Abstract
Objective
Childhood maltreatment increases risk for psychopathology. For some highly prevalent disorders (i.e., major depression, substance abuse, anxiety disorders and posttraumatic stress disorder) there is a substantial subset of individuals with maltreatment histories and a substantial subset without. Do those with maltreatment histories represent a clinically and biologically distinct subtype?
Method
The authors review literature on maltreatment as a risk factor for these disorders and on the clinical differences between individuals with and without maltreatment who share the same diagnoses. Neurobiological findings in maltreated individuals are reviewed and compared to findings reported for these disorders.
Results
Maltreated individuals with depressive, anxiety and substance use disorders show an earlier age of onset, greater symptom severity, more comorbidity, increased risk for suicide and poorer treatment response than non-maltreated individuals with the same diagnoses. Imaging findings associated with these disorders, such as reduced hippocampal volume and amygdala hyperreactivity are more consistently observed in maltreated individuals and may represent a maltreatment-related risk factor. Maltreated individuals also differ from others due to epigenetic modifications and genetic polymorphisms that interact with experience to increase risk for psychopathology.
Conclusions
Phenotypic expression of psychopathology may be strongly influenced by exposure to maltreatment leading to a constellation of ecophenotypes. While these ecophenotypes fit within conventional diagnostic boundaries, they likely represent distinct subtypes. Recognition of this distinction may be essential in determining the biological bases of these disorders. Further, treatment guidelines and algorithms may be enhanced if maltreated and non-maltreated individuals with the same diagnostic labels are differentiated.
Maltreated children are more likely to suffer psychiatric disorder over the course of their lifetime. In particular, they are more likely to develop major depression (1–5), bipolar disorder (6), anxiety disorders (2, 3, 7), posttraumatic stress disorder (2, 3), substance abuse (2, 8, 9), personality disorders (10, 11) and psychoses (12). Further, it appears that survivors of early maltreatment differ from other individuals with the same psychiatric diagnoses in critical ways. Disorders emerge earlier in maltreated individuals, with greater severity, more comorbidity, and show a less favorable response to treatment (13–15). There may also be discernible brain abnormalities in maltreated individuals not present in their non-maltreated counterparts (16, 17). Lastly, childhood maltreatment is also linked to a wide array of medical disorders, shortened life expectancy and reduced telomere length (18, 19). Hence, an understanding of maltreatment as an etiological risk factor is crucial to the development of a science of preventative psychiatry, to the design of effective therapeutic regimens, and to the delineation of an accurate nosology.
Our goal in this review is to advance the thesis (17, 20–23) that affected individuals with childhood maltreatment constitute a critically distinct subtype across depressive, anxiety and substance use disorders. We also propose that the maltreated subtype may be thought of as a phenotypic specialization (phenocopy) resulting from environmental experience or more precisely, an ecophenotype.
Why focus on maltreatment? Because it is maltreatment rather than exposure to other stressors, such as natural disasters, that consistently presents as the antecedent to psychopathology (24, 25). This makes sense. Children are dependent on the adults around them for their survival, and can endure great hardship if they feel protected and cared for. But, when the hardship is the product of their caretakers, and when it is the caretaker who must be protected against, it creates a stressor with far reaching ramifications.
Epidemiology of maltreatment trauma
Maltreatment is characterized by sustained or repeated exposure to events that usually involve a betrayal of trust (20). Active examples include childhood sexual, physical and various forms of emotional abuse. Passive examples include emotional and physical neglect. (See Table 1 for proposed assessment criteria and definitions). As might be expected, parents of maltreated children were often maltreated themselves, and show high rates of untreated or undertreated psychopathology (26). Therefore, intergenerational transmission involves some combination of early life stress, deficient parenting skills, genetic or epigenetic risk and family stressors (27).
Table 1.
Childhood Maltreatment or Abuse Checklist
| Before the age of 18 years: Sustained or repeated exposure to events involving a betrayal of trust by caretakers, or other significant individuals in the child’s life. |
| At least one of the following |
| Active Maltreatment: |
| _____ Emotional abuse |
| _____ Verbal Aggression (communications intended to inflict intense humiliation, denigration or extreme fear) |
| _____ Emotional Manipulation (placing the child in a situation intended to elicit shame, guilt or fear in order to serve the emotional needs of the perpetrator or to persuade the child to perform actions against his/her will or denigrating or destroying things of value to the child) |
| _____ Witnessing Domestic Violence (witnessing adults in the household intentionally humiliating, demeaning, threatening to harm one another or other family members or actively engaged in physically harming family members by shoving, slapping, kicking, throwing objects or using weapons against each other) |
| _____ Physical Abuse (hitting with objects, intentionally inflicting harm that results in bruises, welts or in the need for medical attention, shoving, kicking, dragging child by the hair, approaching the child with a weapon, forcing the child to remove clothing or otherwise humiliate himself/herself in front of others) |
| ____Extreme Corporal Punishment (discipline involving hitting with objects, intentionally inflicting harm that results in bruises, welts or in need for medical attention, forcing child to remove clothing or otherwise humiliate himself/herself in front of others ostensibly for discipline) |
| _____ Sexual Abuse (adults or older children touching or fondling the child’s body in a sexual way or forcing the child to touch or fondle the perpetrator’s body in a sexual way, or forcing the child to engage in other activities with a sexual content or attempted or actual sexual intercourse (oral, anal or vaginal) |
| Passive Maltreatment |
| _____ Emotional Neglect (failure to provide for the child’s basic emotional needs, being emotionally unresponsive to child’s distress, not attending to child’s social and emotional development or not attending to child’s school performance, homework etc, or expecting the child to routinely manage situations that are beyond his/her maturity level or are not safe) |
| _____ Physical Neglect (failure to provide for the child’s basic needs such as for food, clothing, physical safety, adequate supervision, dental health, physical health) |
Differences in definitions make it hard to draw firm conclusions about prevalence. However, retrospective and prospective studies suggest that exposure to one or more forms of childhood maltreatment range from 13.8% in one-year prevalence rates to about 42% in retrospective estimates covering the full 18 years of childhood (28).
Supporting Methodology
Our conceptualization of ecophenotypes emerged from a systematic review of the English literature on the psychiatric and neurobiological consequences of childhood maltreatment. How the review was conducted and tabled results of sexual abuse as a psychiatric risk factor are included in the supplementary materials. Studies selected for citation are representative. No contradictory studies showing a significant protective effect of maltreatment were encountered. In this review, we exclude disorders (e.g., borderline personality and dissociative identity disorder) where research suggests the vast majority of patients were exposed to some type of abuse or neglect (10, 11, 29, 30). On the other end, we also exclude schizophrenia and bipolar disorder, which are known to be highly heritable. Instead, we focus on moderately inheritable disorders in which there are major subsets of patients who can be distinguished by positive or negative histories of childhood maltreatment. These disorders include major depression, anxiety disorders, posttraumatic stress and substance abuse. Childhood maltreatment or early adversity accounts for about 30% – 70% of their population attributable risk fraction (1, 3, 9).
