Abstract
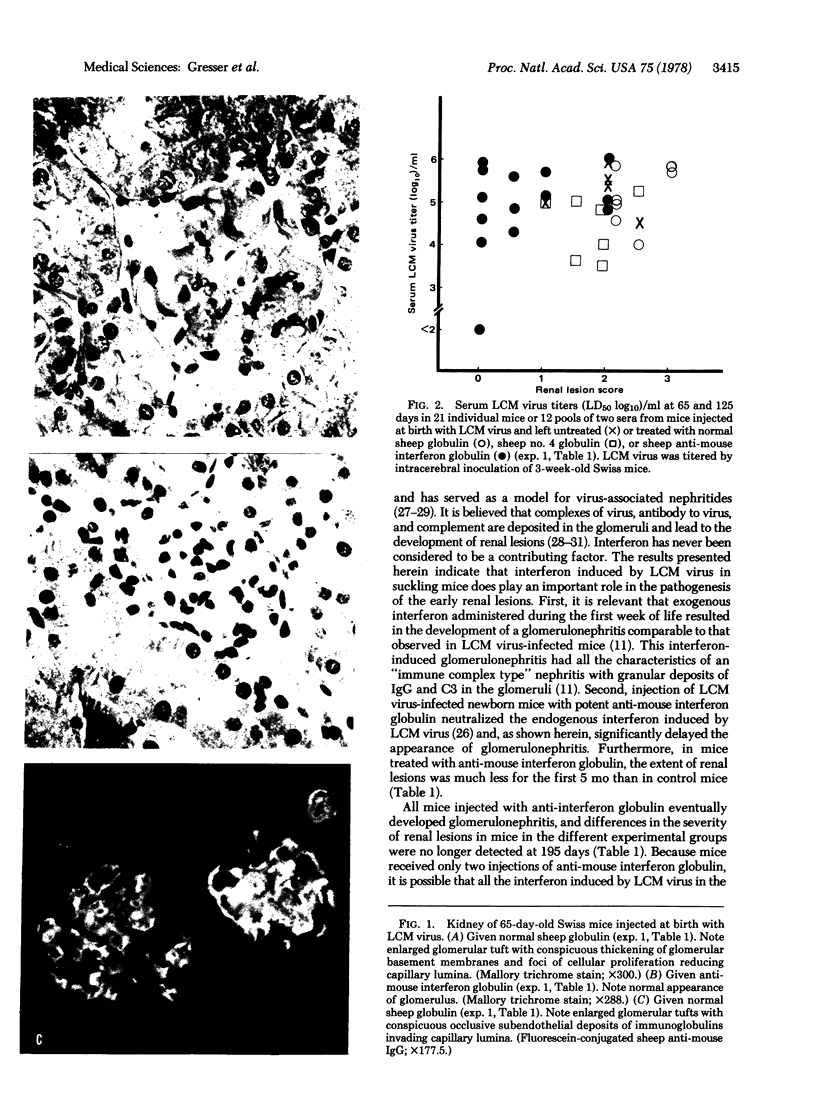
Swiss mice infected at birth with lymphocytic choriomeningitis virus develop glomerulonephritis. Injection of potent anti-mouse interferon globulin at the time of viral infection inhibited the development of these renal lesions. We conclude that the production of endogenous interferon by this virus in the first few days of life plays an important role in the pathogenesis of this glomerulonephritis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker F. D., Hotchin J. Slow virus kidney disease of mice. Science. 1967 Oct 27;158(3800):502–504. doi: 10.1126/science.158.3800.502. [DOI] [PubMed] [Google Scholar]
- Bro-Jorgensen K., Knudtzon S. Changes in hemopoiesis during the course of acute LCM virus infection in mice. Blood. 1977 Jan;49(1):47–57. [PubMed] [Google Scholar]
- Gresser I., Maury C., Tovey M., Morel-Maroger L., Pontillon F. Progressive glomerulonephritis in mice treated with interferon preparations at birth. Nature. 1976 Sep 30;263(5576):420–422. doi: 10.1038/263420a0. [DOI] [PubMed] [Google Scholar]
- Gresser I. On the varied biologic effects of interferon. Cell Immunol. 1977 Dec;34(2):406–415. doi: 10.1016/0008-8749(77)90262-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bourali-Maury C. Efficacy of exogenous interferon treatment initiated after onset of multiplication of vesicular stomatitis virus in the brains of mice. J Gen Virol. 1975 Jun;27(3):395–398. doi: 10.1099/0022-1317-27-3-395. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Bandu M. T. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. II. Studies with herpes simplex, Moloney sarcoma, vesicular stomatitis, Newcastle disease, and influenza viruses. J Exp Med. 1976 Nov 2;144(5):1316–1323. doi: 10.1084/jem.144.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Maury C., Chouroulinkov I. Lethality of interferon preparations for newborn mice. Nature. 1975 Nov 6;258(5530):76–78. doi: 10.1038/258076a0. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E., CINITS M. Lymphocytic choriomeningitis infection of mice as a model for the study of latent virus infection. Can J Microbiol. 1958 Apr;4(2):149–163. doi: 10.1139/m58-016. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J., COLLINS D. N. GLOMERULONEPHRITIS AND LATE ONSET DISEASE OF MICE FOLLOWING NEONATAL VIRUS INFECTION. Nature. 1964 Sep 26;203:1357–1359. doi: 10.1038/2031357a0. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. The biology of lymphocytic choriomeningitis infection: virus-induced immune disease. Cold Spring Harb Symp Quant Biol. 1962;27:479–499. doi: 10.1101/sqb.1962.027.001.046. [DOI] [PubMed] [Google Scholar]
- Huet C., Gresser I., Bandu M. T., Lindahl P. Increased binding of concanavalin A to interferon-treated murine leukemia L 1210 cells. Proc Soc Exp Biol Med. 1974 Oct;147(1):52–57. doi: 10.3181/00379727-147-38279. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. A cell surface alteration in mouse L cells induced by interferon. Biochem Biophys Res Commun. 1977 Jan 24;74(2):707–713. doi: 10.1016/0006-291x(77)90360-6. