Abstract
OBJECTIVES
Preserving physical function with aging may be partially met through modification in dietary protein intake.
DESIGN
Women’s Health Initiative Clinical Trials (CT) and Observational Study (OS).
SETTING & PARTICIPANTS
Women age 50–79 y (n=134,961) with dietary data and ≥ 1 physical function measure.
MEASUREMENTS
Physical function was assessed by short form RAND-36 at baseline and annually beginning in 2005 for all WHI participants, and at closeout for CT participants (average ~7 y after baseline). In a subset of 5346 participants, physical performance measures (grip strength, number of chair stands in 15 seconds, and timed 6-meter walk) were assessed at baseline and years 1, 3, and 6. Calibrated energy and protein intake were derived from regression equations using baseline food frequency questionnaire data collected on the entire cohort and doubly labeled water and 24-hour urinary nitrogen collected from a representative sample as reference measures. Associations between calibrated protein intake and each of the physical function measures were assessed using generalized estimating equations.
RESULTS
Calibrated protein intake ranged from 6.6 to 22.3% energy. Higher calibrated protein intake at baseline was associated with higher self-reported physical function [quintile (Q) 5 vs. Q1: 85.6 (95% CI, 81.9 to 87.5) vs. 75.4 (73.2 to 78.5), Ptrend=0.002] and a slower rate of functional decline [Q5 vs. Q1 annualized change: −0.47 (−0.63 to −0.39) vs. −0.98 (−1.18 to −0.75), Ptrend=0.022]. Women with higher calibrated protein intake also had higher grip strength at baseline [Q5 vs. Q1: 24.7 (24.3 to 25.2) vs. 24.1 (23.6 to 24.5), Ptrend=0.036] and showed slower declines in grip strength [Q5 vs. Q1 annualized change: −0.45 kg (−0.39 to −0.63) vs. −0.59 kg (−0.50 to −0.66), Ptrend=0.028]. Additionally, women with higher calibrated protein intake completed more chair stands at baseline [Q5 vs. Q1: 7.11 (6.91 to 7.26) vs. 6.61 (6.46 to 6.76), Ptrend=0.002].
CONCLUSION
Higher calibrated protein intake is associated with greater physical function and performance and slower rates of decline in postmenopausal women.
Keywords: dietary protein intake, physical performance, physical function, grip strength
INTRODUCTION
Women’s Health Initiative (WHI) investigators previously reported that a 20% higher uncalibrated protein intake (% energy) was associated with a 12% (95% CI, 8 to 16%) decrease in frailty, while 20% higher calibrated protein intake (i.e. corrected for measurement error using biomarkers of energy and protein intake) was associated with a 32% (23 to 44%) decrease in frailty1. Understanding the pathways through which higher dietary protein intake may reduce frailty risk would inform future intervention study designs and could contribute greatly to public health, since frailty is associated with increased risk of falls, fractures, disability, institutionalization, and death2–4. Data from several highly controlled clinical studies support a beneficial role of increased protein intake in reducing loss of lean body mass5–7. Whether the beneficial effect of higher protein intake also translates into clinically important differences in preservation of physical function over time on a population level is less clear.
The WHI is the largest study of postmenopausal women’s health ever undertaken in the U.S. Further, the study provides a particularly robust description of diet and health indicators over a prolonged follow-up period, including data on protein intake and physical function. Here we examined total biomarker-calibrated protein intake in relation to baseline and changes in physical function, as measured by both self-report (short form RAND-36 Physical Function Score) and three objective measures (grip strength, number of chair stands in 15 seconds, and timed 6-meter walk) over a mean follow-up of 11.5 ± 3.1 y in a large sample of postmenopausal women.
METHODS
Study population
The WHI Observational Study (OS) of 93,676 women, along with 68,132 women enrolled in the Clinical Trials (CT), comprised women age 50–79 y when recruited between 1993 and 1998 from 40 clinical centers across the U.S. Over 80% of WHI CT and OS women also enrolled in an Extension Study (WHI Extension) to ascertain additional health outcomes beginning in 2005. Women were eligible for study inclusion if they were postmenopausal, of stable residence, and unlikely to die within 3 y. Additional eligibility criteria were assigned to each CT for reasons of safety, competing risk, and adherence/retention. Further details regarding the design, recruitment strategy, and data collection methods have been published8,9. The study was reviewed and approved by institutional review boards at each participating institution, and all participants signed written, informed consent.
The study population for this analysis includes all WHI OS and CT women with at least one physical function measure (either self-reported or objective). Additionally, women must have completed a food frequency questionnaire (FFQ) at baseline with reported energy intake between 600 and 5000 kcal/d, and they must have had available information necessary to compute calibrated protein intake: age, race/ethnicity, body mass index (BMI), recreational physical activity, and smoking status (n=134,961). Self-reported physical function scores were available for at least two visits for 75.0% and three visits for 70.2% of participants.
Objective physical function measures were available for a subset of the study population. A 25% random sample of CT participants age ≥ 65 y completed measures of grip strength (n=5,331), chair stands (n=5,294), and timed walk (n=5,335), for a total of 5,346 women completing at least one of the three measures at one or more time points. Among the subset of CT women who completed physical performance measures, data were available for at least two visits for 94.8%, 93.6%, and 95.1% and three visits for 84.0%, 81.4%, and 84.6% of participants for grip strength, chair stands, and timed walk, respectively. The subset of women completing physical performance measures were generally older, were more likely to be obese, had a lower income and education, were more likely to live alone, and reported more co-morbidities (i.e. arthritis, diabetes) than the overall study population (data not shown).