Major Depressive Disorder and Maltreatment
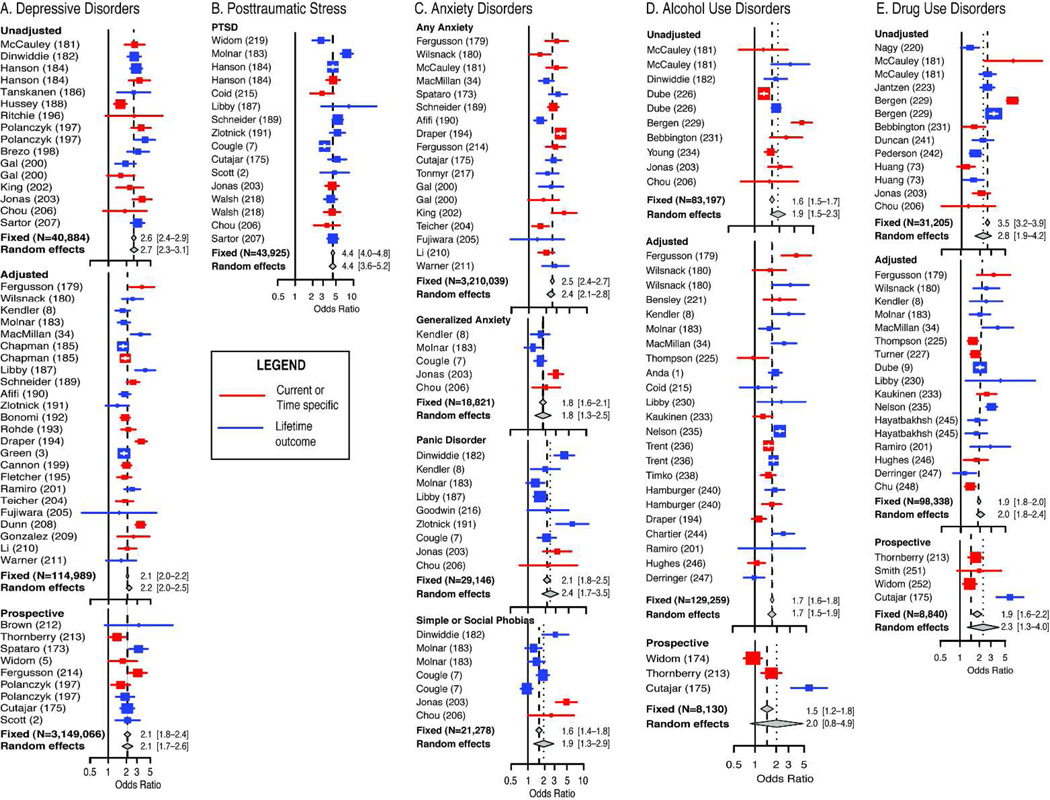
Some of the strongest evidence for an association between exposure to childhood maltreatment and the development of major depression is found in the Adverse Childhood Experiences study (31), which showed that risk for depression increased in a graded dose-dependent fashion with the number of maltreatment-related adverse childhood experiences. Exposure to one or more adverse childhood experiences accounted for 54% of the population attributable risk fraction for current episodes of depression (1) and 67% for suicide attempts (32). Having 5 or more adverse experiences increased the relative risk of receiving a prescription for an antidepressant by 2.9-fold (6). Long-term prospective studies also indicate about a 2-fold increased risk attributable to maltreatment (2, 4, 5) (see Figure 1A). These findings are consistent with results of twin studies showing that heritability plays only a minor role in risk for moderate or even severe depressions (33).
Figure 1.
Forest plots showing odds ratios and 95% confidence interval for psychopathology in individuals exposed to childhood sexual abuse or multiple forms of maltreatment including sexual abuse. A. Diagnoses or suprathreshold symptoms of major depression. B. Diagnoses of posttraumatic stress disorder. C. Diagnoses or suprathreshold symptoms of anxiety disorders including generalized anxiety disorder, panic disorder and simple or social phobias. D. Alcohol related problems including heavy episodic drinking, abuse or dependence. E. Drug related problems including use of illicit drugs, abuse or dependence. Multiple analyses within studies were pooled to provide assessment for overall risk across severity levels and genders. Studies were ordered within each cluster by year of publication. Complete details and citations not included in the main text are provided in supplementary materials.
Maltreatment increases risk for depression in both males and females, though some studies suggest greater risk for depression in physically abused females than males (34, 35). Hence, increased female prevalence may be due, at least in part, to greater sensitivity to physical abuse, and more frequent exposure to childhood sexual abuse (36).
Important clinical differences exist between depressive illnesses with and without childhood maltreatment. Depressions emerge earlier and have a more sustained course (13, 37) in maltreated individuals. These individuals also have more severe mood, neurovegetative and endogenous symptoms and more comobidities, particularly substance abuse (13, 22, 37, 38). Psychotic features are also more common as are suicide attempts and deliberate self-harm (39).
Maltreated depressed patients also differ with respect to treatment response. A recent meta-analysis of depression outcome studies (13) confirmed that childhood maltreatment unequivocally predicts poor treatment outcome. However, it is also possible that maltreated depressed patients may respond preferentially to therapies that are less effective for their nonmaltreated depressed peers. In a large clinical trial (40) chronically depressed subjects received either pharmacotherapy with nefazodone, psychotherapy using the cognitive behavioral analysis system of psychotherapy, or the combination. Psychotherapy was clearly superior to antidepressant monotherapy in the subset with childhood trauma, and nefazodone provided little added benefit. In contrast, chronically depressed individuals without trauma or loss responded more favorably to nefazodone than psychotherapy, and benefitted from the combination. On the other hand, maltreatment was associated in a separate study with a poorer response to interpersonal therapy than cognitive therapy or medication, and with rapid relapse (41). With hindsight we can see that factors found over the years to predict treatment resistance in depression (i.e., early onset, comorbid anxiety and substance use disorders, Axis II diagnoses, presence of psychotic features) are the same factors now known to be characteristic of the maltreatment-related ecophenotype.
Neurobiological studies are beginning to provide compelling reasons for considering depression with maltreatment history as a distinct subtype. Reduced hippocampal size is one of the more prominent neuroimaging findings in major depression. However, Vythilingam et al (16) reported that reduced hippocampal size was only present in the subset of depressed individuals who had maltreatment histories. On balance there is now more consistent evidence for reduced hippocampal size in adults with maltreatment histories than in adults with major depression. Further, reduced hippocampal volume in maltreated individuals in the absence of depression or any psychiatric history has been observed in recent large sample studies (42, 43). In short, what has been regarded as a key finding in major depression may instead be a consequence of early stress that serves in turn as a risk factor. Indeed, reduced hippocampal volume can precede and partially mediate risk for depression with early stress (44).
Amygdala activation during exposure to sad or negative faces is another neuroimaging finding linked to major depression (45), that may be limited to depressed subjects with maltreatment histories (24). Indeed, bilateral amygdala reactivity to emotional expression is enhanced by a history of emotional maltreatment whether or not the subject has depression (46).
