Abstract
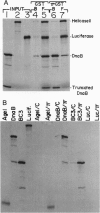
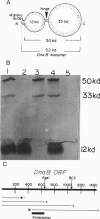
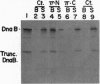
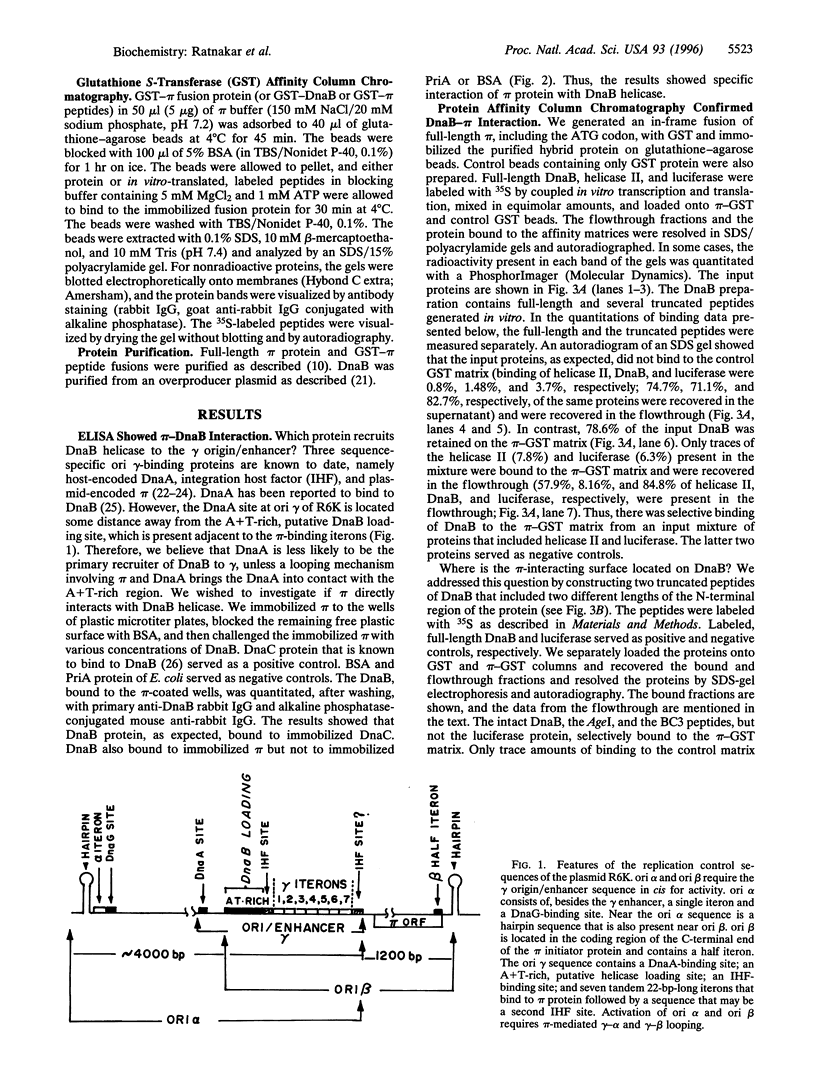
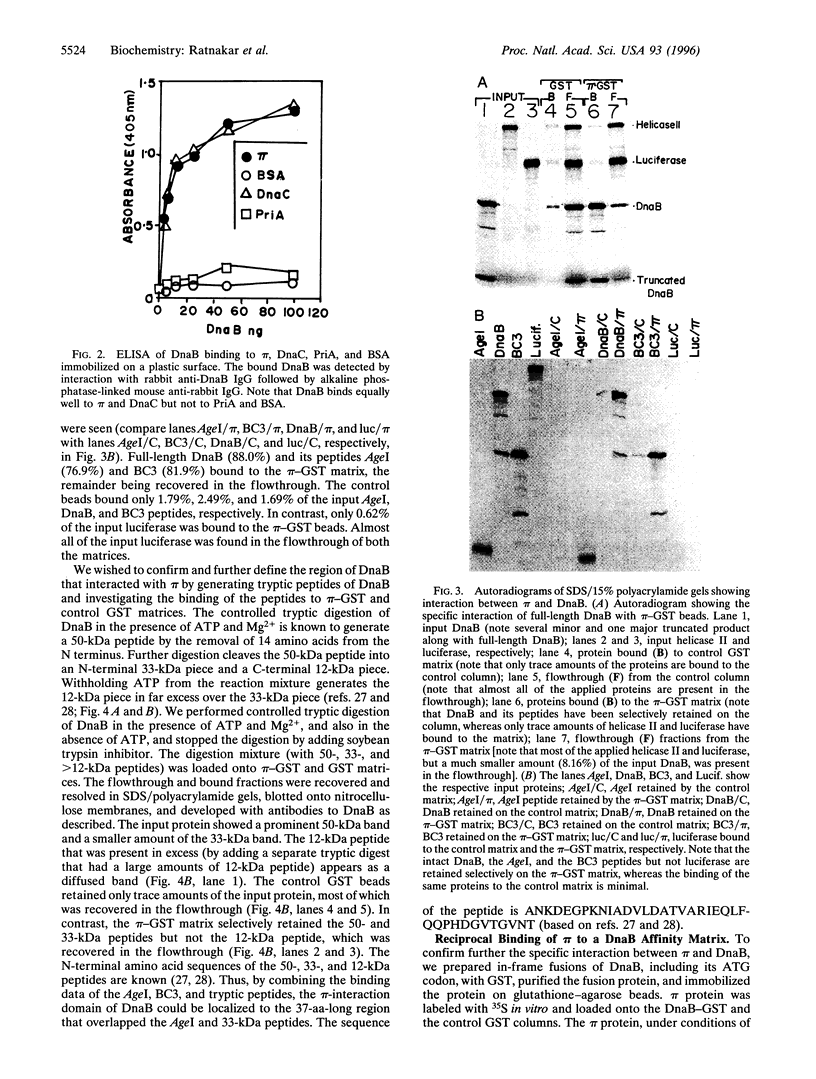
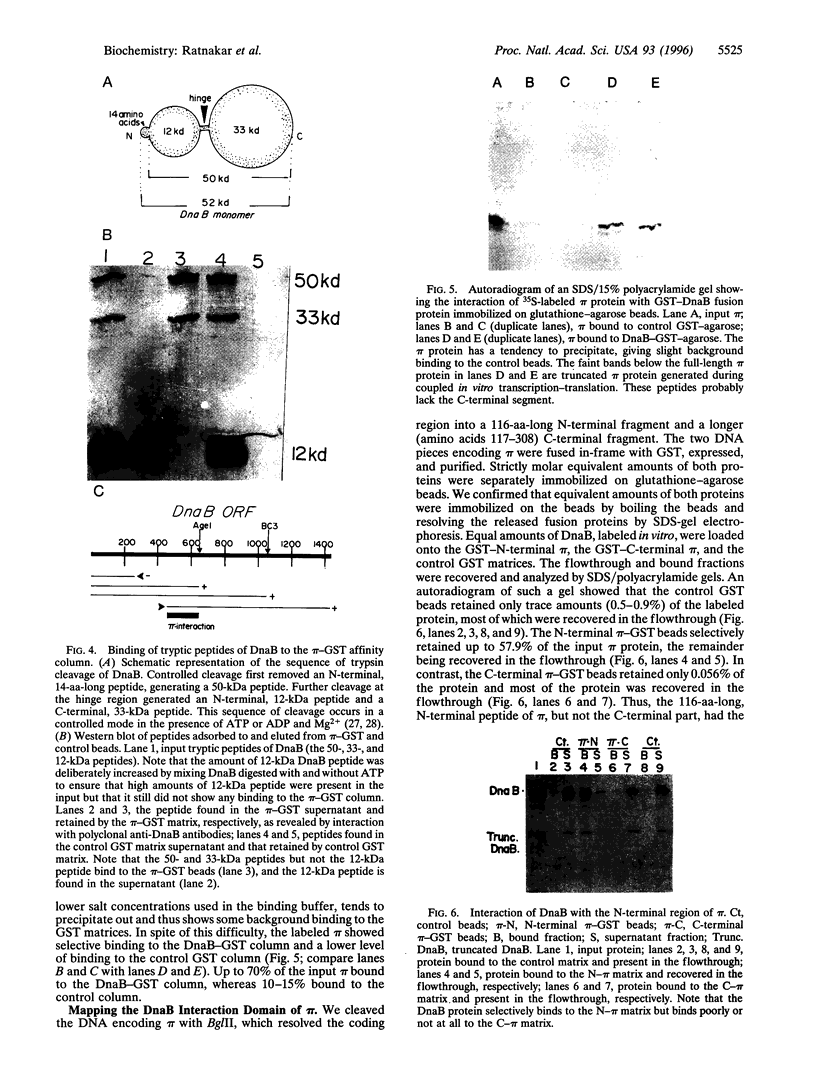
The replication initiator protein pi of plasmid R6K is known to interact with the seven iterons of the gamma origin/enhancer and activate distant replication origins alpha and beta (ori alpha and ori beta) by pi-mediated DNA looping. Here we show that pi protein specifically interacts in vitro with the host-encoded helicase DnaB. The site of interaction of pi on DnaB has been localized to a 37-aa-long region located between amino acids 151 and 189 of DnaB. The surface of pi that interacts with DnaB has been mapped to the N-terminal region of the initiator protein between residues 1 and 116. The results suggest that during initiation of replication, the replicative helicase DnaB is first recruited to the gamma enhancer by the pi protein. In a subsequent step, the helicase probably gets delivered from ori gamma to ori alpha and ori beta by pi-mediated DNA looping.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crosa J. H., Luttropp L. K., Falkow S. Mode of replication of the conjugative R-plasmid RSF1040 in Escherichia coli. J Bacteriol. 1976 Apr;126(1):454–466. doi: 10.1128/jb.126.1.454-466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Molecular cloning of replication and incompatibility regions from the R-plasmid R6K. J Mol Biol. 1978 Sep 25;124(3):443–468. doi: 10.1016/0022-2836(78)90181-x. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Appelt K. The integration host factor of Escherichia coli binds to multiple sites at plasmid R6K gamma origin and is essential for replication. Nucleic Acids Res. 1988 May 11;16(9):3829–3843. doi: 10.1093/nar/16.