Physical function measures
Self-reported physical function was assessed using a summary measure, a subscale from the RAND Short Form-36 (SF-36)10,11, that included 10 items related to the ability to engage in activities (i.e. vigorous and moderate activities, lifting/carrying groceries, climbing stairs, bending, walking, and bathing/dressing oneself) and is scored from 0 to 100, with a higher score indicating greater physical function capacity. The SF-36 was assessed at baseline and annually beginning in 2005 for all WHI participants, and at closeout for CT participants (average ~7 y after baseline). For this analysis, women were followed for a mean total follow-up of 11.5 ± 3.1 y.
Physical performance measures (grip strength, chair stands, and timed walk) were assessed at baseline, at follow-up years 1, 3, and 6 in the CT, and at follow-up year 3 in the OS by trained, certified study staff using standard protocols. Hand grip strength was measured using a handheld dynamometer (Jamar hand dynamometer; Lafayette Instruments, Lafayette, IN). Two measurements were taken using the dominant hand with staff coaching for maximal performance, and the higher score was used in the analysis.
Repeated chair stands and the timed walk, which represent two of three items of the Short Physical Performance Battery (SPPB)12 commonly employed to assess function in aging populations, correlate with disability and/or mortality in older adults13,14. The chair-stand test was conducted if the participant was able to stand at least once without using hands or arms from a straight-backed, non-padded, flat-seated, armless chair. Two 15-second trials of repeated chair stands were performed with arms folded across the chest, with a 1–2 minute rest between trials, and the score with the greater number of chair stands was included in the analysis. The 6-meter timed walk was performed with women walking at usual speed, using ambulatory aids as needed. The test was repeated, and the faster of the two measured times was included in this analysis.
Dietary protein
The 122-item WHI FFQ was self-administered at baseline for all participants15. Daily energy (kcal) and protein (g) intake were estimated for individual foods/food groups, 19 adjustment items, and summary questions (i.e. uncalibrated energy and protein). The WHI Nutritional Biomarkers Study (n=544) was conducted to evaluate accuracy of self-reported energy and protein consumption from the FFQ using biomarkers (doubly labeled water for energy and urinary nitrogen for protein) of “true” intake16.
“Uncalibrated” protein intake refers to the amount calculated directly on the FFQ, whereas “biomarker-calibrated” protein intake refers to the adjusted levels obtained after using regression equations that were developed based upon the NBS. These regression equations incorporate participant characteristics like BMI, age, race/ethnicity, and smoking status17. The biomarker-calibrated values are more accurate than the uncalibrated values, as they acknowledge differential reporting errors in dietary data based upon participant characteristics. Protein intake measures were categorized into quintiles separately for the self-reported (median calibrated protein intake of 14.3% energy) and objective physical function (median calibrated protein intake of 13.7% energy) measures based on distributions among the women included in each analysis.
Potential confounders
Demographic characteristics (age, family income, education, race/ethnicity), medical history [hip fracture, emphysema, treated diabetes, hypertension (on medication and/or blood pressure >140/90 mmHg), arthritis, cancer], and other health-related factors (having a current health care provider, number of falls, living alone, ability to perform activities of daily living, depression) were self-reported at baseline. BMI was computed using measured height (m) and weight (kg) at baseline (kg/m2). Smoking status was self-reported and classified as current, past, or never. Depressive symptoms were assessed by 6-item short form of the Center for Epidemiologic Studies Depression (CESD) Scale24. Postmenopausal hormone therapy was self-reported and categorized as current, past, or never use, with separate indicator variables for unopposed estrogen and estrogen + progestin.
Analytic approach
Baseline characteristics were examined by quintile of calibrated protein intake (% energy). Multivariate adjusted linear generalized estimating equations (GEE) with an exchangeable covariance matrix were used to examine the longitudinal association between baseline protein intake and each outcome (self-reported physical function and three physical performance measures), including a protein-by-time interaction to allow for varying slopes, with time modeled as a continuous variable. The models test whether the mean scores on these outcome measures differ at baseline (intercept) or with respect to annual change over time (slope) according to quintile of protein intake. The reasonableness of linear fit was confirmed by comparing these estimates to results obtained by treating time as a categorical variable. Models were adjusted for previously identified independent predictors of frailty: age, income, education, race/ethnicity, BMI, smoking status, alcohol consumption, physical activity, hormone therapy use, whether the participant lived alone, having a healthcare provider, number of falls, disability, depression, and self-reported history of medical conditions (emphysema, diabetes, hypertension, arthritis, and cancer)3. Models were also adjusted for calibrated total energy intake and clinical trial arm. To evaluate potential effect modification, the relationship between calibrated protein intake and each of the four outcome measures was stratified by median age, recreational physical activity, BMI, or protein source (animal: total protein ratio).