Genetic and epigenetic risk factors may also be distinctly different in major depression with versus without maltreatment. A comprehensive meta-analysis by Karg et al (47) found strong support for a gene × environment interaction involving the serotonin transporter promotor polymorphism and risk for depression when the environmental experience was childhood maltreatment, but only marginal support when the environmental experiences were post-childhood stressful events.
Epigenetic hypermethylation of the Nr3C1 gene results in decreased expression of glucocorticoid receptors and potential hypersecretion of cortisol during stress. Interestingly, Nr3C1 is hypermethylated in autopsy tissue from suicide victims with maltreatment histories, but not in suicide victims without maltreatment or in non-suicidal controls (48).
While some have speculated that non-maltreated individuals who develop depression do so because of a dense family history and high heritable risk, this supposition is not supported by our unpublished data, or by the observation that less severe forms of depression show little evidence of heritability (33). However, non-maltreated depressed individuals may show an array of non-inherited rare copy number variants (CNVs) – short stretches of DNA that are deleted or duplicated between individuals that contribute disproportionately to risk (49).
Finally, depressed patients differ in their risk for autoimmune, metabolic, and cardiovascular disorders based on maltreatment history. This may be related to chronic low-grade inflammation. Longitudinal data show that depression and inflammation are strongly coupled in depressed individuals with maltreatment but not in those without maltreatment (52).
Post-traumatic Stress Disorder
Sexual abuse, physical abuse and witnessing domestic violence are types of maltreatment that may fulfill the DSM A1 criteria for a traumatic event, and are major risk factors for the development of posttraumatic stress disorder (Figure 1B). Scott et al (2) reported adjusted odds ratio of 4.86 for lifetime diagnoses of posttraumatic stress in a prospective study of adults with maltreatment histories. Further, individuals who experience both childhood adversity and adult traumatic events were more likely to develop posttraumatic stress disorder than those who experience either type of adverse event alone (50).
However, there is a growing concern about how well the current DSM conceptualization of posttraumatic stress, which is based on exposure to acute life-threatening events in soldiers, applies to maltreated children. Youngsters often experience traumatic or highly stressful events during a substantial portion of their life, which may be perpetrated by one or more family members rather than a faceless enemy. This has led to two important observations. First, DSM-IV criteria are not sufficiently developmentally sensitive. Severely maltreated children often fail to meet full diagnostic criteria, as they frequently show symptoms in only two of three category clusters, but are as impaired as children meeting full criteria (51). Further, risk for posttraumatic stress in children appears to be influenced by frequency of exposure and multiplicity of exposure types rather than the degree to which they witnessed actual or threatened death or serious injury, or experienced a threat to their physical integrity. Hence, children may be ‘traumatized’ by repeated exposure to types of maltreatment, such as emotional abuse, that do not meet A1 criteria for a traumatic event (52).
Second, as Judy Herman, Bessel Van der Kolk and colleagues articulate, traumatized children also show a complex array of problems, such as affective dysregulation, disturbed attachment patterns, behavioral regression, somatic symptoms, and altered attributions and expectancies that are not included in the current DSM conceptualizations, and often lead to a host of comorbid diagnoses (52). Developmental trauma disorder has been proposed as a diagnostic category that more faithfully captures the critical events and clinical presentation of posttraumatic sequelae in chronically maltreated children (52).
However, developmental trauma disorder is best restricted to maltreated individuals with features of posttraumatic stress (see supplement for further discussion). As noted above, many maltreated individuals are more accurately characterized as depressed, and timing of exposure may be a critical determinant. Schoedl et al (53) found that individuals reporting sexual abuse after age 12 had a 10-fold increase in risk of severe posttraumatic stress disorder in adulthood compared to individuals reporting sexual abuse before age 12. Conversely, more severe depressive symptoms were present in individuals reporting sexual abuse before age 12 then after age 12 (53).
Multiple lines of evidence suggest that maltreated individuals with posttraumatic stress disorder continue to differ from their non-maltreated counterparts in adulthood. They show greater symptom complexity (54), more co-morbid mood disorders (55), more severe dissociation (56, 57) or alexithymia (58) leading to the designation ‘Complex PTSD’ (54, 59, 60). There may also be important neurobiological and genetic differences.
A key neuroimaging finding in posttraumatic stress, particularly in combat veterans (61) has been reduced hippocampal volume. However, a study of monozygotic twins discordant for combat exposure found reduced hippocampal volume in combat exposed individuals with posttraumatic stress as well as in their unexposed twins without posttraumatic stress (62). While these results may be confounded by individual drinking histories or personality factors common to both twins, it is also possible that reduced hippocampal volume resulted from shared early stress, and functioned as a risk factor for posttraumatic stress. As noted above, reduced hippocampal volume has been observed with considerable consistently in adults with maltreatment histories. While some early studies with small sample sizes observed reduced hippocampal size in maltreated adults with but not without posttraumatic stress disorder (63), recent studies with larger samples report reductions that are unrelated to posttraumatic stress (42, 43).
Additional neuroimaging findings in posttraumatic stress disorder, including amygdala hyperreactivity and reduced medial prefrontal and anterior cingulate response (61) have also been observed in individuals with histories of childhood abuse, including subjects without posttraumatic stress or any psychopathology (43). Studies are clearly needed to ascertain to what degree these neuroimaging findings are specific to posttraumatic stress disorder, specific to posttraumatic stress in maltreatment individuals, or are a more general consequence of exposure to childhood maltreatment.
Similar to findings for depressive illness, there are a number of genetic polymorphisms that appear to modulate risk for posttraumatic stress in subjects with maltreatment histories. The most compelling involves polymorphism of FKBP5, which regulates cortisol-binding affinity and the nuclear translocation of the glucocorticoid receptor (64, 65). Interestingly, Xie et al (65) reported that subjects with the TT genotype of rs9470080 had the lowest risk for posttraumatic stress as adults if there was no maltreatment history, but had the highest risk if there was. This suggests that the search for genetic risk factors may be elusive if subjects are not subtyped by maltreatment histories.
Anxiety Disorders
The National Comorbidity Replication Study showed that childhood sexual and/or physical abuse was associated with a 2.03 – 3.83 fold increase in risk for specific phobias, social anxiety disorder, generalized anxiety disorder, as well as panic disorder with or without agoraphobia (7) (Figure. 1C). Childhood adversity accounted for 32.4% of the population attributable risk factor for anxiety disorders (3). Moreover, exposure to multiple types of childhood adversity increased the likelihood of receiving a prescription for an anxiolytic by 2-fold (6).
The impact of exposure to childhood maltreatment on the clinical presentation and treatment of anxiety disorders has been understudied. Anxiety disordered patients with maltreatment histories have significantly higher concurrent rates of major depression (37, 66), more significant impairment in social functioning, higher state and trait anxiety scores (66), greater chronicity (37), symptom severity, and poorer quality of life (67). Severity increases with the number of types of maltreatment experienced and emotional abuse and neglect are especially salient risk factors for social anxiety disorder (67, 68). Lastly, social anxiety patients with a history of emotional abuse were the most likely to drop out of treatment during a clinical trial with paroxetine (68).
