Abstract
Bacillus thuringiensis Cyt proteins are pore-forming toxins that have insecticidal activity mainly against dipteran insects. However, certain Cyt proteins have toxicity to some insect orders, but not toxicity of Cyt1Aa against lepidopteran larvae has been found. Insect specificity has been proposed to rely in specific binding to certain lipids on the brush border membrane of midgut cells since no protein receptors have been described so far. To determine the molecular basis of Cyt1Aa insect specificity we compared different steps of Cyt1Aa mode of action in a susceptible insect as the dipteran Aedes aegypti and also in the non-susceptible lepidopteran Manduca sexta. Our data shows that the lack toxicity of Cyt1Aa to M. sexta larvae does not rely on protoxin processing, membrane binding interaction, and oligomerization of Cyt1Aa since these steps were similar in the two insect species analyzed.
Keywords: Cyt toxins, Bacillus thuringiensis, mode of action, membrane binding, oligomerization
1. Introduction
Bacillus thuringiensis (Bt) form a group of bacteria that upon sporulation produces insecticidal proteins called Cry and Cyt. Different Bt strains produce a variety of Cry or Cyt toxins that give insecticidal specificity to each Bt isolate. Cry and Cyt toxins are pore-forming toxins (PFT) that insert into the cell membrane of their hosts after undergoing structural changes making pores and killing cells by osmotic shock [2, 13, 19].
Among the most used Bt strains for insect control is Bt subs. israelensis (Bti) that is highly effective against dipteran insects such as mosquitoes and black flies that are important vectors of human diseases like malaria or dengue fever. Bti produce four Cry toxins (Cry4Aa, CryBa, Cry10Aa and Cry11Aa) and two Cyt toxins (Cyt1Aa and Cyt2Ba) [2, 10]. Cry toxins produced by different Bt strains show toxicity to a number of dipteran, coleopteran and lepidopteran insects. In the case of Cry toxins insect specificity relies on specific recognition of certain larvae midgut proteins called receptors [21]. In contrast, Cyt toxins are mainly dipteran specific [2, 25, 29]. In the case of Cyt1Aa that is toxic to Aedes aegypti mosquito larvae it was also shown that this protein is toxic to certain coleopteran pest [15]. However, the toxicity of Cyt1Aa against lepidopteran insects is still questionable since it was reported that this toxin may be toxic to Plutella xylostella but a follow up study concluded that Cyt1Aa lacked toxicity to P. xylostella, thus this discrepancy remains to be solved [18, 24]. Interestingly, most Cyt toxins also show hemolytic activity and are toxic to certain mammalian cell lines, to cancer cells and to several bacterial species [7, 27, 28, 32]. In the case of Cyt toxins no protein receptors have been described so far and insect specificity was proposed to rely in specific binding to certain lipids on the brush border membrane of midgut cells although the molecular mechanism of Cyt1Aa insect specificity remains to be determined [12, 22].
One of the most interesting features of Cyt1Aa toxin is its capacity to synergize certain Cry toxins such as Cry4A, Cry4Ba and Cry11Aa [4, 30, 31]. This synergistic effect of Cyt1Aa on Cry4Ba or Cry11Aa depends on their binding interaction and it was proposed that Cyt1Aa is a functional Cry11Aa membrane receptor [3, 20].
The three dimensional structure of three Cyt proteins has been solved showing a single α–β domain composed of two outer layers of α-helix hairpins wrapped around a β-sheet [8, 9, 17]. The α-helices have an amphiphilic character, with the hydrophobic residues packed against the β-sheet [25]. Cyt1Aa is synthesized as a protoxin of 27 kDa that undergoes proteolytic cleavage in the amino- and carboxy-terminal ends yielding an activated toxin of 24 kDa. Cyt toxins interact with non-saturated membrane lipids, such as phosphatidylcholine, phosphatidylethanolamine and sphingomyelin [12]. The proteolytic activation of Cyt1Aa in the presence of membranes results in the formation of high molecular weight oligomers of more than 400 kDa that are proposed to be formed by ~16 Cyt monomers [6, 22]. Mutants of Cyt2Aa that affect oligomer formation have reduced hemolytic and insecticidal activity indicating that oligomerization is a crucial step for Cyt toxin action [23].
To determine the molecular basis of Cyt1Aa insect specificity we compared different steps of Cyt1Aa mode of action in a susceptible insect such as A. aegypti and also in the non-susceptible lepidopteran Manduca sexta. Our data shows that the lack of Cyt1Aa toxicity to M. sexta larvae does not rely on protoxin processing, membrane binding interaction, and oligomerization since these steps were similar in the two insect species analyzed.
2. Materials and Methods
2.1 Production of Cyt1Aa crystals
The Bt acrystalliferous strain 407 was transformed with pWF45 plasmid containing the cloned cyt1Aa gene. Toxin crystals were produced by growing the strain on HCT media plates supplemented with erythromycin (10 μg/ml) for 3 days at 30°C as previously reported [16]. Crystal production was verified by light microscopy. Cultures were recovered and washed three times with 3M NaCl /0.5 M EDTA, pH 8.0, and four times with distilled water and 1mM PMSF. Crystals were purified by discontinuous sucrose gradient as previously described [28]. Cyt1Aa containing fractions were washed and stored in 50 mM Tris, 1mM PMSF, pH 8.0.
2.2 Toxin solubilization
For the analysis of toxin solubilization at different pH’s, 5 μg of Cyt1Aa crystals were centrifuged at 13200 rpm, 4°C, for 10 min of a tabletop centrifuge (Eppendorf, Hamburg, Germany), and the pellet was suspended in either 50 mM phosphate buffer at a pH of 6, 7, 8, and 12 or 50 mM carbonate buffer at pH 9, 10 and 11. DTT was added to a final concentration of 10 mM. Crystals were incubated for 1 hour at 37°C with slight shaking. Soluble protein was recovered by centrifugation for 10 min, at 13200 rpm, 4°C. Five μl of supernatant were separated in 15% SDS-PAGE gel and stained with Coomassie blue. For all other experiments, 15 μg of Cyt1Aa crystals were solubilized with 50 mM carbonate buffer pH 10.5 as described above.