Bootstrapping procedures were conducted to approximate variance around the estimated physical function outcomes across levels of calibrated protein intake. First, variance in the coefficients for the calibration regression equations in the NBS was determined by constructing 1000 bootstrap replicates using their original dataset. Next, using the dataset for the current study16, we constructed 1000 bootstrap replicates, and each new randomly selected sample of participants was paired with a new set of regression coefficients from the first bootstrapping procedure to re-calculate calibrated protein and energy intake. Then, the previously described GEE models were repeated for each sample, and 95% confidence intervals were determined from the distribution of 1000 new estimates. Tolerance (i.e. relative change in the coefficient vector from one iteration to the next) was set to 10−4 during bootstrapping to ensure consistent model convergence. A trend in the relationship between protein quintile (ordinal) and each estimated physical function score at baseline (or annualized change) was assessed by calculating the slope between these variables for each bootstrap replicate. These slopes were used to construct a distribution to determine if there was a significant trend in physical function across levels of protein intake. Statistical analyses were conducted using Stata 12.1 (StataCorp, College Station, TX) and SAS 9.3 (SAS Institute, Cary, NC); all reported P-values are two-sided.
RESULTS
At baseline, mean calibrated protein intake was 14.3 ± 1.4% total energy. Factors associated with higher protein intake included younger age, lower BMI, non-Hispanic white race/ethnicity, higher socioeconomic status, and higher recreational physical activity (Table 1). Similar trends were observed when characterizing protein intake as g/kg body weight and when restricting the study population to the subset of women with physical performance measures (data not shown).
Table 1.
Baseline Characteristics of Women’s Health Initiative Participants, by Quintile of Calibrated Protein Intake (% Energy)
| Characteristic | Quintile 1 6.6–13.1% n = 26,994 |
Quintile 2 13.1–13.9% n = 26,991 |
Quintile 3 13.9–14.6% n = 26,992 |
Quintile 4 14.6–15.4% n = 26,992 |
Quintile 5 15.4–22.3% n = 26,992 |
|---|---|---|---|---|---|
| Age (y), mean ± SD | 66.0 ± 7.2 | 64.9 ± 7.0 | 63.6 ± 6.9 | 61.9 ± 6.7 | 59.5 ± 6.3 |
| Body mass index (kg/m2), mean ± SD | 29.2 ± 7.0 | 28.6 ± 6.1 | 28.0 ± 5.7 | 27.5 ± 5.4 | 26.6 ± 4.9 |
| Underweight (< 18.5), % | 0.93 | 0.78 | 0.68 | 0.79 | 1.11 |
| Normal (18.5 to < 25), % | 28.7 | 30.8 | 33.7 | 36.6 | 42.4 |
| Overweight (25.0 to < 30), % | 33.0 | 34.4 | 35.2 | 35.6 | 35.2 |
| Obese (≥ 30), % | 37.4 | 34.0 | 30.5 | 27.1 | 21.3 |
| Ethnicity, % | |||||
| White | 78.1 | 83.4 | 85.4 | 86.5 | 85.6 |
| Black | 14.1 | 9.04 | 7.28 | 6.09 | 5.90 |
| Hispanic | 3.70 | 3.29 | 3.18 | 3.63 | 3.86 |
| American Indian | 0.54 | 0.46 | 0.34 | 0.31 | 0.41 |
| Asian/Pacific Islander | 2.32 | 2.66 | 2.71 | 2.54 | 3.15 |
| Other | 1.27 | 1.15 | 1.11 | 0.96 | 1.08 |
| Income, % | |||||
| < $20,000 | 25.5 | 18.2 | 15.0 | 12.0 | 9.40 |
| $20,000 to $34,999 | 29.0 | 27.4 | 24.9 | 21.9 | 18.0 |
| $35,000 to $49,999 | 19.7 | 21.1 | 21.4 | 21.2 | 19.7 |