Neuroimaging studies in subjects with anxiety disorders, particularly those involving intense fear and panic, such as panic disorder, specific phobias and social anxiety, report evidence for amygdala hyperreactivity, which may stem from underactivity of the prefrontal cortex and insufficient inhibition of the amygdala (69, 70). Overactivation of the insula, a paralimbic region associated with perception of somatic sensations, has also been observed (70, 71). However, as indicated above, heightened amygdala activation has been observed in fMRI studies of adults without psychopathology if they were exposed to childhood maltreatment (43, 46). Moreover, a recent report (72) found that threatening faces produced overactivity in both the amygdala and anterior insula in maltreated children with normal levels of anxiety. Hence, amygdala and insula findings are not specific to subjects with anxiety disorders. An alternative hypothesis is that enhanced amygdala and insula response to threat emerges as a consequence of exposure to childhood maltreatment, and serves as a risk factor for the later development of anxiety disorders.
Substance Use Disorders
A substantial body of research shows the important role of maltreatment on risk for drug abuse and dependence (8, 9) (Fig. 1D–E), though the nature of the association may be complicated by high rates of substance abuse in maltreating parents and by the possibility of prenatal exposure, prenatal malnutrition and prematurity. A well-controlled epidemiological and co-twin study of women (8) reported that non-genital childhood sexual abuse was associated with a 2.9-fold increase in risk for drug-dependence and that sexual abuse involving intercourse was associated with a 5.7-fold increase. Risk was related to the number of different types of maltreatment an individual experienced. Compared with individuals with no adverse childhood events, adults with 5 or more adverse childhood events are 7- to 10-fold more likely to report illicit drug use problems, addiction to illicit drugs, and parenteral drug use (9). The population attributable risk fractions for these outcomes were 56%, 64%, and 67%, respectively (9). Results from the National Longitudinal Study of Adolescent Health and National Youth Survey provide prospective evidence for a causal relationship between physical abuse and early adult substance abuse (73, 74).
A moderate number of studies report differences between substance abusing individuals with and without maltreatment. The maltreated ecophenotype is associated with an earlier age of initiation, increased likelihood of engagement in risky sexual behaviors (75), increased risk for recent incarceration (76), greater ratings of psychological distress (77) and increased risk for co-morbid personality disorders (78). Physical maltreatment appears to be a particularly salient risk factor for development of substance abuse (35) and progression to parenteral drug use (79).
Substance abusers with maltreatment histories respond more poorly to treatment, with greater use of substances during treatment, and more persistence of substance-related problems post-discharge (80–82). Integrative therapies designed to address the combined impact of substance abuse and trauma-related psychopathology have been developed (83).
Key neuroimaging findings in substance abusers suggest the possibility of a ‘dopamine deficiency’ that may manifest as reduced activation of the ventral striatum (nucleus accumbens) during rewarding or pleasurable tasks (84, 85). Further, deficits in brain regions implicated in salience attribution (orbitofrontal cortex) and inhibitory control (anterior cingulate gyrus) may underlie the patterns of compulsive and impulsive behaviors that characterize addiction (86). Although these factors have not been well-studied in maltreated individuals, the few relevant studies report reduced sensitivity to reward and decreased basal ganglia response (87), as well as structural and resting blood flow deficits in ventral striatum, anterior cingulate and orbitofrontal cortex (43, 88, 89). Further research is needed to ascertain whether these deficits are common to substance abusers in general or more specific to the subset with histories of childhood maltreatment.
How does maltreatment increase the likelihood of developing so many different psychiatric disorders?
Could maltreatment be a nonspecific amplifying factor that ‘tips the balance” so that individuals at hereditary risk for one disorder or another become more likely to express it? In essence then, could maltreatment act to enhance the “penetrance” of inherited genetic susceptibilities? This could provide an explanation for both the increase in prevalence and associated comorbidities.
A richer and more compelling alternative is that the myriad possible outcomes of exposure to childhood maltreatment depend on the timing, type and severity of exposure plus a host of genetic factors that influence susceptibility and resilience, and an array of protective factors that attenuate risk. Epigenetic modifications in stress-response systems and neurotrophic factors regulating trajectories of brain development may be the driving force producing the various ecophenotypes. We believe that this explanation best accounts for the presently available data and suggest that psychiatric disorders presenting in individuals with substantial histories of childhood maltreatment be thought of as ecophenotypic variants or ecophenocopies (see supplement for strategies for capturing this in our nosology).
Neurobiological correlates of childhood maltreatment
As indicated above there is a growing body of reproducible findings linking childhood maltreatment to structural and functional brain differences. The most consistent finding is that of alterations in the corpus callosum, characterized by reduced midsaggital area (90–94) or decreased fractional anisotropy (diminished integrity) on diffusion tensor scans (95, 96) (Table 2). Another reasonably consistent finding is reduction in hippocampal volume in adults (16, 93, 97–105) but not in younger children (91, 92, 106, 107) with maltreatment histories (Table 3). The hippocampus is likely the most stress-sensitive structure in the brain, and translational studies show that stress or glucorticoids act on the hippocampus to suppress neurogenesis in the dentate gyrus and provoke remodeling of pyramidal cells in portions of the Cornu Ammonis, particularly CA3. We recently reported that childhood maltreatment was associated with volume reductions in the same subfields in a relatively large population of young adults (105), suggesting that the same mechanisms may be at work.
Table 2.
Childhood maltreatment and area or integrity of the corpus callosum.