2.3 Preparation of brush border membrane vesicles (BBMV)
For BBMV preparation midgut tissue of either 4th instar A. aegypti larvae or 3rd instar M. sexta larvae were dissected. Midguts and caeca were recovered, intestinal content cleared and the tissue washed and stored in cold MET buffer (300 mM Mannitol, 5 mM EGTA, 1 M Tris-HCL, pH 7.4), supplemented with 1 mM PMSF and 5 mM DTT (buffer A). A. aegypti midgut tissue was homogenized in 5 ml buffer A and then 4.5 ml of cold buffer A were added with 500 μl of 240 mM MgCl2 and let stand on ice for 20 min. The mixture was then centrifuged at 3,000 xg for 15 min at 4°C. The supernatant was recovered and transferred to a fresh tube. The membrane pellet was homogenized and centrifuged twice as described above. The three supernatants were pooled and centrifuged at 100,000 xg for 10 min, at 4°C. The supernatant from this centrifugation was discarded and pellet collected in buffer A, aliquots made and stored at −70°C until used. For M. sexta, 3 g of midgut tissue were homogenized in a 1:10 ratio with cold buffer A. After that, an equal volume of 24 mM MgCl2 was added and let stand on ice for 15 minutes. The supernatant was recovered by centrifugation at 2,500 xg for 15 min at 4°C and transferred to a fresh tube. It was again centrifuged at 30,000 xg for 30 min at 4°C. The pellet was suspended in 1:1 volume of cold buffer A and 24 mM MgCl2. Centrifugation steps were repeated once more. The final pellet was suspended in 1 ml cold buffer A and aliquoted. BBMV were quantified by Lowry method (Bio-RAD, USA). M. sexta midgut juice was obtained from 3rd instar larvae by recovering food content from dissected larvae and centrifugating for 5 min at 9,300 xg on a tabletop centrifuge. The supernatant was stored at −20°C.
2.4 Liposome preparation
All lipids were obtained from Avanti Polar (Alabaster, Alabama, USA). For liposome preparation a total of 1.3 μmol of lipids were mixed in a 10:3:1 ratio of phosphatidilcholine, cholesterol and stearylamine. Lipids were carefully spread by rotation on the inside of crystal tube washed with chloroform, forming a lipid film by evaporation of solvent with the help of a nitrogen flow. Afterwards, the lipid film was completely dried in vacuum for 12 h on a Speed Vac SVC100 (Savant Instruments Inc, Holbrook, USA). Liposomes were hydrated with 1.3 ml of 10 mM CHES, 150 mM KCl, pH 9.0, letting them stand for 10-30 min, and removing oxygen with a nitrogen flow. The mixture was capped and briefly vortexed a couple of times to obtain multilamellar vesicles. Small unilamellar vesicles (SUV) were obtained after sonication the mixture for few min, using 1 min pulse in a Branson 1200 waterbath (Branson, USA), with 1 min rest on ice between each pulse. Liposomes were used immediately or stored at 4°C at most 2 days.
2.5 Cyt1Aa activation
Soluble Cyt1Aa protein was quantified by the Bradford method (Bio-RAD, USA) using bovine serum albumin as standard. Either proteinase K (Sigma-Aldrich) was added at 1:10 m/m ratio (proteinase K/Cyt1Aa) or M. sexta midgut juice at 1% or 10% v/v ratio of midgut juice/Cyt1Aa to 40 μg of solubilized Cyt1Aa. Incubation at 30°C was done for 30, 60 or 120 minutes. The reaction was inhibited by the addition of 1mM PMSF and immediately centrifuged for 10 min at 16,000 xg, 4°C. The supernatant was recovered and quantified and 3 μg of each digestion were loaded and run in a 15% SDS-PAGE gel and stained with Coomassie Blue. N-terminal sequencing was performed at the facilities of Instituto de Biotecnología UNAM, after SDS-PAGE 7 % and transfer onto polyvinylidene difluoride membranes.
2.6 Cyt1Aa oligomerization
For oligomerization of Cyt1Aa 400 ng of soluble Cyt1Aa protoxin were activated with proteinase K 1:10 (w/w), 10% or 1% M. sexta gastric extract in the presence or absence of 90 ul synthetic liposomes for a final volume of 100 ul, adjusted with 50 mM carbonate buffer pH 10.5. Protoxin was incubated 1 hr at 37°C, and reaction stopped with 1mM PMSF. 20 ul of reaction was heated at 65 °C and were separated on a 15% SDS-PAGE gel and transferred to a PVDF membrane (Millipore) for 16 h at 4 °C and 150 mA. The membrane was blocked for 1 h at room temperature with 5% skim milk in 0.1% Tween-20/PBS. The membrane was washed with 0.1% Tween-20/PBS. Cyt1Aa was detected by incubating 1 h with a polyclonal antibody for Cyt1Aa at 1:70,000 in 0.1% Tween-20/PBS. Washing was done as before, followed by incubation with 1:10,000 HRP-anti rabbit antibody (Sigma-Aldrich) in 0.1% Tween-20/PBS. After washing, the membrane was revealed with SuperSignal reagent (Pierce). Cyt1Aa oligomerization was also analyzed by activation of 200 ng of soluble protoxin with 40 ng proteinase K in the presence of A. aegypti or M. sexta 20 μg BBMV protein in 100 μl of 50 mM Na2CO3 pH 10.5 for 2 h at 37°C. Finally, 1 mM PMSF was added to stop proteolysis. Membrane fractions were separated by centrifugation 30 min at 60 000 rpm and each sample was heated 3 min at 50 °C before loading SDS-PAGE and visualized by western blot using polyclonal anti-Cyt1A antibody as described above.