| $50,000 to $74,999 | 15.1 | 18.4 | 20.4 | 22.6 | 24.1 |
| ≥ $75,000 | 10.8 | 14.9 | 18.2 | 22.3 | 28.9 |
| Education, % | |||||
| ≤ High school diploma or GED | 28.4 | 23.7 | 21.2 | 18.6 | 15.6 |
| Some college | 40.5 | 38.8 | 37.7 | 36.7 | 36.0 |
| ≥ College degree | 31.1 | 37.5 | 41.2 | 44.8 | 48.5 |
| Physical activity (MET-hr/wk.), mean ± SD | 9.9 ± 12.5 | 11.5 ± 13.1 | 12.5 ± 13.6 | 13.4 ± 13.7 | 14.9 ± 14.8 |
| Live alone, % | 38.2 | 32.7 | 29.5 | 26.3 | 23.3 |
| Have current healthcare provider, % | 92.6 | 94.3 | 94.7 | 94.9 | 94.5 |
| Number of falls in past year, % | |||||
| 0 | 67.5 | 67.3 | 67.8 | 67.7 | 69.2 |
| 1 | 19.7 | 20.3 | 19.9 | 20.1 | 19.3 |
| 2 | 8.44 | 8.37 | 8.35 | 8.18 | 7.64 |
| 3+ | 4.35 | 4.04 | 3.94 | 4.02 | 3.85 |
| ≥ 1 activity of daily living disability, % | 2.21 | 1.65 | 1.63 | 1.49 | 1.28 |
| Depression score, % | |||||
| 0 | 26.7 | 27.1 | 28.3 | 28.3 | 29.0 |
| 1–2 | 38.4 | 41.0 | 40.9 | 41.5 | 41.1 |
| 3–4 | 17.9 | 17.3 | 17.5 | 17.0 | 16.8 |
| 5–18 | 17.1 | 14.5 | 13.3 | 13.3 | 13.2 |
| Smoking, % | |||||
| Never | 41.6 | 51.6 | 53.2 | 53.4 | 53.3 |
| Past | 35.1 | 41.6 | 44.0 | 45.3 | 46.2 |
| Current | 23.3 | 6.79 | 2.79 | 1.30 | 0.50 |
| Unopposed estrogen use, % | |||||
| Never | 64.6 | 63.6 | 63.8 | 63.4 | 65.3 |
| Past | 15.2 | 13.8 | 12.5 | 12.1 | 10.3 |
| Current | 20.2 | 22.6 | 23.7 | 24.5 | 24.4 |
| Estrogen + progesterone use, % | |||||
| Never | 81.6 | 76.5 | 72.7 | 69.6 | 65.6 |
| Past | 6.96 | 8.18 | 9.09 | 9.38 | 9.89 |
| Current | 11.4 | 15.3 | 18.2 | 21.0 | 24.5 |
| Medical history, % | |||||
| Arthritis | 53.0 | 49.9 | 48.5 | 46.0 | 40.6 |
| Diabetes (treated with pills/shots) | 4.05 | 4.30 | 4.45 | 4.42 | 4.46 |
| Cancer | 10.4 | 9.81 | 9.34 | 8.84 | 8.95 |
| Hypertension | 39.4 | 36.7 | 34.3 | 31.2 | 27.1 |
| Emphysema | 5.40 | 3.83 | 3.47 | 2.96 | 2.76 |
| Hip fracture | 1.05 | 1.02 | 0.91 | 0.86 | 0.57 |
| Dietary intake, mean ± SD | |||||
| Calibrated energy (kcal/d) | 2069 ± 245 | 2069 ± 218 | 2071 ± 203 | 2075 ± 188 | 2071 ± 171 |
| Calibrated Protein (g/d), | 71.5 ± 12.1 | 74.7 ± 11.0 | 76.7 ± 10.5 | 79.0 ± 10.1 | 81.7 ± 9.9 |
| Calibrated Protein (g/kg body weight/d) | 0.97 ± 0.17 | 1.03 ± 0.17 | 1.07 ± 0.17 | 1.12 ± 0.18 | 1.19 ± 0.20 |
| Animal protein (g/d) | 35.5 ± 19.0 | 43.2 ± 20.7 | 48.1 ± 21.4 | 52.9 ± 22.2 | 60.5 ± 25.2 |
| Vegetable protein (g/d) | 19.3 ± 8.8 | 20.8 ± 8.9 | 21.0 ± 8.8 | 20.9 ± 8.6 | 19.7 ± 8.4 |
| Fruits/vegetables (servings/d) | 3.8 ± 2.2 | 4.1 ± 2.1 | 4.2 ± 2.1 | 4.2 ± 2.1 | 4.2 ± 2.1 |
| Calcium (mg/d) | 666 ± 359 | 777 ± 400 | 839 ± 424 | 896 ± 458 | 960 ± 535 |
| Vitamin D (mcg/d) | 3.3 ± 2.3 | 3.9 ± 2.6 | 4.4 ± 2.8 | 4.8 ± 3.1 | 5.5 ± 3.8 |
| Alcohol (g/d) | 7.0 ± 15.2 | 5.5 ± 10.7 | 5.2 ± 9.4 | 4.8 ± 8.6 | 4.0 ± 7.2 |
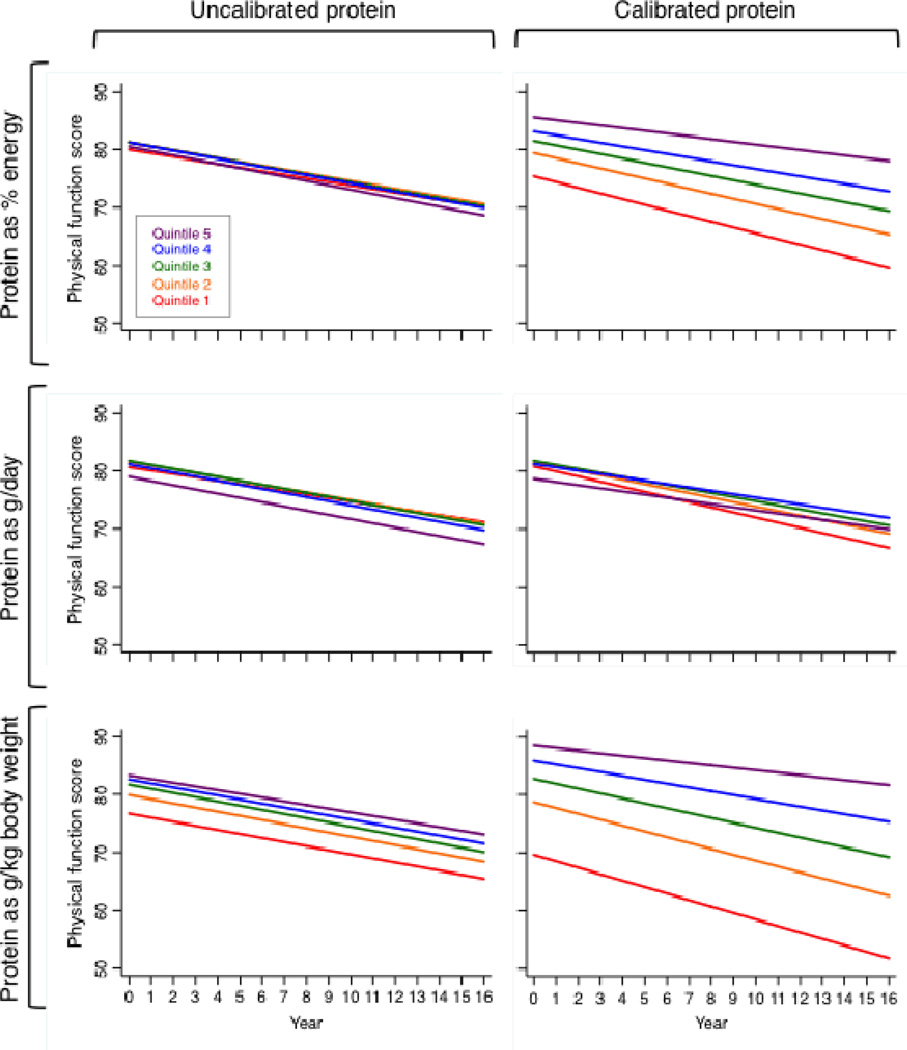