| Number of Subjects | Age (years, Mean±SD, Range) |
|||||||
|---|---|---|---|---|---|---|---|---|
| First Author (Reference) |
Types of Maltreatment |
Diagnostic Requirement |
Exposed | Comparison | Sex | Meds | Main Corpus Callosum Findings* |
|
| Teicher (90) | Sexual, physical or neglect | Inpatients with v. wo abuse | 28 | 23 | 12.9±2.9 4–17 | Both | No | Decr. regions IV, III. Males more affected than females |
| De Bellis (91) | Sexual, physical or WDV | PTSD v. typical controls | 44 | 61 | 12.1±2.3 6–17 | Both | No | Decr. IV, V-VII. Males more affected than females |
| De Bellis (92) | Sexual, physical or WDV | PTSD v. SES matched controls | 28 | 66 | 11.5±2.9 4–17 |
Both | No | Decr. VII, IV-VI |
| De Bellis (144) | Sexual, physical or WDV | PTSD v. SES or Typical controls | 61 | 122 | 11.7± 2.6 4–17 |
Both | No | Decr. VII, I, VI. Males more affected than females. Reanalysis |
| Teicher (94) | Sexual, physical or neglect | Inpatients with v. wo abuse and controls | 28 | 23 inpatients 115 controls | 12.2±3.4 4–17 | Both | No | Decr. IV, V-VII. Males affected by neglect, females by sexual abuse. Partial reanalysis |
| Zanetti (145) | Physical or sexual | BPD** with v. wo PA/SA and controls | 10 (4 wo PA/SA) | 20 controls | 29.1±9.1 18–45 |
Both | No | NS BPD v. controls, Increased V, VII BPD with v. wo abuse |
| Rusch (146) | Sexual | BPD with v. wo SA and controls | 20 (10 wo PA/ SA) | 20 controls | 27.6±6.8 | Female | No | Decr. V BPD v. control. Decr. V, VI BPD with v. wo abuse |
| Kitayama (147) | Sexual, physical or WDV | PTSD v. typical controls | 9 | 9 | 37.3±9.4 | Female | Yes | Decr. V and total area |
| Jackowski (95) | Sexual, physical or WDV | PTSD v. typical controls | 17 | 15 | 10.6±2.3 6–14 |
Both | No | Decr. FA middle and posterior |
| Andersen (93) | Sexual | No diagnosis required 27% history PTSD | 26 | 17 | 19.8±1.4 18–22 |
Female | No | Decr. III. Sensitive period 9–10 years |
| Carrion (148) | Sexual, physical or WDV | PTSD symptoms v. controls | 24 | 24 | 11.0±2.2 7–14 |
Both | Yes | NS 8.7% decrease in VII |
| Mehta (120) | Early deprivation 24 months | Romanian orphans v. controls | 14 | 11 | 16.1±0.8 | Both | No | NS 6.5% decrease in absolute volume |
| Teicher (96) | Peer verbal abuse | No psychopathology | 63 | Used ratings not groups | 21.9±1.9 18–25 |
Both | No | Decr. FA VII, males and females affected to the same degree |
| Frodl (149) | CTQ scores | Unaffected relatives MDD, controls | 6 relatives, 4 controls | 15 relatives, 20 controls | 36.3±12.9 | Both | No | Decr FA in VII controls with v. wo abuse, Incr. FA in VII, relatives with v. wo abuse |
Statistically significant differences noted in one or more of the following regions: I-rostrum, II-genu, III-rostral body, IV-anterior midbody, V-posterior midbody, VI-isthmus, VII-splenium.
Note subject with borderline personality disorder without physical or sexual abuse were not considered to be unexposed to maltreatment given the likelihood that they experienced emotional abuse or neglect (11). References not cited in text are included in the Supplementary materials
BPD-borderline personality disorder, CTQ-childhood trauma questionnaire, Decr.-decreased, FA-fractional anisotropy from diffusion tensor imaging scans, Incr.-increased, MDD-major depressive disorder, NS-non-significant, PA – physical abuse, PTSD-posttraumatic stress disorder, SA – sexual abuse, SES-socioeconomic status, Sx-symptoms, WDV-witnessing domestic violence, wo-without.
Table 3.
Childhood maltreatment and structure and function of the hippocampus.
| Number of Subjects | Age (years, Mean±SD, Range) |
|||||||
|---|---|---|---|---|---|---|---|---|
| First Author (Reference) |
Types of Maltreatment |
Diagnostic Requirement |
Exposed | Comparison | Sex | Meds | Main Corpus Callosum Findings* |
|
| Adults | ||||||||
| Bremner (97) | Physical or sexual | PTSD v. healthy controls | 17 | 17 | 41.3±6.6 25–52 |
Female | Yes | Decr. 12% L |
| Stein (99) | Sexual | PTSD or DID v. SES controls | 21 | 21 | 31.1±6.4 | Female | Yes | Decr. 5% L |
| Driessen (103) | CTQ score | BPD v. healthy controls | 21 | 21 | 29.6±6.5 21–40 |
Female | Yes | Decr. 16% L, R |
| Vythilingam (16) | Physical or sexual | MDD with v. wo abuse and controls | 21 | 11 MDD 14 controls | 31.4±6.9 | Female | No | Decr. 15% L MDD with PA/SA v. control. NS MDD wo PA/SA v. control |
| Schmahl (104) | Physical or sexual | BPD with abuse v. comparison without BPD | 10 | 23 | 30.3±8.0 | Female | Yes | Decr, 11% L, 16% R |
| Bremner (63) | Sexual | Abuse with PTSD, abuse wo PTSD and controls | 10 with PTSD, 12 wo PTSD | 11 | 34.9±7.5 | Female | No | Decr. 19% L, R CSA with PTSD v. control. NS CSA without PTSD v. control |
| Brambilla (102) | Physical or sexual | BPD v. healthy controls | 10 | 20 | 33.0±8.9 | Both | No | Decr. 6.8% L, R most marked in BPD with abuse |
| Pederson (150) | CTQ severe to extreme pubertal | Abuse with PTSD, abuse wo PTSD, controls | 17 with PTSD, 17 wo PTSD | 17 | 25±6 20–40 |
Female | ? | NS 2.8% decr L abuse with PTSD v. control NS 6.3% decr. L abuse wo PTSD v. control. |
| Vermetten (100) | Physical or sexual | DID with PTSD v comparison | 15 | 23 | 37.8±9.0 | Female | Yes | Decr. 19.2% L, R |
| Cohen (114) | ELSQ high v. low 0–12 y | No psychopathology | 122 | 84 | 39.9±17.2 18–70 |
Both | No | Decr. L p=0.07, R p=0.06 |
| Zetzsche (151) | Physical or sexual | BPD** with v. wo PA/SA and controls | 14 BPD with 11 BPD wo PA/ SA | 25 | 26.7±6.7 | Female | Yes | Decr. 5% L p=0.07, 6% R p=0.03 BPD v. control NS BPD with v. wo PA/ SA |
| Andersen (93) | Sexual | No diagnosis required 27% history PTSD | 26 | 17 | 19.8±1.4 18–22 |
Female | No | Decr. 6.8% bilateral, sensitive period 3–5, 11– 13 years |
| Bonne (152) | Sexual, physical/ emotional | PTSD with v. without abuse, and controls | 11 | 11 PTSD 22 controls | 35.9±10.4 | Both | No | Decr. 9% bilateral PTSD v. control. NS PTSD with v. wo abuse |
| Weniger (153) | Physical or sexual | PTSD, DD and controls | 10 PTSD 13 DD | 25 | 32.±7.1 | Female | Yes | Decr. 18% bilateral PTSD v. control NS DD V. control |
