Abstract
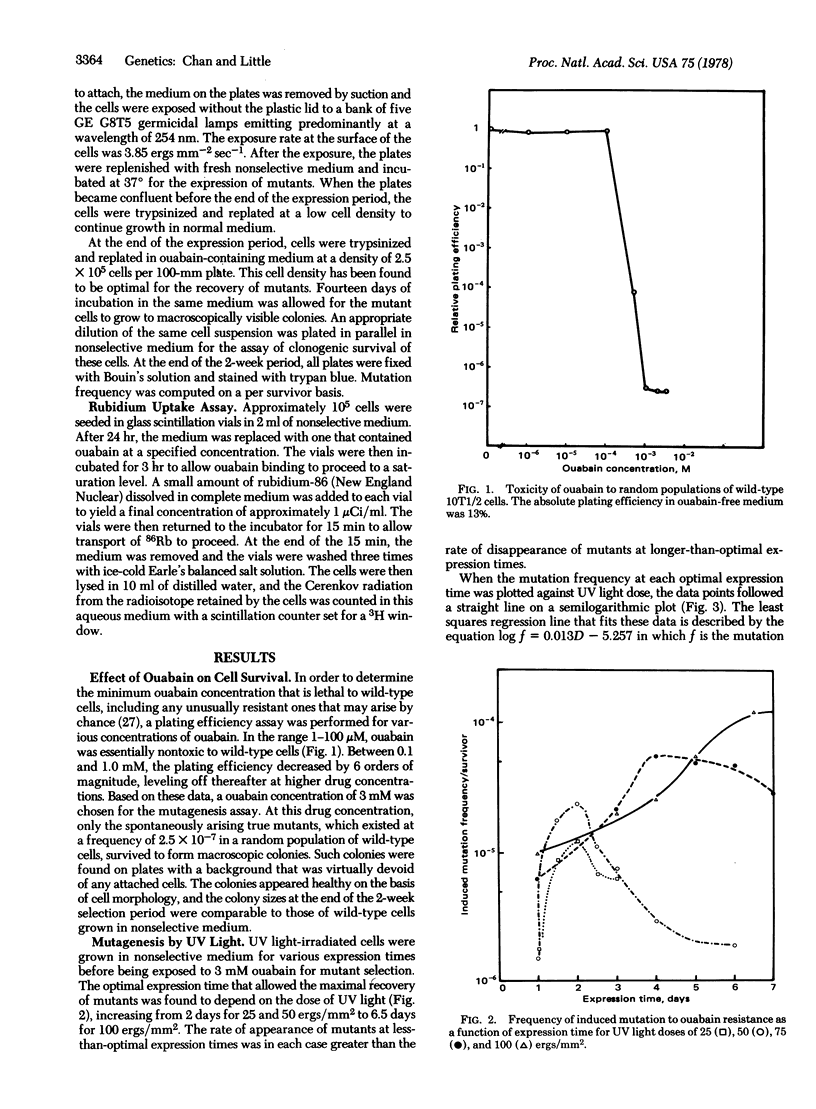
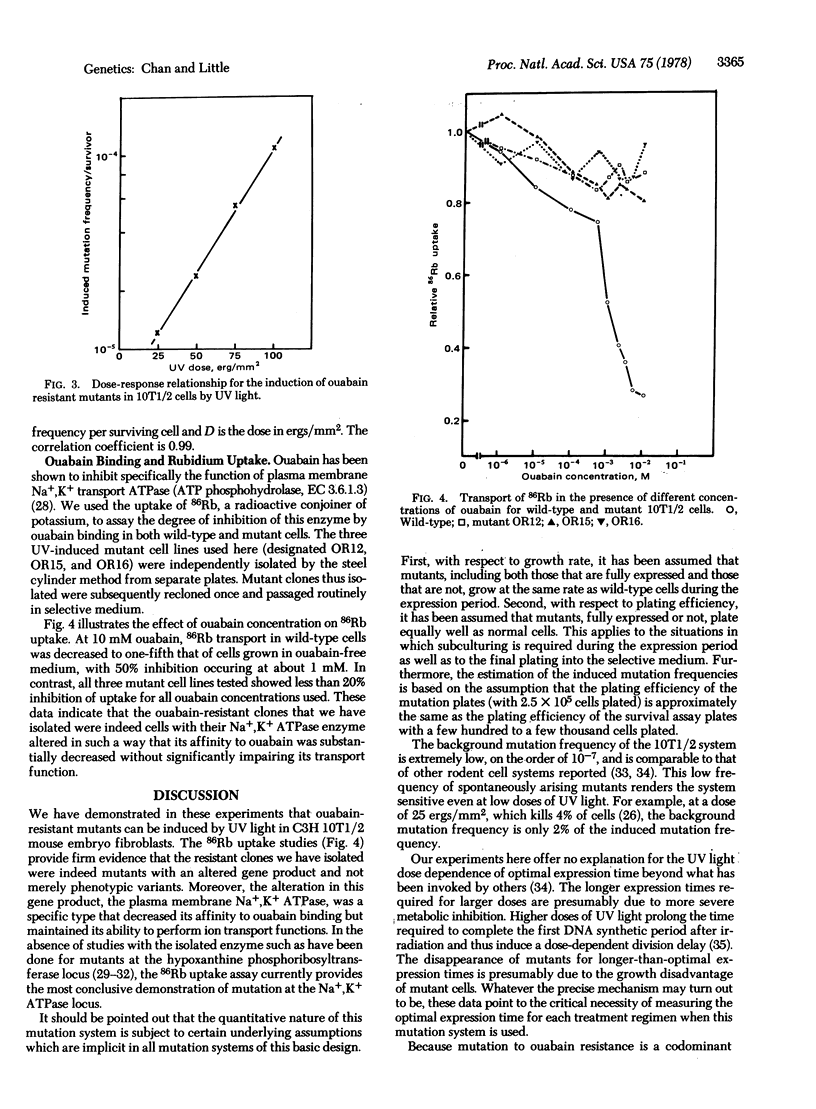
Ouabain-resistant mutants were induced in C3H mouse embryo 10T1/2 fibroblasts by exposure to ultraviolet light, thus making available an in vitro system for studying mutagenesis and oncogenic transformation in parallel. 86Rb uptake studies showed that biochemical mutants at the plasma membrane Na+,K+ transport ATPase (EC 3.6.1.3) locus were being selected for in this system. The optimal expression time for the mutants was found to depend on the dose of ultraviolet light, as was the induced mutation frequency. The ratio of transformation to mutation frequencies was found to be on the order of 10 for four different doses, suggesting that the target size in the cellular genome for transformation may be approximately 10 times the size of the Na+,K+ ATPase gene. We propose that both transformation and mutation induction can now be quantitatively studied in this single system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlett C. F., Turnbull D., Harcourt S. A., Lehmann A. R., Colella C. M. A comparison of the 8-azaguanine and ouabain-resistance systems for the selection of induced mutant Chinese hamster cells. Mutat Res. 1975 Dec;33(2-3):261–278. doi: 10.1016/0027-5107(75)90202-x. [DOI] [PubMed] [Google Scholar]
- Beaudet A. L., Roufa D. J., Caskey C. T. Mutations affecting the structure of hypoxanthine: guanine phosphoribosyltransferase in cultured Chinese hamster cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):320–324. doi: 10.1073/pnas.70.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Chan G. L., Little J. B. Induction of oncogenic transformation in vitro by ultraviolet light. Nature. 1976 Dec 2;264(5585):442–444. doi: 10.1038/264442a0. [DOI] [PubMed] [Google Scholar]