Women in the highest quintile of calibrated protein intake reported a 14% higher level of physical function at baseline than women in the lowest quintile (Table 2). Further, the rate of self-reported annual decline in physical function was 52% lower in women in the highest compared with the lowest quintile of calibrated protein intake. The positive association between self-reported physical function and protein intake was consistent across the continuum of calibrated protein intake. Results were similar when characterizing protein intake as g/kg body weight (consistent with dietary reference intake estimates) instead of % energy; however, there was no association for grams of absolute protein intake and self-reported physical function (Figure 1). Furthermore, the positive associations between protein intake and physical function were evident using either uncalibrated or calibrated protein intake as g/kg body weight; however, the strength of the associations was substantially weaker for uncalibrated protein intake.
Table 2.
Baseline Physical Function Measures and Annualized Change (and 95% Confidence Intervals) by Quintile of Calibrated Protein Intakea
| Physical function measure | Quintile of calibrated protein intake (% energy) | Ptrend | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Self-reported score (n = 112,213) | ||||||
| Baseline | 75.4 (73.2 to 78.5) | 79.4 (78.4 to 80.7) | 81.5 (81.2 to 81.9) | 83.1 (81.8 to 84.4) | 85.6 (81.9 to 87.5) | 0.002 |
| Annualized change | −0.98 (−1.18 to −0.75) | −0.88 (−1.03 to −0.70) | −0.75 (−0.82 to −0.70) | −0.64 (−0.69 to −0.59) | −0.47 (−0.63 to −0.39) | 0.022 |
| Grip strength, kg (n = 4,527) | ||||||
| Baseline | 24.1 (23.6 to 24.5) | 24.2 (23.7 to 24.5) | 24.3 (24.0 to 24.9) | 24.8 (24.3 to 25.2) | 24.7 (24.3 to 25.2) | 0.036 |
| Annualized change | −0.59 (−0.66 to −0.50) | −0.57 (−0.66 to −0.50) | −0.56 (−0.65 to −0.47) | −0.56 (−0.63 to −0.49) | −0.45 (−0.53 to −0.38) | 0.028 |
| Chair stands, n (n = 4,497) | ||||||
| Baseline | 6.61 (6.46 to 6.76) | 6.67 (6.55 to 6.86) | 6.83 (6.71 to 6.99) | 7.02 (6.79 to 7.13) | 7.11 (6.91 to 7.26) | 0.002 |
| Annualized change | −0.09 (−0.11 to −0.05) | −0.05 (−0.08 to −0.03) | −0.07 (−0.10 to −0.04) | −0.06 (−0.09 to −0.03) | −0.05 (−0.08 to −0.03) | 0.306 |
| Timed walk, seconds (n = 4,533) | ||||||
| Baseline | 6.02 (5.81 to 6.37) | 6.10 (5.70 to 6.27) | 5.89 (5.67 to 6.31) | 6.25 (5.87 to 6.50) | 5.84 (5.62 to 6.22) | 0.748 |
| Annualized change | 0.16 (0.05 to 0.22) | 0.05 (0.01 to 0.17) | 0.12 (0.00 to 0.18) | 0.00 (−0.06 to 0.09) | 0.07 (0.00 to 0.14) | 0.080 |
Estimates derived from GEE adjusted for age, income, education, race/ethnicity, BMI, smoking status, alcohol consumption, physical activity, hormone therapy use, whether the participant lived alone, having a healthcare provider, number of falls, disability, depression, self-reported history of medical conditions (emphysema, diabetes, hypertension, arthritis, and cancer), calibrated total energy intake, and clinical trial arm. Confidence intervals were calculated using 1000 bootstrap replicates to account for uncertainty in the calibrated estimates of protein intake.
Figure 1.
Self-reported physical function score over time, by quintiles of protein intake, calculated using GEE. Models were adjusted for age, income, education, race/ethnicity, BMI (height only for g/kg models), smoking status, alcohol consumption, physical activity, hormone therapy use, whether the participant lived alone, having a healthcare provider, number of falls, disability, depression, and self-reported history of medical conditions (emphysema, diabetes, hypertension, arthritis, and cancer), calibrated total energy intake, and clinical trial arm.