| Lenze (154) | CECA scores | Remitted MDD with v. without abuse, controls | 19 | 12 Remitted MDD, 24 controls | 48.5±14.9 23–86 |
Female | Yes | Decr. L Remitted MDD v control. Abuse NS contribution |
| Soloff (155) | Physical or sexual | BPD with v. without PA/SA and controls | 20 with 14 without PA/ SA | 30 | 26.6±7.9 | Both | No | Decr. R,L BPD v. control NS BPD with v without PA/SA |
| Weniger (101) | Physical or sexual | BPD and controls | 24 | 25 | 32.5±6.5 21–45 |
Female | Yes | Decr. 12% bilateral, (with or wo comorbid PTSD) |
| Gatt (156) | ELSQ | No psychopathology | 89 | Used ratings not groups | 36.2±12.7 | Both | No | Decr. GMV R, L with ELS ratings and MET polymorphism BDNF |
| Frodl (98) | CTQ scores | MDD and healthy controls | 43 | 42 | 44.1±12.4 18–65 |
Both | Yes | NS GMV. EN: Decr. WMV L-females, L & R-males |
| Thomaes (157) | Physical or sexual | Complex PTSD and controls | 33 | 30 | 35.5±11.0 | Female | Yes | Decr. R p < 0.04, R inverse correlation with abuse severity p < 0.02 |
| Landré (158) | Sexual | PTSD and unexposed controls | 17 | 17 | 24.8±4.7 18–40 |
Female | No | NS |
| Sala (159) | Physical or sexual | BPD and matched controls | 15 BPD (6 PA/SA) | 15 | 33.5±7.9 | Both | Yes | Decr 12.7% R, BPD v. control. Decr. R & L, BPD with v. without PA SA |
| Everaerd (160) | List of Threatening Life Events | No psycho- pathology. 5HTTLPR genotyping | 357 | Used ratings not groups | 23.7±5.6 | Both | No | Gene × abuse × gender. Males w S’-allele and severe adversity had decr R L p<0.002 |
| Teicher (42) | CTQ and ACE scores | No diagnosis required 46% exposed history MDD | 104 | 89 | 21.9±2.1 18–25 |
Both | No | Decr 6% L subfields dentate gyrus and CA3. Not related to MDD or PTSD |
| Dannlowski (43) | CTQ scores | No psychopathology | 148 | Used ratings not groups | 33.8±10.4 [20–57 |
Both | No | Decr. R p < 0.05 |
| Carballedo (161) | CTQ scores | No psychopathology, with v. without family history MDD | 20 positive 20 negative family history | Used median split ratings | 36.5±13.1 18–65 |
Both | No | Decr. L R hippocampus heads in subjects with emotional abuse and positive family history |
| Children and adolescents | ||||||||
| De Bellis (91) | Sexual, physical or WDV | PTSD v. typical controls | 44 | 61 | 12.1±2.3 6–17 |
Both | No | NS 2.2% increase |
| Carrion (148) | Sexual, physical or WDV | PTSD symptoms v. controls | 24 | 24 | 11.0±2.2 7–14 |
Both | Yes | NS 7.6% decrease |
| De Bellis (162) | Sexual, physical or WDV | PTSD v. typical controls | 9 | 9 | 10.6±1.6 | Both | Yes | NS baseline or followed longitudinally for > 2 years |
| Chugani (163) | Early deprivation mean 38 months | Romanian orphans v. epilepsy control | 10 | 7 | 10.3±3.9 7–13 |
Both | No | Decr. PET glucose metabolism L temporal region including hippocampus |
| De Bellis (92) | Sexual, physical or WDV | PTSD v. SES matched controls | 28 | 66 | 11.5±2.9 4–17 |
Both | No | NS 1.8% decrease |
| Tupler (107) | Sexual, physical or WDV | PTSD v. SES or typical controls | 61 | 122 | 11.7± 2.6 4–17 |
Both | No | NS GMV. Increased WMV. Reanalysis |
| Carrion (106) | Sexual, physical or WDV | PTSD symptoms | 15 | 0 | 10.4 8–14 |
Both | Yes | Inverse correlation r = − 0.48 volume and cortisol 12–18 months |
| Mehta (120) | Early deprivation 24 months | Romanian orphans v. controls | 14 | 11 | 16.1±0.8 | Both | No | Decr 16% L, R absolute, NS after adjusted brain volume |
| Rao (44) | Early life adversity (ELA) | MDD, high risk and controls | 30 MDD 22 high risk, 35 controls | Ratings of exposure within each group | 14.9±1.8 12–20 |
Both | No | Decr. R & L w ELA in high risk and controls. Hipp volume partially mediated risk for MDD with ELA |
| Carrion (164) | Sexual, physical or WDV | PTSD symptoms v. controls | 16 | 11 | 13.9±2.0 10–17 |
Both | Yes | Abnormal (decr.) R BOLD response verbal memory task |
| Maheu (165) | Caregiver deprivation – emotional neglect | Orphans or foster care v. controls | 11 | 19 | 13.5±2.6 9–18 |
Both | No | Abnormal (incr.) L BOLD response fearful and angry v. neutral faces |
| Tottenham (121) | Early deprivation 63 months | Orphans v. healthy controls | 34 | 28 | 8.9±2.1 5–15 |
Both | ? | NS 2.5% decrease L in late adoptees (after 15 months) |
| Edmiston (166) | CTQ scores | No psychopathology | 42 | Used ratings not groups | 15.33±1.37 12–17 |
Both | No | Decr with total scores R, L females. Decr with emotional neglect R, L males and females |
| Lupien (122) | Mothers w chronic MDD | Exposed v. controls | 17 | 21 | 10 | Both | No | NS |
Statistically significant differences (percent reduction) observed in right or left hippocampal volume, gray matter volume, white matter volume, or function. In most studies measures of hippocampal volume were adjusted for differences in total brain volume.
Note subject with borderline personality disorder but without physical or sexual abuse were not considered to be unexposed to maltreatment given the likelihood that they experienced emotional abuse or neglect (11). References not cited in text are included in the Supplementary materials.
5HTTLPR-Serotonin Transporter Promoter Polymorphism, BPD-borderline personality disorder, CECA- Childhood Experience of Care and Abuse, CTQ-childhood trauma questionnaire, DD-Dissociative Disorders, Decr.-decreased, DID-Dissociative Idenrity Disorder, Incr.-increased, ELSQ-Early Life Stress Questionnaire, EN-Emotional neglect, GMV-Gray Matter Volume, L-left, MDD-major depressive disorder, NS-non-significant, PA – physical abuse, PET-Positron Emission Tomography, PTSD-posttraumatic stress disorder, R-right, SA – sexual abuse, SES-socioeconomic status, Sx-symptoms, WDV-witnessing domestic violence, WMV-White Matter Volume, wo-without.
There are also associations between exposure to early maltreatment and the attenuated structural or functional development of the neocortex (93, 108–113) (including anterior cingulate (109, 114–116), orbitofrontal (89, 116, 117) and dorsolateral prefrontal cortex (88, 115)), as well as visual and auditory cortex (Table 4).
Table 4.