2.7 Cyt1Aa labeling and detection
For biotinylation of Cyt1Aa, the proteinase K activated Cyt1Aa was dialyzed overnight at 4 °C against a borate buffered solution at pH 8.6 (0.05M boric acid, 0.05M NaOH, 0.15M NaCl) at a 1:1000 ratio of toxin/solution. Dialyzed toxin was recovered and quantified by UV absorbance on a Nanodrop 2000 equipment (Thermo Scientific, Rockford, USA). Biotinylation reagent, 20 μl, (Amersham Biosciences, Sweden) was added for each 600 μg of activated Cyt1Aa and incubated at room temperature for 1 hour. Excess biotin was removed by passing through a Sephadex G25 column equilibrated and washed with PBS by centrifugation for 2 min at 2000 rpm on a free angle rotor. The biotin was detected by immunoblotting. Briefly, samples were loaded and run in a 15% SDS-PAGE gel and transferred to PVDF membrane (Millipore, USA). The membrane was blocked for 20 min with 2% Tween-20/PBS solution and washed twice with 0.1% Tween-20/PBS. HRP-streptavidin (GE Healthcare) was added at 1:5000 in 1% Tween 20/PBS and incubated for 1 h at room temperature, washed twice as before and two times with PBS. Finally the membrane was revealed with SuperSignal reagent (Pierce, USA).
For fluorescent labeling of the Cys190 of Cyt1Aa with Alexa Fluor 350 (Life Technologies, USA), proteinase K activated Cyt1Aa was purified by ion exchange chromatography on a DEAE column (Toyopearl, Germany) washed and equilibrated with 20 mM Tris at pH 7.5. Elution of Cyt1Aa fractions were monitored by UV absorbance and Coomassie blue staining. Collected Cyt1Aa fractions were concentrated with Amicon Ultra 4 NMWL 5000 filters (Millipore, USA) and quantified by UV absorbance on a Nanodrop 2000 as before. Before labeling, activated Cyt1Aa was incubated for 5 min with 1 mM DTT. Reduced Cyt1Aa was washed with 10 volumes of degassed PBS on an Amicon Ultra-4 column. For labeling of Cyt1Aa, a 50 molar excess of Alexa-350 was added in the presence of 1mM EDTA and degassed PBS in a total reaction volume of 400 μl. Incubation was done for 2 h at 37°C, in the dark. Excess label in the sample was removed by dialyzing exhaustively with degassed PBS and then passing through a Sephadex G25 column equilibrated with degassed PBS. Labeled Cyt1Aa was eluted and labeling efficiency verified by UV absorbance at 280 nm and 364 nm on a Nanodrop 2000 equipment. Absorbance at 364 nm of labeled Cyt1Aa was corrected by determining the factor of absorbance of non-labeled Cyt1Aa at this wavelength. The following formula was used to determine labeling stoichiometry considering that activated Cyt1Aa at a concentration of 1 g/L will have an absorbance of 0.898 at 280 nm (calculated from Cyt1Aa sequence), an extinction coefficient (ε) of 19,000 for AlexaFluor 350, and a molecular weight of 24 kDa for activated Cyt1Aa.
2.8 Cyt1A binding competition
For binding competition of Cyt1Aa, 10 μg of A. aegypti or M. sexta BBMV were incubated for 30 min at 37 °C with 0, 100, 500, and 1000 molar excesses of non-biotinylated Cyt1Aa. Afterwards, 5 nM of biotinylated Cyt1Aa was added to the samples and incubated for a further 30 min at 37°C. Samples were centrifuged for 1 h at 90000 rpm, 4 °C. Pellets were suspended in 15 μl of PBS, loaded on a 15% SDS-PAGE gel and transferred to a PVDF membrane. Biotinylated Cyt1Aa was detected as before.
2.9 Fluorescence quenching on BBMV
All readings were done using an AMINCO Bowman Series 2 spectrofluorometer. Four μg of AlexaFluor 350 labelled Cyt1Aa were incubated for 1 h in the presence or absence of 10 μg of BBMV derived from A. aegypti or M. sexta midguts. Afterwards, samples were centrifuged for 1 h at 90000 rpm, 4 °C. The supernatant was recovered and the pellets were suspended in 50 mM carbonate buffer, pH 10.5. Supernatant of Cyt1Aa without BBMV and pellets obtained after incubations with BBMV were analyzed. KI was added to samples at final concentration of 0, 50, 100, 250 and 500 mM. KCl was used to maintain ionic strength at 500 mM in all samples. Na2S2O3 is added at 8 mM to the sample to avoid production iodine ions. Volumes were adjusted to 250 μl with sample buffer. Equivalent samples without labeled Cyt1Aa but with KI were used as a control. Quenching efficiency was calculated as the ratio of F/F0, where F and F0 are the AlexaFluor 350 fluorescence measurements in the presence or absence of KI, respectively. The Stern-Volmer constant was calculated as the slope of the linear regression of the quenching curves.