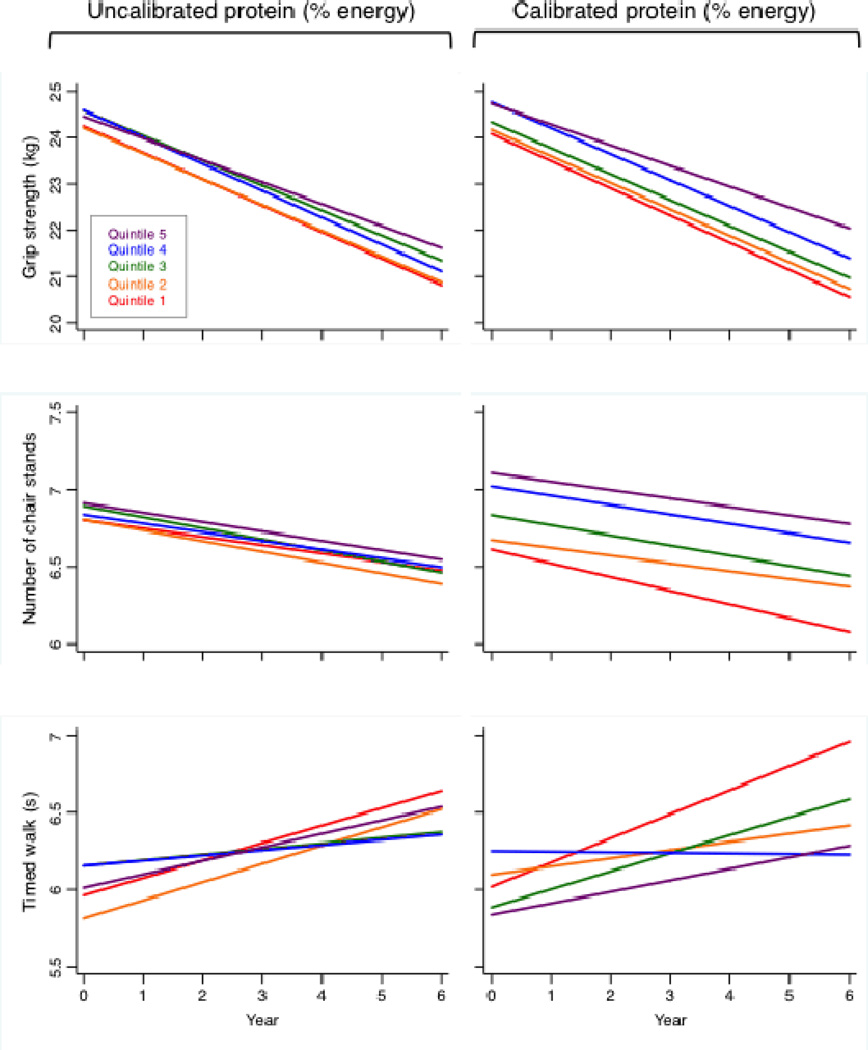
With respect to objective measures of physical function, mean grip strength at baseline was slightly higher among women with higher calibrated protein intake (P=0.036), and these women experienced smaller declines in grip strength over time than those with low calibrated protein intake (P=0.028) (Table 2). Additionally, women in the highest quintile of calibrated protein intake completed on average 0.5 more chair stands at baseline than women in the lowest quintile (P=0.002). In contrast, there was no significant association between calibrated protein intake and the timed 6-meter walk in either cross-sectional or longitudinal analyses (P=0.748 and 0.080, respectively). Consistent with the self-reported analyses, estimates for the association between protein intake and objective measures of physical function did not change substantively when characterizing protein intake as g/kg body weight instead of % energy, but grams of absolute protein intake was not significantly associated with any of the objective measures (data not shown). Furthermore, associations between protein intake and grip strength or chair stands were substantially stronger using calibrated versus uncalibrated protein intake (Figure 2). For self-reported and objective measures, no substantive interactions were detected between calibrated protein intake and age, BMI, or physical activity (data not shown).
Figure 2.
Physical performance measures over time, by quintiles of protein intake, calculated using GEE. Models were adjusted for age, income, education, race/ethnicity, BMI, smoking status, alcohol consumption, physical activity, hormone therapy use, whether the participant lived alone, having a healthcare provider, number of falls, disability, depression, and self-reported history of medical conditions (emphysema, diabetes, hypertension, arthritis, and cancer), calibrated total energy intake, and clinical trial arm.
DISCUSSION
In this large, prospective study of postmenopausal women with a mean 11.5 y of follow-up, women with higher calibrated protein intake demonstrated smaller declines in self-reported physical function as well as two physical performance measures (grip strength and repeated chair stands). There was no association between calibrated protein intake and gait speed, suggesting that factors related to preserving mobility may be less closely related to protein intake. Therefore, improvements in physical function, particularly with respect to factors related to strength, may partially explain the protein-frailty association. Over a 4-y follow-up period, a one-point lower SPPB12 score, which is frequently used as a summary measure of physical function, was associated with 15% (95% CI, 6 to 24%) higher risk of frailty and 25% (95% CI, 6 to 47%) higher risk of mortality in the Einstein Aging Study17. Two randomized, controlled trials of protein supplementation (30 g/day) and protein supplementation (30 g/day) plus exercise observed ~1 point increases in SPPB after 24 intervention weeks compared to no changes in the placebo group18,19. These data support the hypothesis that greater protein intake as % energy reduces the risk of frailty by maintaining functional status, muscle strength, and balance in postmenopausal women.
