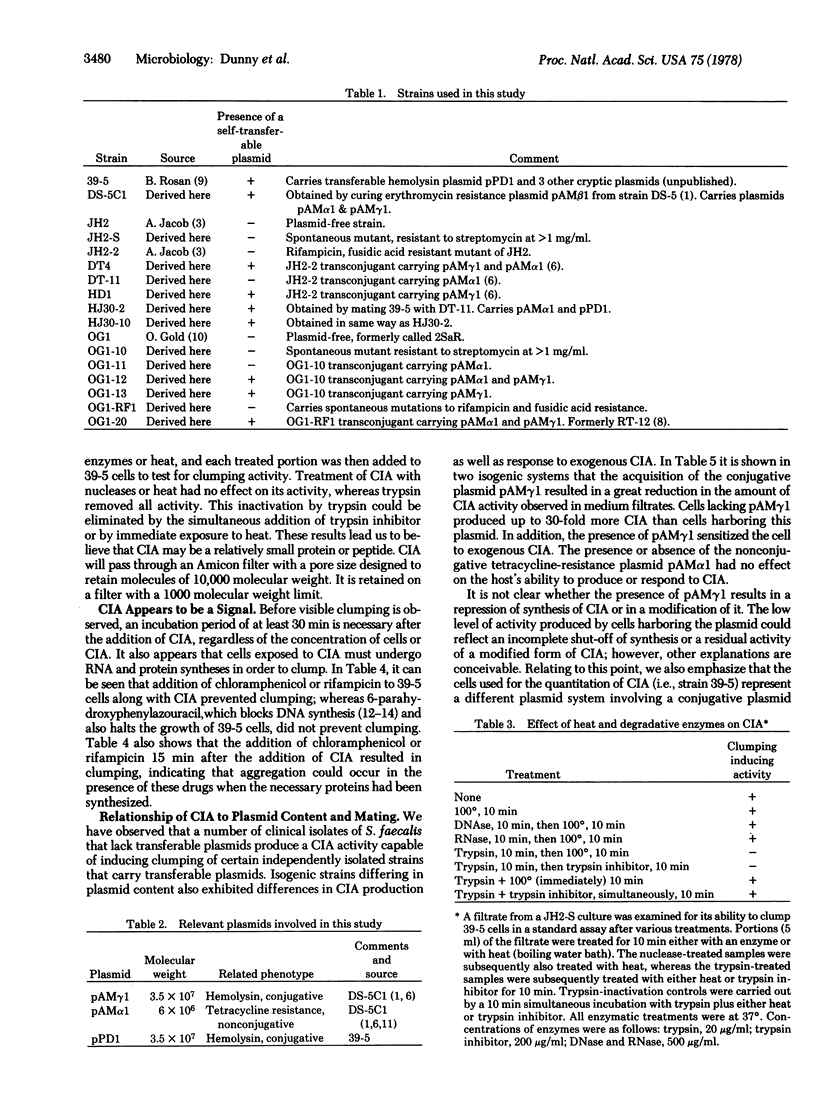
Abstract
Recipient strains of Streptococcus faecalis produce a trypsin sensitive, heat resistant, nuclease resistant factor, designated clumping-inducing agent (CIA) which causes strains carrying certain conjugative plasmids to aggregate. RNA and protein synthesis but not DNA synthesis are required for aggregation to occur. Recipient filtrates that contain CIA activity also induce donors to mate at high frequencies. Introduction of a transferable plasmid into strains producing CIA dramatically reduces the amount of CIA activity produced by the strain but allows the strain to respond to exogenously added CIA. Our data suggest that CIA represents a bacterial sex hormone (pheromone).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. C., Handschumacher R. E. Inhibition of the synthesis of deoxyribonucleic acid in bacteria by 6-(p-hydroxyphenylazo)-2,4-dihydroxypyrimidine. I. Metabolic studies in Streptococcus fecalis. J Biol Chem. 1966 Jul 10;241(13):3083–3089. [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Cozzarelli N. R. The mechanism of action of inhibitors of DNA synthesis. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetello C., Van Larebeke N., Holsters M., De Picker A., Van Montagu M., Schell J. Ti plasmids of Agrobacterium as conjugative plasmids. Nature. 1977 Feb 10;265(5594):561–563. doi: 10.1038/265561a0. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Gooday G. W. Fungal sex hormones. Annu Rev Biochem. 1974;43(0):35–87. doi: 10.1146/annurev.bi.43.070174.000343. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A., Manigault P., Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977 Feb 10;265(5594):560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- Kohoutová M., Braná H., Holubová I. Isolation and purification of a substance inducing competence and inactivating transforming DNA in Pneumococcus. Biochem Biophys Res Commun. 1968 Jan 25;30(2):124–129. doi: 10.1016/0006-291x(68)90458-0. [DOI] [PubMed] [Google Scholar]
- Mackenzie J. M., Neville M. M., Wright G. E., Brown N. C. Hydroxyphenylazopyrimidines: characterization of the active forms and their inhibitory action on a DNA polymerase from Bacillus subtilis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):512–516. doi: 10.1073/pnas.70.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. R., Brown B. L., Clewell D. B. Characterization of plasmids determining hemolysin and bacteriocin production in Streptococcus faecalis 5952. J Bacteriol. 1977 May;130(2):948–950. doi: 10.1128/jb.130.2.948-950.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osowiecki H., Nalecz J., Dobrzański W. T. The mechanism of competence in the transformation of Streptococci of serological group H. Purification and some properties of the competence factor. Mol Gen Genet. 1969;105(1):16–20. doi: 10.1007/BF00750310. [DOI] [PubMed] [Google Scholar]
- Ou J. T. Mating signal and DNA penetration deficiency in conjugation between male Escherichia coli and minicells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3721–3725. doi: 10.1073/pnas.72.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSAN B., WILLIAMS N. B. HYALURONIDASE PRODUCTION BY ORAL ENTEROCOCCI. Arch Oral Biol. 1964 May-Jun;9:291–298. doi: 10.1016/0003-9969(64)90061-5. [DOI] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]