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germino J., Bastia D. Interaction of the plasmid R6K-encoded replication initiator protein with its binding sites on DNA. Cell. 1983 Aug;34(1):125–134. doi: 10.1016/0092-8674(83)90142-3. [DOI] [PubMed] [Google Scholar]
- Germino J., Bastia D. The replication initiator protein of plasmid R6K tagged with beta-galactosidase shows sequence-specific DNA-binding. Cell. 1983 Jan;32(1):131–140. doi: 10.1016/0092-8674(83)90503-2. [DOI] [PubMed] [Google Scholar]
- Inuzuka N., Inuzuka M., Helinski D. R. Activity in vitro of three replication origins of the antibiotic resistance plasmid RSF1040. J Biol Chem. 1980 Dec 10;255(23):11071–11074. [PubMed] [Google Scholar]
- Kelley W. L., Bastia D. Activation in vivo of the minimal replication origin beta of plasmid R6K requires a small target sequence essential for DNA looping. New Biol. 1992 May;4(5):569–580. [PubMed] [Google Scholar]
- Kelley W. L., Bastia D. Conformational changes induced by integration host factor at origin gamma of R6K and copy number control. J Biol Chem. 1991 Aug 25;266(24):15924–15937. [PubMed] [Google Scholar]
- Kelley W. L., Patel I., Bastia D. Structural and functional analysis of a replication enhancer: separation of the enhancer activity from origin function by mutational dissection of the replication origin gamma of plasmid R6K. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5078–5082. doi: 10.1073/pnas.89.11.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri G. S., MacAllister T., Sista P. R., Bastia D. The replication terminator protein of E. coli is a DNA sequence-specific contra-helicase. Cell. 1989 Nov 17;59(4):667–674. doi: 10.1016/0092-8674(89)90012-3. [DOI] [PubMed] [Google Scholar]