Childhood maltreatment and structure and function of the cerebral cortex.
| Number of Subjects | Age (years, Mean±SD, Range) |
|||||||
|---|---|---|---|---|---|---|---|---|
| First Author (Reference) |
Types of Maltreatment |
Diagnostic Requirement |
Exposed | Comparison | Sex | Meds | Main Corpus Callosum Findings* |
|
| De Bellis (91) | Sexual, physical or WDV | PTSD v. typical controls | 44 | 61 | 12.1±2.3 6–17 |
Both | No | Increased prefrontal cerebrospinal fluid (volume loss) |
| De Bellis (109) | Sexual, physical or WDV | PTSD v. typical controls | 11 | 11 | 10.2±29 4–14 |
Both | No | Decreased N-acetyl aspartate/ creatine ratio anterior cingulate |
| Carrion (108) | Sexual, physical or WDV | PTSD symptoms v. controls | 24 | 24 | 11.0±2.2 7–14 |
Both | Yes | Decreased frontal asymmetry |
| Chugani (163) | Early deprivation mean 38 months | Romanian orphans v. epilepsy control | 10 | 7 | 10.3±3.9 7–13 |
Both | No | Decr. PET glucose metabolism R, L orbital frontal gyrus, infralimbic prefrontal cortex |
| De Bellis (92) | Sexual, physical or WDV | PTSD v. SES matched controls | 28 | 66 | 11.5±2.9 4–17 |
Both | No | Increased prefrontal cerebrospinal fluid (volume loss) |
| De Bellis (167) | Sexual, physical or WDV | PTSD v. typical controls | 43 | 61 | 12.1±2.3 6–17 |
Both | No | Incr. R L Superior temporal gyrus GMV reanalysis |
| De Bellis (144) | Sexual, physical or WDV | PTSD v. SES or typical controls | 61 | 122 | 11.7±2.6 4–17 |
Both | No | Increased prefrontal cerebrospinal fluid (volume loss) reanalysis |
| Brambilla (102) | Physical or sexual | BPD v. healthy controls | 10 | 20 | 33.0±8.9 | Both | No | NS in temporal lobes and dorsolateral prefrontal cortex |
| Richert (168) | Sexual, physical or WDV | PTSD symptoms v. controls | 23 | 24 | 11.0±2.2 7–14 |
Both | Yes | Incr. middle inferior ventral prefrontal GMV reanalysis (108) |
| Cohen (114) | ELSQ high v. low 0–12 y | No psychopathology | 122 | 84 | 39.9±17.2 18–70 |
Both | No | Decreased anterior cingulate total volume |
| Kitayama (169) | Physical sexual | PTSD v. healthy controls | 8 | 13 | 39.3±8.2 | Both | ? | Decreased R anterior cingulate volume |
| Andersen (93) | Sexual v. healthy controls | No diagnosis required 27% history PTSD | 26 | 17 | 19.8±1.4 18–22 |
Female | No | Decreased total frontal GMV sensitive period 14–16 years |
| Tomada (111) | Sexual v. healthy controls | No diagnosis required most wo Axis I, II disorder | 23 | 14 | 19.7±1.4 18–22 |
Female | No | Decreased occipital GMV BA17–18 sensitive period before 12 years partial reanalysis (93) |
| Tomoda (115) | Harsh corporal punishment v. healthy controls | No diagnosis required most wo Axis I, II disorder | 23 | 22 | 21.7±2.0 18–25 |
Both | No | Decreased GMV dorsolateral, anterior cingulate and medial prefrontal |
| Carrion (148) | Sexual, physical or WDV | PTSD symptoms v. controls | 24 | 24 | 11.0±2.2 7–14 |
Both | Yes | Increased R, L inferior and superior prefrontal GMV reanalysis (108) |
| Carrion (170) | Sexual, physical or WDV | PTSD symptoms v. controls | 30 | 15 | 13.2±2.1 10–16 |
Both | No | Decr. L ventral & inferior prefrontal GMV. Inverse correlation prebedtime cortisol L ventral GMV |
| van Harmelen (171) | Emotional abuse or neglect | MDD or anxiety disorders v. controls | 84 | 97 | 37.5±10.4 18–65 |
Both | Yes | Decreased L dorsomedial prefrontal GMV independent of psychopathology |
| Sheu (88) | Harsh corporal punishment v. controls | No diagnosis required 63% no lifetime history | 19 | 23 | 21.9±2.1 18–25 |
Both | No | Incr. T2 relaxation time (decr regional cerebral blood volume) R, L dorsolateral prefrontal |
| Hanson (89) | Physical v. health controls | No diagnosis required | 31 | 41 | 11.8±1.1 | Both | Yes | Decr R right orbital frontal, dorsolateral, temporal, and bilateral parietal lobes |
| Frodl (98) | CTQ scores | MDD and healthy controls | 43 | 42 | 44.1±12.4 18–65 |
Both | Yes | Physical neglect: decreased prefrontal GMV |
| Thomaes (157) | Physical or sexual | Complex PTSD and controls | 33 | 30 | 35.5±11.0 | Female | Yes | Decreased R dorsal anterior cingulate, R orbitofrontal |
| Landré (158) | Sexual | PTSD and unexposed controls | 17 | 17 | 24.8±4.7 18–40 |
Female | No | NS regional measures of cortical thickness |
| Tomoda (112) | Parental verbal abuse v. healthy controls | No diagnosis required, 48% history mood do | 21 | 19 | 21.2±2.2 18–25 |
Both | No | Increased L superior temporal gyrus GMV |
| Edmiston (166) | CTQ scores | No psychopathology | 42 | Used ratings not groups | 15.33±1.37 12–17 |
Both | No | Decreased dorsolateral, orbitofrontal, subgenual prefrontal GMV |
| Gerritsen (172) | List of Threatening Life Events | No psychopathology. BDNF polymorphism | 568 | Used ratings not groups | 23.4±5.4 18–50 |
Both | No | Decr. anterior cingulated & medial orbitofrontal at 1.5T not 3T. GxE BDNF v. events subgenual anterior cingulate |
| Carballedo (161) | CTQ scores | No psychopathology, with v. wo family history MDD | 20 positive 20 negative family history | Used median split ratings | 36.5±13.1 18–65 |
Both | No | Decr. L dorsolateral & medial prefrontal, R anterior cingulate with emotional abuse and positive family history |
| Tomoda (128) | WDV v. healthy controls | No diagnosis required, 59% past psychiatric history | 22 | 30 | 21.7±2.2 18–25 |
Both | No | Decr GMV thickness R lingual gyrus BA18, decr. thickness R,L V2, L occipital pole. Sensitive period 11–13 years |
Statistically significant differences observed in right or left regional corticall volume, gray matter volume, white matter volume, thickness or function. In most studies measures of cortical volume were adjusted for differences in total brain volume.
References not cited in text are included in the Supplementary materials.
5HTTLPR-Serotonin Transporter Promoter Polymorphism, BPD-borderline personality disorder, CECA- Childhood Experience of Care and Abuse, CTQ-childhood trauma questionnaire, DD-Dissociative Disorders, Decr.-decreased, DID-Dissociative Idenrity Disorder, Incr.-increased, ELSQ-Early Life Stress Questionnaire, EN-Emotional neglect, FHN-Family history negative, FHP-family history positive, GMV-Gray Matter Volume, L-left, MDD-major depressive disorder, NS-non- significant, PET-Positron Emission Tomography, PTSD-posttraumatic stress disorder, R-right, SES-socioeconomic status, Sx-symptoms, w-with, WDV-witnessing domestic violence, WMV-White Matter Volume, wo-without.