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon and cell division. VI. Inhibitory effect of interferon on the multiplication of mouse embryo and mouse kidney cells in primary cultures. Proc Soc Exp Biol Med. 1971 Dec;138(3):1044–1050. doi: 10.3181/00379727-138-36047. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Gresser I., Leary P., Tovey M. Interferon treatment of mice: enhanced expression of histocompatibility antigens on lymphoid cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1284–1287. doi: 10.1073/pnas.73.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Leary P., Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Oldstone M. B., Welsh R. M. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature. 1977 Jul 7;268(5615):67–68. doi: 10.1038/268067a0. [DOI] [PubMed] [Google Scholar]
- Mims C. A. Observations on mice infected congenitally or neonatally with lymphocytic choriomeningitis (LCM) virus. Arch Gesamte Virusforsch. 1970;30(1):67–74. doi: 10.1007/BF01262584. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Subrahmanyan T. P. Immunofluorescence study of the mechanism of resistance to superinfection in mice carrying the lymphocytic choriomeningitis virus. J Pathol Bacteriol. 1966 Apr;91(2):403–415. doi: 10.1002/path.1700910215. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Immune complex disease in chronic viral infections. J Exp Med. 1971 Sep 1;134(3 Pt 2):32s–40s. [PubMed] [Google Scholar]
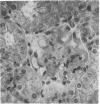
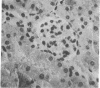
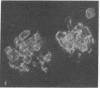
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Persistent lymphocytic choriomeningitis viral infection. 3. Virus-anti-viral antibody complexes and associated chronic disease following transplacental infection. J Immunol. 1970 Oct;105(4):829–837. [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Rivière Y., Bandu M. T. Induction d'interféron par le virus de la chorioméningite lymphocytaire chez la souris. Ann Microbiol (Paris) 1977 Apr;128A(3):323–329. [PubMed] [Google Scholar]
- Rivière Y., Gresser I., Guillon J. C., Tovey M. G. Inhibition by anti-interferon serum of lymphocytic choriomeningitis virus disease in suckling mice. Proc Natl Acad Sci U S A. 1977 May;74(5):2135–2139. doi: 10.1073/pnas.74.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUB E. Interference with eastern equine encephalomyelitis (EEE) virus in the brains of mice immune to lymphocytic choriomeningitis (LCM). Arch Gesamte Virusforsch. 1961;11:419–427. doi: 10.1007/BF01249595. [DOI] [PubMed] [Google Scholar]
- TRAUB E., KESTING F. EXPERIMENTS ON HETEROLOGOUS AND HOMOLOGOUS INTERFERENCE IN LCM-INFECTED CULTURES OF MURINE LYMPH NODE CELLS. Arch Gesamte Virusforsch. 1963 Oct 7;14:55–64. doi: 10.1007/BF01555163. [DOI] [PubMed] [Google Scholar]
- TRAUB E. Observations on immunological tolerance and "immunity" in mice infected congenitally with the virus of lymphocytic choriomeningitis (LCM). Arch Gesamte Virusforsch. 1960;10:303–304. doi: 10.1007/BF01250677. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., SNYDER R. M. Viral interference induced in mice by acute or persistent infection with the virus of lymphocytic choriomeningitis. Nature. 1962 Oct 27;196:393–394. doi: 10.1038/196393a0. [DOI] [PubMed] [Google Scholar]