- Kolter R., Inuzuka M., Helinski D. R. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978 Dec;15(4):1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- MacAllister T. W., Kelley W. L., Miron A., Stenzel T. T., Bastia D. Replication of plasmid R6K origin gamma in vitro. Dependence on dual initiator proteins and inhibition by transcription. J Biol Chem. 1991 Aug 25;266(24):16056–16062. [PubMed] [Google Scholar]
- Mallory J. B., Alfano C., McMacken R. Host virus interactions in the initiation of bacteriophage lambda DNA replication. Recruitment of Escherichia coli DnaB helicase by lambda P replication protein. J Biol Chem. 1990 Aug 5;265(22):13297–13307. [PubMed] [Google Scholar]
- Marszalek J., Kaguni J. M. DnaA protein directs the binding of DnaB protein in initiation of DNA replication in Escherichia coli. J Biol Chem. 1994 Feb 18;269(7):4883–4890. [PubMed] [Google Scholar]
- Masai H., Nomura N., Kubota Y., Arai K. Roles of phi X174 type primosome- and G4 type primase-dependent primings in initiation of lagging and leading strand syntheses of DNA replication. J Biol Chem. 1990 Sep 5;265(25):15124–15133. [PubMed] [Google Scholar]
- McEachern M. J., Bott M. A., Tooker P. A., Helinski D. R. Negative control of plasmid R6K replication: possible role of intermolecular coupling of replication origins. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7942–7946. doi: 10.1073/pnas.86.20.7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron A., Mukherjee S., Bastia D. Activation of distant replication origins in vivo by DNA looping as revealed by a novel mutant form of an initiator protein defective in cooperativity at a distance. EMBO J. 1992 Mar;11(3):1205–1216. doi: 10.1002/j.1460-2075.1992.tb05161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron A., Patel I., Bastia D. Multiple pathways of copy control of gamma replicon of R6K: mechanisms both dependent on and independent of cooperativity of interaction of tau protein with DNA affect the copy number. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6438–6442. doi: 10.1073/pnas.91.14.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Erickson H., Bastia D. Enhancer-origin interaction in plasmid R6K involves a DNA loop mediated by initiator protein. Cell. 1988 Feb 12;52(3):375–383. doi: 10.1016/s0092-8674(88)80030-8. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Patel I., Bastia D. Conformational changes in a replication origin induced by an initiator protein. Cell. 1985 Nov;43(1):189–197. doi: 10.1016/0092-8674(85)90023-6. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Arai N., Bond M. W., Kaziro Y., Arai K. Nucleotide sequence of dnaB and the primary structure of the dnaB protein from Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):97–101. [PubMed] [Google Scholar]
- Nakayama N., Arai N., Kaziro Y., Arai K. Structural and functional studies of the dnaB protein using limited proteolysis. Characterization of domains for DNA-dependent ATP hydrolysis and for protein association in the primosome. J Biol Chem. 1984 Jan 10;259(1):88–96. [PubMed] [Google Scholar]
- Shafferman A., Helinski D. R. Structural properties of the beta origin of replication of plasmid R6K. J Biol Chem. 1983 Apr 10;258(7):4083–4090. [PubMed] [Google Scholar]
- Shon M., Germino J., Bastia D. The nucleotide sequence of the replication origin beta of the plasmid R6K. J Biol Chem. 1982 Nov 25;257(22):13823–13827. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tougu K., Peng H., Marians K. J. Identification of a domain of Escherichia coli primase required for functional interaction with the DnaB helicase at the replication fork. J Biol Chem. 1994 Feb 11;269(6):4675–4682. [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]