3. Results
3.1 Solubilization of Cyt1Aa at different pH’s
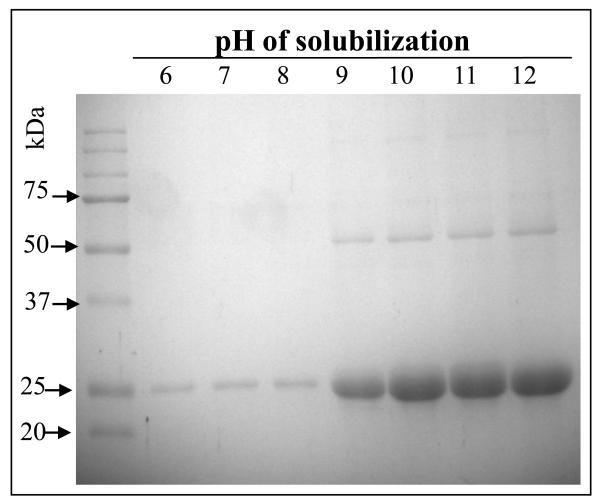
Cyt1Aa showed a LC50 toxicity value of 925.5 (602.5-1824.3) ng/ml to 4th instar A. aegypti larvae and no toxicity to M. sexta neonate larvae at the highest toxin concentration tested of 2000 ng/cm2. In comparison, previously reported toxicity of Cry1Ab toxin showed a LC50 value of 1 ng/cm2 to M. sexta [16]. The first step in the mode of action of Cyt1Aa is its solubilization in the alkaline pH of the midgut of susceptible larvae [25]. Lepidopteran insects have alkaline pH from 8 to 10 in the different midgut regions similar to that in dipteran larvae, although the pH gradients encountered throughout the larval gut is different in both insect orders [11, 26]. To determine if Cyt1Aa solubilization could be a limiting step at certain pH’s, Cyt1Aa crystals were solubilized at different pH’s. Figure 1 shows that Cyt1Aa was readily solubilized from pH 9 up to pH 12 indicating that toxin solubilization should not be a limiting step in toxicity of Cyt1Aa to M. sexta.
Figure 1.
Cyt1Aa protoxin is efficiently solubilized at alkaline pH’s. Spore crystals suspensions were incubated at different pH’s as indicated in Materials and Methods and the soluble proteins were separated by SDS-PAGE after removal of the insoluble material by centrifugation.
3.2 Cyt1Aa activation by M. sexta midgut juice
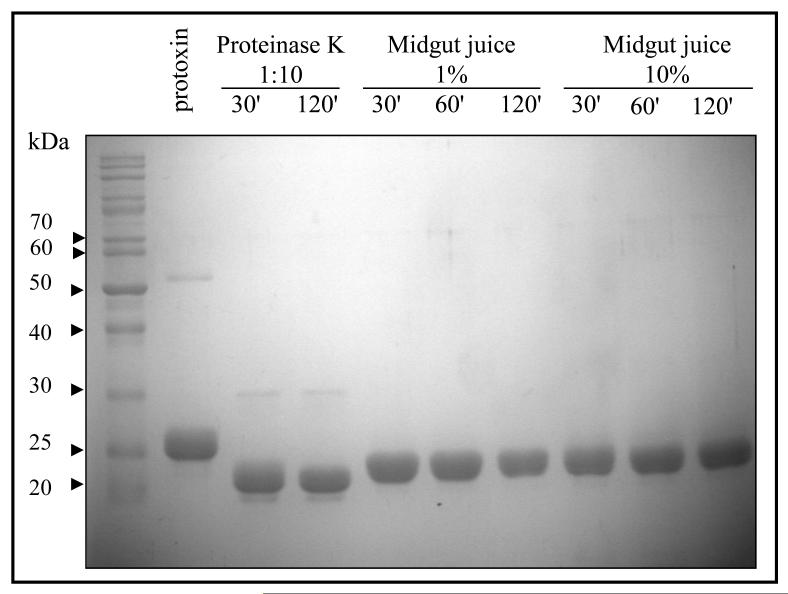
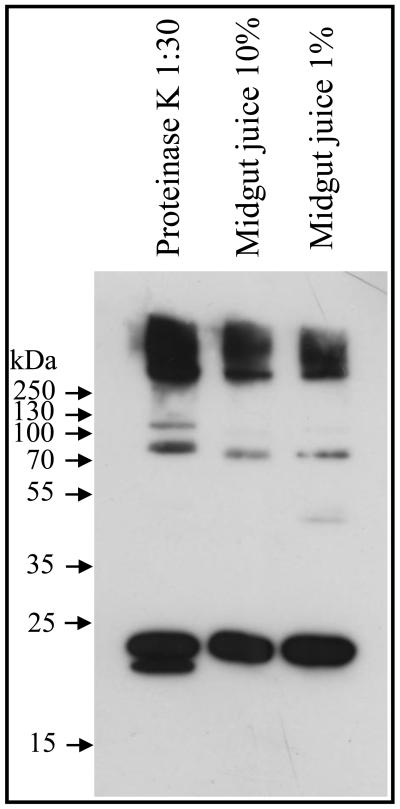
For toxicity, Cyt1Aa is activated by midgut proteases by cleavages in both the amino- and carboxy-terminal ends [1, 25]. In vitro, Cyt1Aa can also be activated by trypsin or proteinase K treatment although both proteases show different sites for activation: while trypsin induced a cleavage at the N-terminal end at residue Arg25, activation with proteinase K induced cleavages at Arg30 and Val31 [1]. These differences in the processing of the toxin have a slight effect in toxicity since Cyt1Aa trypsin activated toxin showed less efficient hemolysis activity than proteinase K activated toxin [1]. To analyze if M. sexta midgut proteases could affect Cyt1Aa activation and toxicity, Cyt1Aa protoxin was activated with M. sexta midgut juice at two different concentrations and different time points and the activated toxin was analyzed by SDS-PAGE electrophoresis. For comparison Cyt1Aa protoxin was also activated with proteinase K. Figure 2 shows that Cyt1Aa protoxin activated with midgut juice from M. sexta larvae yielded a band of 24 kDa. Bands of slightly smaller size (~ 23 kDa) were obtained after proteinase K treatment. Importantly, the Cyt1Aa activated toxin resists high M. sexta midgut juice concentrations indicating that toxin degradation could not account for the lack of toxicity of Cyt1Aa in M. sexta (Fig. 2). The N-terminal end sequence of Cyt1Aa after proteinase K or M. sexta midgut juice treatment was determined. The Cyt1Aa protein activated with M. sexta midgut juice showed the same sequence as the Cyt1Aa activated with proteinase K (RVEDPNIDDL) indicating that M. sexta midgut juice properly activated Cyt1Aa toxin.
Figure 2.
