press; 1989. pp. 1–14. [Google Scholar]
- 47.Mansfield MA. In: Lateral Flow Immunoassay. Wong RC, Tse HY, editors. New York: Humana Press; 2009. p. 96. [Google Scholar]