- Chu E. H., Trosko J. E., Chang C. C. Mutational approaches to the study of carcinogenesis. J Toxicol Environ Health. 1977 Jul;2(6):1317–1334. doi: 10.1080/15287397709529533. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Hokin L. E. The sodium-potassium adenosinetriphosphatase. Annu Rev Biochem. 1974;43(0):327–356. doi: 10.1146/annurev.bi.43.070174.001551. [DOI] [PubMed] [Google Scholar]
- DeMars R. Resistance of cultured human fibroblasts and other cells to purine and pyrimidine analogues in relation to mutagenesis detection. Mutat Res. 1974 Sep;24(3):335–364. doi: 10.1016/0027-5107(74)90180-8. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Fenwick R. G., Jr, Caskey C. T. Mutant chinese hamster cells with a thermosensitive hypoxanthine-guanine phosphoribosyltransferase. Cell. 1975 Jun;5(2):115–122. doi: 10.1016/0092-8674(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Heidelberger C. Chemical carcinogenesis. Annu Rev Biochem. 1975;44:79–121. doi: 10.1146/annurev.bi.44.070175.000455. [DOI] [PubMed] [Google Scholar]
- Huberman E., Donovan P. J., DiPaolo J. A. Mutation and transformation of cultured mammalian cells by N-acetoxy-N-2-fluorenylacetamide. J Natl Cancer Inst. 1972 Mar;48(3):837–840. [PubMed] [Google Scholar]
- Huberman E., Mager R., Sachs L. Mutagenesis and transformation of normal cells by chemical carcinogens. Nature. 1976 Nov 25;264(5584):360–361. doi: 10.1038/264360a0. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Cell-mediated mutagenesis of mammalian cells with chemical carcinogens. Int J Cancer. 1974 Mar 15;13(3):326–333. doi: 10.1002/ijc.2910130308. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Mutability of different genetic loci in mammalian cells by metabolically activated carcinogenic polycyclic hydrocarbons. Proc Natl Acad Sci U S A. 1976 Jan;73(1):188–192. doi: 10.1073/pnas.73.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S. A test for mutation theory of cancer: carcinogenesis by misrepair of DNA damaged by 4-nitroquinoline 1-oxide. Br J Cancer. 1977 May;35(5):595–601. doi: 10.1038/bjc.1977.