Given the large sample size of the current study, it is important to consider whether our statistically significant findings are also clinically relevant. Over a 5-y period, average self-reported physical function declined by 2 versus 5 points among women in the highest versus lowest quintile of calibrated protein intake, respectively. Likewise, over a 5-y period, average grip strength declined by 2.3 kg versus 3.0 kg in the highest versus lowest quintile of calibrated protein intake. These differences are larger than what has been defined as substantially clinically important changes in a cohort using somewhat different (i.e. SPPB, gait speed, timed walk) but analogous measures of physical function20,21.
One issue to be resolved is determining the optimal percentage of energy as protein associated with greater physical function in aging women22,23. In our study higher self-reported physical function was demonstrated with higher protein intake through the upper bound of intake (top quintile median of 16.0% energy or 1.18 g/kg body weight). While there remains insufficient evidence overall in regard to specific levels of protein intake required to optimize health status in aging women, our findings are supported by those from available epidemiological studies and clinical trials24–27. A recent meta-analysis of randomized, controlled trials for protein supplementation reported that participants consuming high-protein diets (mean intake 1.25 ± 0.17 g/kg body weight) increased grip strength by 1.76 kg (95% CI, 0.36 to 3.17) compared with control groups (four studies, n=219, mean age 65 y, age range 53–85 y, 60% female)28. Similar to our findings, however, prior studies of protein intake and mobility reported no significant effects29–31. Mean intake in the high-protein diets in the meta-analysis was slightly higher than the mean protein intake in the top quintile for this study (1.19 ± 0.20 g/kg body weight), and the mean intake for the comparison group was 0.72 ± 0.09 g/kg body weight, which was lower than the bottom quintile for this study (0.97 ± 0.17 g/kg body weight).
Low grip strength is a predictor of disability, mortality, and other poor outcomes in older adults32. Evidence suggests that reductions in frailty risk can be achieved through physical activity interventions by directly targeting inactivity and improving strength and motor performance33. In the current study, each MET-hr./week of recreational physical activity was associated with a 0.2-unit increase in self-reported physical function (P<0.001), no significant difference in grip strength, 0.02-unit increase in the number of chair stands completed (P<0.001), and 0.01-second decrease in the timed walk (P=0.001). However, a 3-y prospective study of 92 women (mean age 71 ± 4 y) enrolled in a twice-weekly fitness program documented declines in grip strength (–3.2 ± 5.0 kg), increases in walking time (0.71 ± 0.9 seconds), and declines in energy (–345 ± 533 kcal/d) and protein (–9.5 ± 14.7 g/d) intake34. A stratified analysis of protein intake (% energy) by median recreational physical activity level in our sample showed that the protein associations with physical function were independent of activity (data not shown), suggesting that women may benefit from consuming higher amounts of protein irrespective of physical activity level. An intervention combining protein and physical activity would exclude older adults who are on bed rest, yet evidence from clinical trials suggests that essential amino acid supplementation helps to preserve muscle mass and improve function among individuals during bed rest25,35.
Strengths of this study include the prospective design, wide age range in this well characterized sample of postmenopausal women, ability to correct for measurement error in protein and energy intake using biomarker-derived calibration equations, availability of multiple standardized physical performance measures over a 6-y period as well as annual repeat measures of self-reported physical function spanning more than a decade, and ability to adjust for a large number of covariates that may be confounders. However, similar to the general U.S. population36, the range of protein intake was not highly variable and cannot inform on aging women with chronic intake outside this range (e.g. high-protein diets or animal product-restricted vegetarian diets). Furthermore, given the differences in participant characteristics among women consuming low versus high protein intake, it is possible factors other than protein intake are partially explaining observed associations, though we did our best to account for potential confounders. Additionally, there is substantial measurement error in self-reported physical activity, physical activity measurement was restricted to recreational/ leisure time, and protein intake is positively correlated with physical activity. Thus, we cannot rule out the possibility of residual confounding by physical activity. Analyses incorporating objective measures of physical activity could better elucidate the relationship between protein intake and physical function. The possibility of selection bias also must be considered, as participants enrolled in WHI for up to almost 20 y may differ from the general population of postmenopausal women. However, women in this study had considerable variation in physical function measures at baseline, and the change over time was sufficient in magnitude, to detect significant differences with respect to protein intake.
In summary, this prospective study of well functioning postmenopausal women showed that higher biomarker-calibrated protein intake was associated with smaller declines in self-reported physical function and objective measures of physical performance over an average 11.5-y time period. Given the multi-factorial nature of age and disease-related functional decline, modification of one potential factor may not be sufficient to delay decline. However, our data suggest that efforts to intervene in relation to protein intake in this subgroup of the population are warranted and should be rigorously evaluated. Randomized, controlled trials could also provide important information regarding optimal dose, composition, and effects of protein intake on aging.
ACKNOWLEDGMENTS
Drs. Joel O. Wertheim and Shankar Viswanathan provided valuable input into the analytic approach.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. This work was also supported by 4R00AG035002, PO1 CA53996, and 5R01AG025441-03. JB is employed by Fred Hutchinson Cancer Research Center, Seattle WA. JB has funding from the National Institutes of Health for research in the health of postmenopausal women and for dietary biomarker development and application.