Cyt1Aa protoxin is efficiently processed with Manduca sexta midgut juice. Cyt1Aa protoxin was treated with Proteinase K or 1% or 10 % M. sexta midgut juice as described in Materials and Methods and the protease treatment was stopped at different time points. Samples were separated by SDS-PAGE and stained with Coomasie blue.
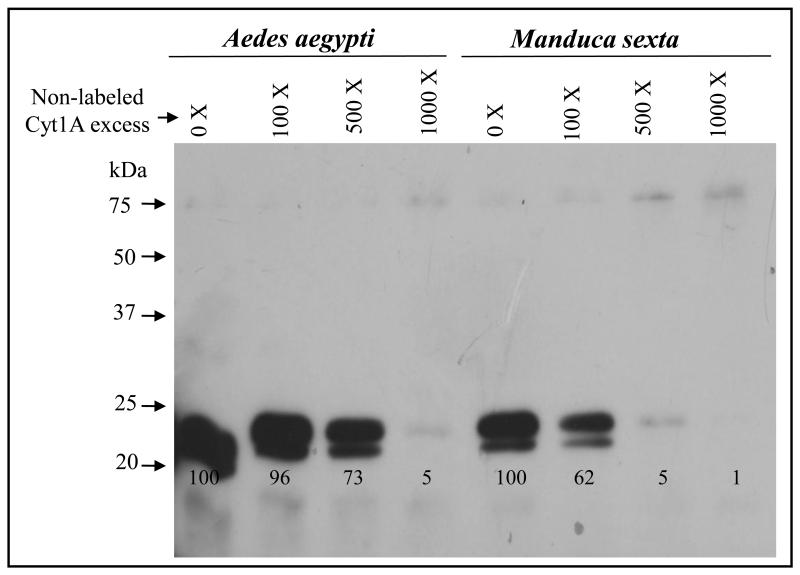
3.3 Binding of Cyt1Aa to M. sexta and A. aegypti BBMV
To analyze if the binding of Cyt1Aa to BBMV of M. sexta could be a limiting step in toxicity, a qualitative binding competition assay was performed with biotin-labeled Cyt1Aa. To determine if the binding to BBMV was specific, we analyzed the binding of biotin-labeled Cyt1Aa to BBMV previously incubated with different molar excess of unlabeled Cyt1Aa. For comparison, we performed a similar binding experiment with A. aegypti BBMV. Figure 3 shows that Cyt1Aa labeled toxin bound to M. sexta BBMV in a specific manner since the binding of labeled Cyt1Aa was efficiently competed by unlabeled Cyt1Aa. Similar results were obtained in the binding experiment with A. aegypti BBMV, although the labeled Cyt1Aa toxin was competed with less unlabeled toxin in the case of M. sexta BBMV (Fig. 3).
Figure 3.
Binding competition experiments shows that Manduca sexta BBMV have lower Cyt1Aa binding sites than Aedes aegypti BBMV. M. sexta or A. aegypti BBMV were incubated with different molar excess of unlabelled and biotin labeled Cyt1Aa was then bound to BBMV samples. BBMV were obtained by centrifugation and separated by SDS-PAGE. Bound biotin-Cyt1Aa was revealed with streptavidin coupled to peroxidase as indicated in Materials and Methods.
3.4 Oligomerization of Cyt1Aa
Activation of Cyt1Aa in the presence of membrane lipids results in the formation of high molecular weight oligomers [6]. To determine if activation of Cyt1Aa with M. sexta midgut juice results in Cyt1Aa oligomerization, we analyzed the effect of Cyt1Aa protoxin activation with M. sexta midgut juice in the presence of small unilamellar vesicules (SUV) composed of phosphatidylcholine, cholesterol and stearylamine as described in Materials and Methods. Figure 4 shows that in the presence of SUV, treatment of Cyt1Aa protoxin with M. sexta midgut juice resulted in the formation of high molecular weight oligomers. Similar size oligomers were formed with proteinase K treatment (Fig. 4).
Figure 4.
Activation of Cyt1Aa protoxin by Manduca sexta midgut juice in the presence of synthetic membranes induces Cyt1Aa oligomerization. Cyt1Aa protoxin was treated with Proteinase K or two different M. sexta midgut juice concentrations in the presence of small unilaminar vesicles and the samples were loaded on SDS-PAGE and revealed by western blot with anti-Cyt1Aa antibody.
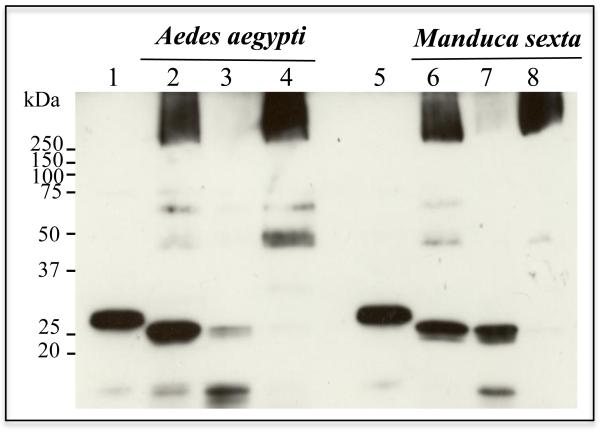
To determine if Cyt1Aa could form oligomers and insert into M. sexta BBMV we performed proteolytic activation of Cyt1Aa with proteinase K in the presence of BBMV and the membrane pellet was separated by centrifugation to analyze oligomer formation by western blot using an anti-Cyt1Aa polyclonal antibody. For comparison, a similar experiment was performed with A. aegypti BBMV. Figure 5 shows that Cyt1Aa could form oligomers of high molecular weight that inserted into M. sexta BBMV since oligomers were observed in the membrane pellet whereas monomers were detected in the supernatant. A similar result was obtained with A. aegypti BBMV indicating that oligomers insert into BBMV of both the susceptible and non-susceptible insects.