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankovitz R., Buchwald M., Baker R. M. Isolation of ouabain-resistant human diploid fibroblasts. Cell. 1974 Nov;3(3):221–226. doi: 10.1016/0092-8674(74)90135-4. [DOI] [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Krauss S. W., Olsen A. S. Tryptic peptide analysis of normal and mutant forms of hypoxanthine phosphoribosyltransferase from HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):926–930. doi: 10.1073/pnas.74.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienta R. J., Poiley J. A., Lebherz W. B., 3rd Morphological transformation of early passage golden Syrian hamster embryo cells derived from cryopreserved primary cultures as a reliable in vitro bioassay for identifying diverse carcinogens. Int J Cancer. 1977 May 15;19(5):642–655. doi: 10.1002/ijc.2910190508. [DOI] [PubMed] [Google Scholar]
- Pienta R. J., Shah M. J., Lebherz W. B., 3rd, Andrews A. W. Correlation of bacterial mutagenicity and hamster cell transformation with tumorigenicity induced by 2,4-toluenediamine. Cancer Lett. 1977 Jul;3(1-2):45–52. doi: 10.1016/s0304-3835(77)94028-9. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Robbins A. R., Baker R. M. (Na, K)ATPase activity in membrane preparations of ouabain-resistant HeLa cells. Biochemistry. 1977 Nov 15;16(23):5163–5168. doi: 10.1021/bi00642a600. [DOI] [PubMed] [Google Scholar]
- Ruoho A., Kyte J. Photoaffinity labeling of the ouabain-binding site on (Na+ plus K+) adenosinetriphosphatase. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2352–2356. doi: 10.1073/pnas.71.6.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Terzaghi M., Little J. B. Repair of potentially lethal radiation damage in mammalian cells is associated with enhancement of malignant transformation. Nature. 1975 Feb 13;253(5492):548–549. doi: 10.1038/253548a0. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]


