Abstract
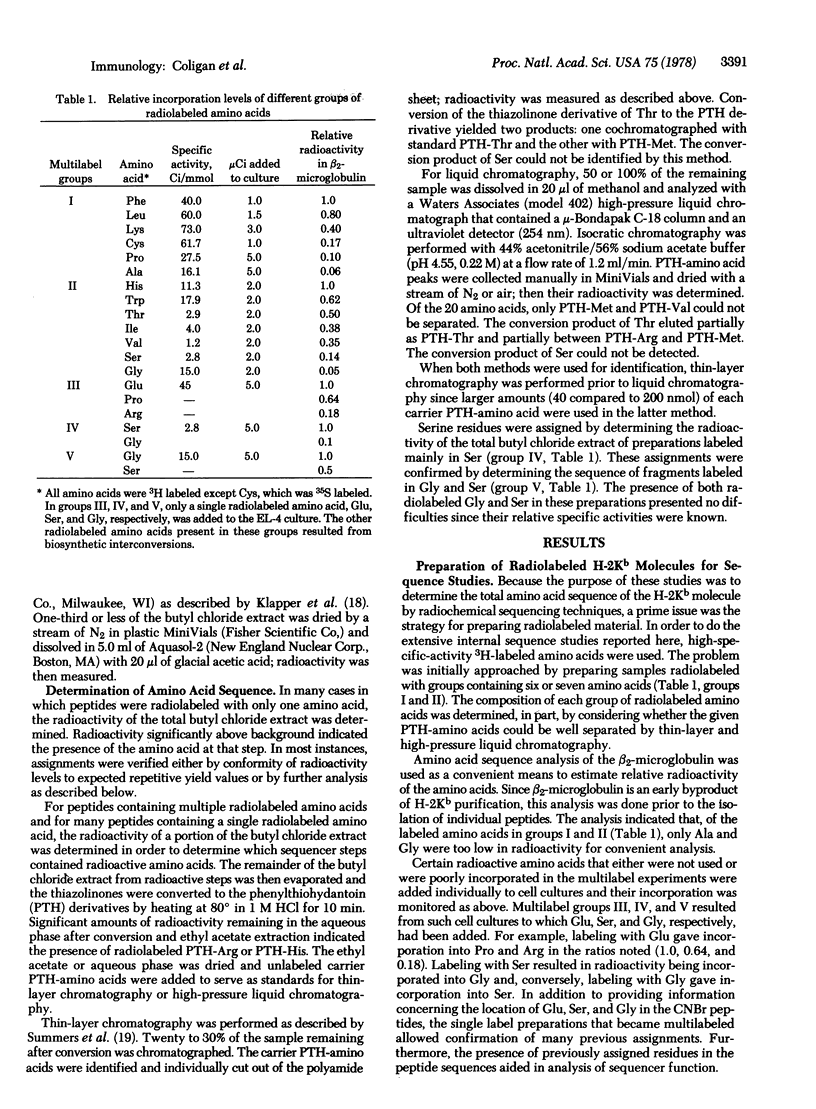
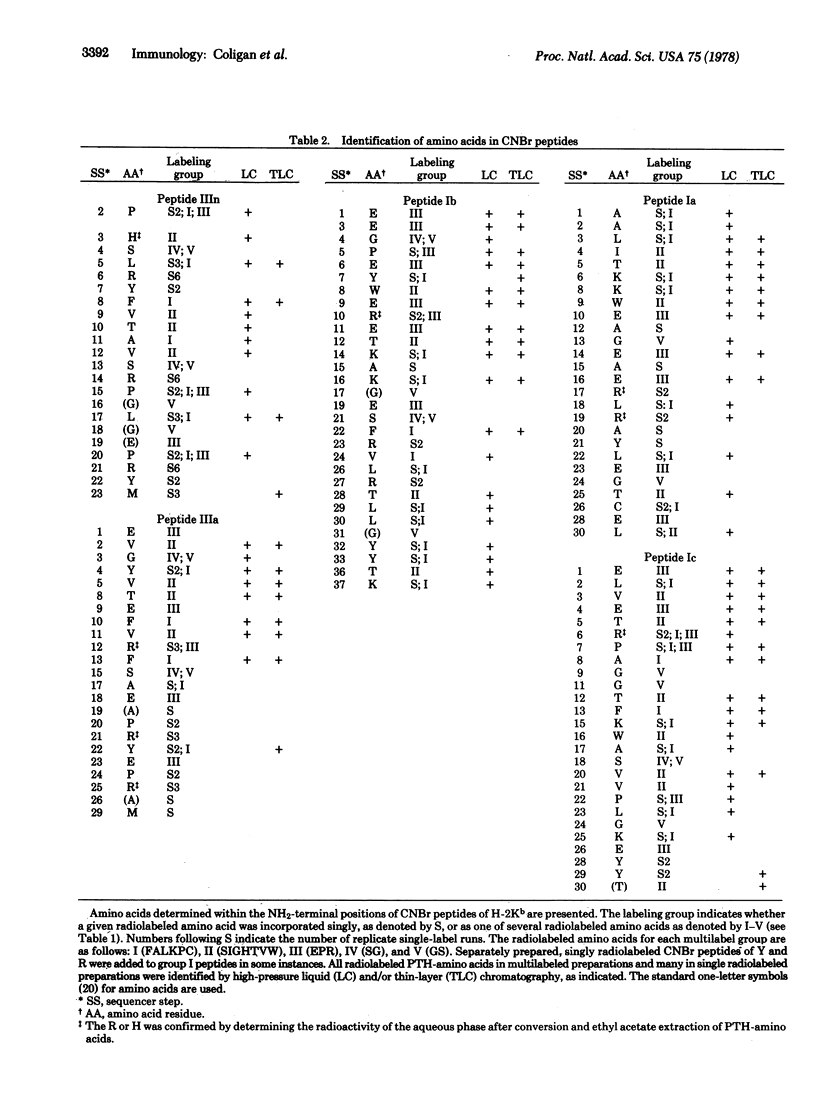
Radiochemical microtechniques have been used in the amino acid sequence analysis of five major CNBr fragments of the glycoprotein specified by the murine major histocompatibility complex gene H-2kb. These fragments have been tentatively aligned and represent the NH2-terminal 80% of the intact molecule. All amino acids except Asp, Asn, and Gln have been assigned in 128 out of 149 possible positions in the NH2-terminal portions of each of these fragments. These assignments, which represent approximately 50% of the total sequence from these fragments, are listed below in the order of their alignment in the intact H-2Kb molecule: IIIn, -PHSLRYFVTAVSRP(G)L(G)(E)PRYM; IIIa, EVGYV--TEFVRF-S-AE(A)PRYEPR(A)--M; Ib, E-EGPEYWERET-KAK(G)-E-SFR--LRTLL(G)YY--TK; Ia, AALITK-KWE-AGEAERLRAYLEGTC-E-L; Ic, ELVETRPAG-GTF-KWAS-VVPLGKE-YY(T). The unassigned positions represented by dashes in the above sequences may be tentatively assigned as Asp, Asn, or Gln.
The NH2-terminal sequence obtained for the H-2Kb molecule was compared to the limited sequence information available for other major histocompatibility complex gene products. An 84% homology (16 of 19 residues) to the H-2Kq and H-2Kk molecules, which are identical to one another in the positions compared, was observed. A similar comparison with 28 of the 31 NH2-terminal residues of HLA-B7 indicated 68% homology. Furthermore, significant homology was observed between H-Kb and HLA-B7 in a region of glycosylation, which occurs between positions 85 and 100 in the two molecules.