Figure 5.
Cyt1Aa oligomers insert into BBMV of Aedes aegypti and Manduca sexta. Cyt1Aa protoxin was activated with proteinase K in the presence of BBMV from both insects, finally the BBMV membranes were separated by centrifugation. The membrane pellets and the supernatants were loaded on SDS-PAGE and Cyt1Aa was revealed by western blot by anti-Cyt1Aa antibody. Lanes 2 to 4 correspond to A. aegypti BBMV while lanes 6 to 8 to M. sexta BBMV. Lanes 1 and 5 are Cyt1Aa protoxin samples, lanes 2 and 6 the correspond to the BBMV samples without separation by centrifugation, lanes 3 and 7 are the supernatants after separation of the membranes by centrifugation and lanes 4 and 8 are the BBMV membrane pellets after separation by centrifugation.
3.5 Analysis of Cyt1Aa BBMV insertion by fluorescence quenching
To determine if the inserted Cyt1Aa in BBMV from the two insect species have a similar conformation we took advantage of the fact that activated Cyt1Aa contains a single cysteine residue 190 located in β7 at the carboxy-terminal end. Cys190 was labeled with fluorescent dye Alexa-350 to perform quenching experiments with KI and determine the exposure of this residue to the solvent after BBMV membrane insertion. It has been proposed that β7 inserts into the membrane in the pore formed by Cyt1Aa [9, 25]. Alexa-350 dye shows low sensitivity to changes in the polar environment but is efficiently quenched by KI [33], which is a soluble quencher. Activated Cyt1Aa was labeled with Alexa-350 as described in materials and methods and the labeling of the toxin was analyzed directly on the SDS-PAGE, visualizing the labeled protein by excitation with UV light transilluminator (data not shown). The labeled toxin was then incubated with BBMV from both insects. To determine the exposure of Cys190 to the solvent the BBMV samples were incubated with increasing concentrations of KI. As control labeled Cyt1Aa was similarly incubated in solution. The KI quencher gave a linear Stern-Volmer plot (Fig. S1). The apparent dynamic quenching constants KSV derived from the slopes of these plots are presented in Table S1. The data shows that Cys190 was less exposed to the solvent after Cyt1Aa binding to BBMV from both insects since the value of Sterm Volver constant was greatly reduced in comparison to that obtained from Cyt1Aa in solution. Furthermore, the fluorescence of Cys190-Alexa350 showed a similar shift of 7 nanometers to the blue region of the Cyt1Aa bound to BBMV of both insects (Table S1). These results show a similar change in the polar environment of Cys190 when Cyt1Aa inserts into the membranes of both insect species.
4. Discussion
It has been proposed that Cyt1Aa insect specificity relies on specific recognition of certain unsaturated lipids in the membranes of gut cells of susceptible insects [12, 25]. Here we show that Cyt1Aa binds to BBMV of the non-susceptible M. sexta larvae in a manner that is similar to that observed in BBMV of the susceptible insect A. aegypti. Activation of Cyt1Aa with M. sexta midgut proteases resulted in an activated toxin that showed similar amino-terminal as the proteinase K treated protein and could oligomerize in the presence of synthetic lipids as well as in the presence of M. sexta and A. aegypti BBMV. Furthermore, the oligomer formed inserted into BBMV since it was obtained in membrane pellets in contrast to monomeric toxin that was always found in supernatants. Also, we performed qualitative binding assays that showed that Cyt1Aa bound to the M. sexta BBMV in a specific way since previous binding of non-labeled toxin inhibited the binding of the biotin-labeled toxin, suggesting that binding is specific and binding sites were saturated. Finally, fluorescence quenching assays of Cyt1Aa labeled toxin with Alexa-350 dye showed that the inserted Cyt1Aa toxin in BBMV from both insect species was protected from quenching by KI and suffered a similar shift in fluorescence maximal wavelength, suggesting a similar conformation upon insertion into membranes of both insect species. Two subtle differences were noticed in the processing of Cyt1Aa with M. sexta midgut juice and in the binding competition experiments. Regarding processing of Cyt1Aa, processing with M. sexta midgut juice resulted in processed Cyt1Aa protein with a similar amino terminal end as the proteinase k treated protein but a slight higher molecular weight than the protein processed with protease K (Fig. 2). As mentioned previously, differences in the processing of the toxin have a slight effect in toxicity since Cyt1Aa trypsin activated toxin showed less efficient hemolysis activity than proteinase K activated toxin [1]. It remains to be determined if processing with M. sexta midgut juice affects Cyt1Aa toxicity although processing with M. sexta midgut juice triggered efficient Cyt1Aa oligomerization (Fig. 4). Regarding binding competition experiment, binding of Cyt1Aa was saturated with less excess concentration of unlabeled Cyt1Aa in M. sexta BBMV in comparison to A. aegypti. This could suggest that M. sexta BBMV have lower Cyt1Aa binding sites than A. aegypti. We confirmed 30-50 % less binding sites of Cyt1Aa in M. sexta BBMV in comparison to A. aegypti by ELISA competitive binding assays (data not shown). However, the lower amount of binding sites in M. sexta membranes does not explain by itself the complete lack of toxicity. Recently, it has been shown that toxicity of Cyt2Aa could be targeted to non-susceptible aphids by introducing a peptide sequence that showed binding affinity to an aminopetidase-N present in the aphid gut into certain exposed loop regions of Cyt2Aa [5]. The engineered Cyt2Aa showed enhance binding to aphid BBMV and toxicity indicating that the number of binding sites in the gut is a limiting step for Cyt2Aa toxicity to aphids [5]. Thus, it is possible that a small amount of Cyt1Aa that could reach M. sexta brush border membrane could be limited in the number of binding sites needed to trigger toxicity. Nevertheless, we cannot rule out other possibilities like an enhanced immune response in lepidopteran gut that could be sufficient to cope with Cyt1Aa toxicity. It is important to note that although Cyt1Aa is toxic to A. aegypti larvae, toxicity is low compared with other Cry mosquitocidal proteins [10]. Also, it was recently shown that in other mosquito species as Anopheles albimanus Cyt1Aa is not toxic but it still synergized Cry4Ba and Cry11Aa in this mosquito species [14]. It could be possible that the major role of Cyt1Aa in toxicity against dipteran larvae is its synergistic capacity to enhance Cry toxicity rather than its toxicity itself.