Sponsor’s Role: NHLBI as WHI sponsor was represented on key study committees. The NBS sponsor (NCI) has not been involved in operational aspects of this work, or in the development of this manuscript.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
| Elements of Financial/Personal Conflicts |
JB | all other authors | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Employment or Affiliation | x | X | ||
| Grants/Funds | x | X | ||
| Honoraria | x | X | ||
| Speaker Forum | x | X | ||
| Consultant | x | X | ||
| Stocks | x | X | ||
| Royalties | x | X | ||
| Expert Testimony | x | X | ||
| Board Member | x | X | ||
| Patents | x | X | ||
| Personal Relationship | x | X | ||
Author Contributions: Each author contributed significantly to the formulation of the research question, data analysis, or interpretation of the data. Everyone who contributed significantly to the work is listed as an author, and written consent was obtained from all named contributors.
All authors contributed significantly to the manuscript.
REFERENCES
- 1.Beasley JM, LaCroix AZ, Neuhouser ML, et al. Protein intake and incident frailty in the Women's Health Initiative observational study. J. Am. Geriatr. Soc. 2010 Jun;58(6):1063–1071. doi: 10.1111/j.1532-5415.2010.02866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans WJ, Paolisso G, Abbatecola AM, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010 Oct;11(5):527–536. doi: 10.1007/s10522-010-9297-0. [DOI] [PubMed] [Google Scholar]
- 3.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J. Am. Geriatr. Soc. 2005 Aug;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology.Series A, Biological sciences and medical sciences. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Drummond MJ, Dreyer HC, Pennings B, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008 Mar 6; doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab. 2009 Jan;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 2007 Aug;86(2):451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 8.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control. Clin. Trials. 1998 Feb;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 9.Anderson G. Implementation of the women's health initiative study design. Ann. Epidemiol. 2003;13(9):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 10.Brunner RL, Cochrane B, Jackson RD, et al. Calcium, vitamin D supplementation, and physical function in the Women's Health Initiative. J. Am. Diet. Assoc. 2008 Sep;108(9):1472–1479. doi: 10.1016/j.jada.2008.06.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993 Oct;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A. Biol. Sci. Med. Sci. 2000 Apr;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 15.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann. Epidemiol. 1999 Apr;9(3):178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am. J. Epidemiol. 2008 May 15;167(10):1247–1259. doi: 10.1093/aje/kwn026. [DOI] [PubMed] [Google Scholar]
- 17.Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J. Am. Geriatr. Soc. 2012 Oct;60(10):1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tieland M, Dirks ML, van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. Journal of the American Medical Directors Association. 2012 Oct;13(8):713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. Journal of the American Medical Directors Association. 2012 Oct;13(8):720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) The journal of nutrition, health & aging. 2009 Jun;13(6):538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006 May;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 22.Millward DJ. Amino acid scoring patterns for protein quality assessment. Br. J. Nutr. 2012 Aug;108(Suppl 2):S31–S43. doi: 10.1017/S0007114512002462. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br. J. Nutr. 2012 Aug;108(Suppl 2):S88–S93. doi: 10.1017/S0007114512002590. [DOI] [PubMed] [Google Scholar]
- 24.Filion ME, Barbat-Artigas S, Dupontgand S, Fex A, Karelis AD, Aubertin-Leheudre M. Relationship between protein intake and dynapenia in postmenopausal women. The journal of nutrition, health & aging. 2012 Jul;16(7):616–619. doi: 10.1007/s12603-012-0054-8. [DOI] [PubMed] [Google Scholar]
- 25.Ferrando AA, Paddon-Jones D, Hays NP, et al. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010 Feb;29(1):18–23. doi: 10.1016/j.clnu.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. The American Journal of Clinical Nutrition. 2008 May;87(5):1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 27.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Current opinion in clinical nutrition and metabolic care. 2009 Jan;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing research reviews. 2012 Apr;11(2):278–296. doi: 10.1016/j.arr.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am. Rev. Respir. Dis. 1988 May;137(5):1075–1082. doi: 10.1164/ajrccm/137.5.1075. [DOI] [PubMed] [Google Scholar]
- 30.Bonnefoy M, Cornu C, Normand S, et al. The effects of exercise and protein-energy supplements on body composition and muscle function in frail elderly individuals: a long-term controlled randomised study. Br. J. Nutr. 2003 May;89(5):731–739. doi: 10.1079/BJN2003836. [DOI] [PubMed] [Google Scholar]
- 31.Otte K, Ahlburg P, D'Amore F, Stellfeld M. Nutritional repletion in malnourished patients with emphysema. Journal of Parenteral and Enteral Nutrition. 1989;13(2):152–156. doi: 10.1177/0148607189013002152. [DOI] [PubMed] [Google Scholar]
- 32.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 33.Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin. Geriatr. Med. 2011 Feb;27(1):101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarti S, Ruggiero E, Coin A, et al. Dietary intake and physical performance in healthy elderly women: A 3-year follow-up. Exp. Gerontol. 2012 Oct 11; doi: 10.1016/j.exger.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 35.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Current opinion in clinical nutrition and metabolic care. 2010 Jan;13(1):34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fulgoni VL., 3rd Current protein intake in America: analysis of the National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008 May;87(5):1554S–1557S. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]