Keywords: histocompatibility antigens, radiolabeling, immunoprecipitation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. L., Kato K., Silver J., Nathenson S. G. Notable diversity in peptide composition of murine H-2K and H-2D alloantigens. Biochemistry. 1974 Jul 16;13(15):3174–3178. doi: 10.1021/bi00712a027. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Freed J. H., Nathenson S. G. Structural and serological properties of murine Ia alloantigens. Transplant Rev. 1976;30:236–270. doi: 10.1111/j.1600-065x.1976.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Nisizawa T., Uehara H., Nathenson S. G., Coligan J. E., Kindt T. J. Primary structure of murine major histocompatibility complex alloantigens: isolation, biochemical characterization, and preliminary alignment of CNBr fragments from the H-2Ib glycoprotein. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2909–2913. doi: 10.1073/pnas.75.6.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- McMillan M., Cecka J. M., Murphy D. B., McDevitt H. O., Hood L. Structure of murine Ia antigens: partial NH2-terminal amino acid sequences of products of the I-E or I-C subregion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5135–5139. doi: 10.1073/pnas.74.11.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Brown J. L., Ewenstein B. M., Nisizawa T., Sears D. W., Freed J. H. Structural differences between parent and variant H-2K glycoproteins from mouse strains carrying H-2 gene mutations. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):343–349. doi: 10.1101/sqb.1977.041.01.041. [DOI] [PubMed] [Google Scholar]
- Ostberg L., Rask L., Wigzell H., Peterson P. A. Thymus leukaemia antigen contains beta2-microglobulin. Nature. 1975 Feb 27;253(5494):735–737. doi: 10.1038/253735a0. [DOI] [PubMed] [Google Scholar]
- Parham P., Alpert B. N., Orr H. T., Strominger J. L. Carbohydrate moiety of HLA antigens. Antigenic properties and amino acid sequences around the site of glycosylation. J Biol Chem. 1977 Nov 10;252(21):7555–7567. [PubMed] [Google Scholar]
- Paul W. E., Benacerraf B. Functional specificity of thymus- dependent lymphocytes. Science. 1977 Mar 25;195(4284):1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Kato K., Cullen S. E., Nathenson S. G. H-2 histocompatibility alloantigens. Some biochemical properties of the molecules solubilized by NP-40 detergent. Biochemistry. 1973 May 22;12(11):2157–2164. doi: 10.1021/bi00735a023. [DOI] [PubMed] [Google Scholar]
- Schwartz B. D., Nathenson S. G. Isolation of H-2 alloantigens solubilized by the detergent NP-40. J Immunol. 1971 Nov;107(5):1363–1367. [PubMed] [Google Scholar]
- Shimada A., Nathenson S. G. Murine histocompatibility-2 (H-2) alloantigens. Purification and some chemical properties of soluble products from H-2b and H-2d genotypes released by papain digestion of membrane fractions. Biochemistry. 1969 Oct;8(10):4048–4062. doi: 10.1021/bi00838a023. [DOI] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Silver J., Russell W. A., Reis B. L., Frelinger J. A. Chemical characterization of murine Ia alloantigens determined by the i-E/i-C subregions of the H-2 complex. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5131–5134. doi: 10.1073/pnas.74.11.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Terhorst C., Robb R., Jones C., Strominger J. L. Further structural studies of the heavy chain of HLA antigens and its similarity to immunoglobulins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4002–4006. doi: 10.1073/pnas.74.9.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Capra J. D. The protein products of the murine 17th chromosome: genetics and structure. Adv Immunol. 1978;26:147–193. doi: 10.1016/s0065-2776(08)60230-8. [DOI] [PubMed] [Google Scholar]
- Vitetta E., Uhr J. W., Boyse E. A. Isolation and characterization of H-2 and TL alloantigens from the surface of mouse lymphocytes. Cell Immunol. 1972 Jun;4(2):187–191. doi: 10.1016/0008-8749(72)90019-6. [DOI] [PubMed] [Google Scholar]