Supplementary Material
Figure S1. Stern-Volmer plots of labeled Cyt1Aa toxin in the membrane inserted state in Aedes aegypti or Manduca sexta BBMV
Alexa Fluor 350 labeled protein quenched with I−. Quenching of the fluorescence of labeled mutant toxins was analyzed in the membrane-inserted state after insertion into BBMV. The quenching with KI was analyzed in Cyt1Aa labeled with Alexa Fluor-350. White circles show the result of KI quenching in solution in the absence of BBMV; white squares show the result of KI quenching in the presence of BBMV from A. aegypti larvae; black triangles show the result of KI quenching in the presence of BBMV from M. sexta larvae.
Highlights.
Cyt1Aa is properly activated by Manduca sexta midgut proteases
Cyt1Aa shows specific binding to Manduca sexta BBMV
Manduca sexta midgut proteases facilitates Cyt1Aa oligomer formation in the presence of membrane lipids
A similar region containing Cys residue of Cyt1Aa inserts into Manduca sexta and Aedes aegypti midgut membranes
Acknowledgments
We thank Dr. Lourival Possani and Dr. Fernando Zamudio for N-terminal sequence determination. Lizbeth Cabrera and Jorge Sanchez for technical assistance. This work was supported by CONACyT No. 179977, NIH No. 2R01 AI066014-06 and PAPIIT IN201113.
Abbreviations
- Cry
crystal proteins
- Bt
Bacillus thuringiensis
- PFT
pore forming toxins
- Bti
Bacillus thuringiensis subs israelensis
- BBMV
brush border membrane vesicles
- LC50
median lethal concentration
- SUV
small unilamellar vesicles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Al-yahyaee SAS, Ellar DJ. Maximal toxicity of cloned CytA δ-endotoxin from Bacillus thuringiensis subsp. israelensis requires proteolytic processing from both the N- and C-termini. Microbiol. 1995;141:3141–3148. [Google Scholar]
- [2].Bravo A, Likitvivatanavong S, Gill SS, Soberón M. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol. 2011;41:423–31. doi: 10.1016/j.ibmb.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cantón PE, Reyes EZ, Ruiz de Escudero I, Bravo A, Soberón M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides. 2011;32:595–600. doi: 10.1016/j.peptides.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chang C, Yu YM, Dai SM, Law SK, Gill SS. High-level cryIVD and cytA gene expression in Bacillus thuringiensis does not require the 20-kilodalton protein and the coexpressed gene products are synergistic in their toxicity to mosquitoes. Appl Environ Microbiol. 1993;59:815–821. doi: 10.1128/aem.59.3.815-821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chougule NP, Li H, Liu S, Linz LB, Narva KE, Meade T, Bonning C. Retargeting of the Bacillus thuringiensis toxin Cyt2Aa against hemipteran insect pests. Proc Natl Acad Sci USA. 2013;110:8465–8470. doi: 10.1073/pnas.1222144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chow E, Singh GJP, Gill SS. Binding and aggregation of the 24 kDa toxin of Bacillus thuringiensis subsp. israelensis to cell membranes and alteration by monoclonal antibodies and amino acid modifiers. Appl Environ Microbiol. 1989;55:2779–2788. doi: 10.1128/aem.55.11.2779-2788.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cohen S, Cahan R, Ben-Dov E, Nisnevitch M, Zaritsky A, Firer MA. Specific targeting to murine myeloma cells of Cyt1aa toxin from Bacillus thuringiensis subspecies israelensis. J Biol Chem. 2007;282:28301–28308. doi: 10.1074/jbc.M703567200. [DOI] [PubMed] [Google Scholar]
- [8].Cohen S, Dym O, Albeck S, Ben-Dov E, Cahan R, Firer M, Zaritsky A. High-resolution crystal structure of activated Cyt2Ba monomer from Bacillus thuringiensis subsp. israelensis. J Mol Biol. 2008;380:820–827. doi: 10.1016/j.jmb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [9].Cohen S, Albeck S, Ben-Dov E, Cahan R, Firer M, Zaritsky A, Dym O. Cyt1Aa toxin: crystal structure reveals implications for its membrane perforating function. J Mol Biol. 2011;413:804–814. doi: 10.1016/j.jmb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- [10].Crickmore N, Bone EJ, Williams JA, Ellar DJ. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol Lett. 1995;131:249–254. [Google Scholar]
- [11].Dow JA. pH gradients in lepidopteran midgut. J Exp Biol. 1992;172:355–375. doi: 10.1242/jeb.172.1.355. [DOI] [PubMed] [Google Scholar]
- [12].Du J, Knowles BH, Li J, Ellar DJ. Biochemical characterization of Bacillus thuringiensis cytolytic toxins in association with phospholipid bilayer. Biochem J. 1999;338:185–193. [PMC free article] [PubMed] [Google Scholar]
- [13].de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE. Structure, diversity and evolution of protein toxins from spore-forming entomopathogenic bacteria. Ann Rev Genet. 2003;37:409–433. doi: 10.1146/annurev.genet.37.110801.143042. [DOI] [PubMed] [Google Scholar]
- [14].Fernández-Luna MT, Tabashnik B, Lanz-Mendoza H, Bravo A, Soberón M, Miranda-Rios J. Single-Concentration tests show synergism among Bacillus thuringiensis subsp. israelensis toxins against the malaria vector mosquito Anopheles albimanus. J Invertebr Pathol. 2010;104:231–233. doi: 10.1016/j.jip.2010.03.007. [DOI] [PubMed] [Google Scholar]
- [15].Federici BA, Bauer LS. Cyt1Aa protein of Bacillus thuringiensis is toxic to the cottonwood leaf beetle, Chrysomela scripta, and suppresses high levels of resistance to Cry3Aa. Appl Environ Microbiol. 1998;64:4368–4371. doi: 10.1128/aem.64.11.4368-4371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gómez I, Sánchez J, Miranda R, Bravo A, Soberón M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix α–1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS lett. 2002;513:242–246. doi: 10.1016/s0014-5793(02)02321-9. [DOI] [PubMed] [Google Scholar]
- [17].Li J, Koni PA, Ellar DJ. Structure of the mosquitocidal delta-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- [18].Meyer SK, Tabashnik BE, Liu Y-B, Wirth MC, Federici BA. Cyt1A from Bacillus thuringiensis lacks toxicity to susceptible and resistant larvae of diamnondback moth (Plutella xylostella) and pink bollworm (Pectinophora gossypiella) Appl Environ Microbiol. 2001;67:462–463. doi: 10.1128/AEM.67.1.462-463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Parker MW, Feil SC. Pore-forming protein toxins: from structure to function. Progr Biophys Mol Biol. 2005;88:91–142. doi: 10.1016/j.pbiomolbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- [20].Pérez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberón M, Bravo A. Bacillus thuringiensis subsp. israeliensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc Natl Acad Sci USA. 2005;102:18303–18308. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev. 2007;71:255–281. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Promdonkoy B, Ellar DJ. Membrane pore architecture of a cytolytic toxin from Bacillus thuringiensis. Biochem J. 2000;350:275–282. [PMC free article] [PubMed] [Google Scholar]
- [23].Promdonkoy B, Rungrod A, Promdonkoy P, Pathaichindachote W, Krittanai Ch, Panyim S. Amino acid substitutions in α-A and α-C of Cyt2Aa2 alter hemolytic activity and mosquito-larvicidal specificity. J Biotechnol. 2007;133:287–293. doi: 10.1016/j.jbiotec.2007.10.007. [DOI] [PubMed] [Google Scholar]
- [24].Sayyed A, Crickmore N, Wright DJ. Cyt1Aa from Bacillus thuringiensis subsp. israelensis is toxic to the diamondback moth, Plutella xylostella, and synergizes the activity of Cry1Ac towards a resistant strain. Appl Environ Microbiol. 2001;67:5859–5861. doi: 10.1128/AEM.67.12.5859-5861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soberón M, López-Díaz JA, Bravo A. Cyt toxins produced by Bacillus thuringiensis: A protein fold conserved in several pathogenic microorganisms. Peptides. 2012;41:87–93. doi: 10.1016/j.peptides.2012.05.023. [DOI] [PubMed] [Google Scholar]
- [26].Souza-Neto JA, Gusmao DS, Lemos FJ. Chitinolytic activities in the gut of Aedes aegypti (Diptera:Culicidae) larvae and their role in digestion of chitin rich structures. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:717–724. doi: 10.1016/s1095-6433(03)00224-1. [DOI] [PubMed] [Google Scholar]
- [27].Teixera Corrêa RF, Ardisson-Araújo DMP, Monnerat RG, Ribeiro BM. Cytotoxicity analysis of three Bacillus thuringiensis subsp. israelensis δ-endotoxins towards insect and mammalian cells. PloS ONE. 2012:e46121. doi: 10.1371/journal.pone.0046121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thomas WE, Ellar DJ. Bacillus thuringiensis var. israelensis crystal δ-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- [29].van Frankenhuyzed K. Cros-order and cross-phylum activity of Bacillus thuringiensis pesticidal proteins. J Invertebr Pathol. 2013;114:76–85. doi: 10.1016/j.jip.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [30].Wirth CM, Federici BA, Walton WE. Cyt1Aa from Bacillus thuringiensis synergizes activity of Bacillus sphaericus against Aedes aegypti. (Diptera: Culicidae) Appl Environ Microbiol. 2000;66:1093–1097. doi: 10.1128/aem.66.3.1093-1097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wu D, Johnson JJ, Federeci BA. Synergism of mosquitocidal toxicity between CytA and CryIV proteins using inclusions produced from cloned genes of Bacillus thuringiensis subsp. israelensis. Mol Microbiol. 1994;13:965–972. doi: 10.1111/j.1365-2958.1994.tb00488.x. [DOI] [PubMed] [Google Scholar]
- [32].Yudina TG, Konukhova AV, Revina LP, Kostina LI, Zalunin IA, Chestukhina GG. Antibacterial activity of Cry- and Cyt-proteins from Bacillus thuringiensis ssp. israelensis. Ca J Microbiol. 2003;49:37–44. doi: 10.1139/w03-007. [DOI] [PubMed] [Google Scholar]
- [33].Zavala LE, Pardo-López L, Cantón PE, Gómez I, Soberón M, Bravo A. Domains II and III of Bacillus thuringiensis Cry1Ab toxin remain exposed to the solvent after insertion of part of domain I into the membrane. J Biol Chem. 2011;286:19109–19117. doi: 10.1074/jbc.M110.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Stern-Volmer plots of labeled Cyt1Aa toxin in the membrane inserted state in Aedes aegypti or Manduca sexta BBMV
Alexa Fluor 350 labeled protein quenched with I−. Quenching of the fluorescence of labeled mutant toxins was analyzed in the membrane-inserted state after insertion into BBMV. The quenching with KI was analyzed in Cyt1Aa labeled with Alexa Fluor-350. White circles show the result of KI quenching in solution in the absence of BBMV; white squares show the result of KI quenching in the presence of BBMV from A. aegypti larvae; black triangles show the result of KI quenching in the presence of BBMV from M. sexta larvae.