While maltreatment may be associated with alterations in the striatum/basal ganglia (87, 88, 114) and cerebellum (118, 119), most studies have not reported structural differences in the amygdala (91–93, 97, 102, 114). However, increased amygdala volume has been reported in children with institutional deprivation or rearing by chronically depressed mothers (120–122), while smaller amygdala volumes have been observed in adults with childhood trauma and borderline personality disorder or dissociative identity disorder (100, 101, 103, 104). Nevertheless, there is good evidence of enhanced amygdala reactivity in maltreated individuals (17, 43, 46, 72).
Secondly, there appear to be sensitive periods when these regions are maximally susceptible to the effects of stress. Following on this path of inquiry, we examined the relationship between age at exposure to sexual abuse and observed alterations in brain morphology in a preliminary sample of young adult women. Our findings show the hippocampus to be maximally susceptible to maltreatment in women exposed between the ages of 3 and 5 years. However, when maltreatment occurred at ages 9 to 10 years, the midportion of the corpus callosum was maximally susceptible and at 14–16 years (93), the prefrontal cortex was affected. Thus, there appear to be specific windows of vulnerability in development that determine the negative effects of exposure. These observations are supported by translational research showing that synaptic density in hippocampus but not prefrontal cortex of rats is sensitive to the effects of early (preweaning) stress, while the opposite is true with regard to peripubertal stress (123, 124). Rao et al (125) provided additional support for an early hippocampal sensitive period, reporting that degree of parental nurturance at 4 years of age, but not at 8 years of age, predicted hippocampal volume at age 14.
Thirdly, the effects of maltreatment on brain functioning may not appear immediately following exposure (124). Several studies have reported reductions in the gray matter volume of the hippocampus in adults with maltreatment histories but not in maltreated children (Table 3). This pattern of results is consistent with translational studies showing that effects of early stress on the hippocampus first emerge during the transition between puberty and adulthood (124). The delay between exposure and neurobiological change may be particularly relevant, as a comparable time lag often occurs between exposure and emergence of depression or posttraumatic stress (126).
Fourth, maltreatment also appears to affect the development of sensory systems and pathways that process and convey the adverse experience. For example, parental verbal abuse is associated with decreased fractional anisotropy (FA) in the arcuate fasciculus, which interconnects Wernike’s and Broca’s area (127), and with alterations in gray matter volume in auditory cortex (112). Conversely, witnessing domestic violence is associated with a reduction in gray matter volume in primary and secondary visual cortex (128) and with decreased FA in the inferior longitudinal fasciculus, which interconnects visual cortex to limbic system to shape our emotional and memory response to things that we see (129).
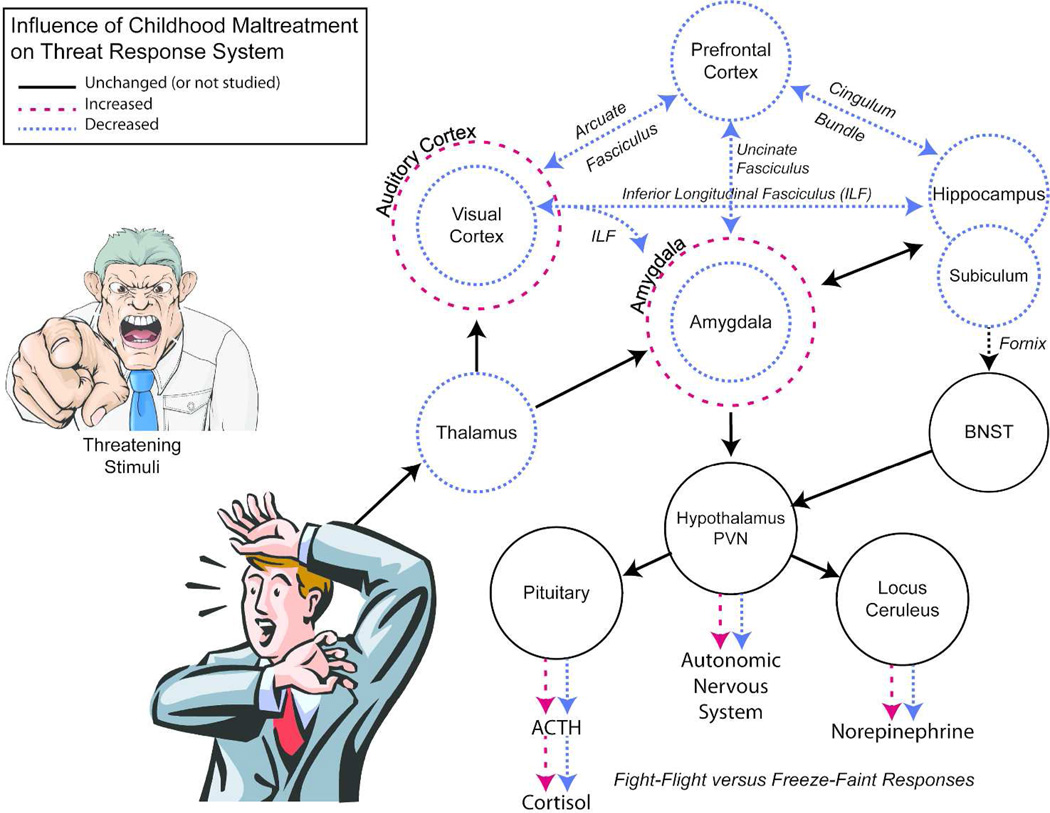
Figure 2 places these findings in context by showing that many of the identified neuroanatomical abnormalities are interconnected and comprise components of a circuit regulating response to potentially threatening stimuli. Briefly, thalamus and sensory cortex process threatening sights and sounds and convey this information to the amygdala (130). Prefrontal regions, particularly ventromedial and orbitofrontal cortex modulate amygdala response, perhaps turning it down with the realization that something is not actually a threat, or irrationally amplifying it in other cases (130). The hippocampus also processes this information and plays a key role in retrieving relevant explicit memories (130). The amygdala integrates this information and signals the paraventricular nucleus of the hypothalamus, which in turn regulates autonomic (e.g., heart rate) and pituitary/adrenal hormonal responses and signals the locus ceruleus, which regulates the intracerebral noradrenergic response. The hippocampus, through the subiculum and bed nucleus of the stria terminalis also modulates paraventricular response, particularly to psychological stressors (131).
Figure 2.
Neurocircuit regulating stress response to threatening or salient stimuli. Childhood maltreatment alters development of regions and pathways within this circuit, which serves to reprogram response to subsequent stressors; resulting in either exaggerated or blunted responses. Based primarily on LeDoux (130). BST-bed nucleus of stria terminals, PVN – paraventricular nucleus of hypothalamus.
Hence, childhood maltreatment, by affecting the development of key components of this system, reprograms response to subsequent stressors. The influence of maltreatment on autonomic and hypothalamic-pituitary-adrenal response to psychological stressors has been evaluated in a series of studies using the Trier Social Stress Test. Heim et al (132) first reported that women with a history of physical and/or sexual abuse had heightened cortisol, ACTH and heart rate response to stress challenge. Subsequent studies have generally painted a different picture with evidence emerging for a blunting of cortisol response in adults with maltreatment histories (133–136). Nevertheless, some individuals show an augmented response, consistent with an enhanced fight-flight reaction and others show a blunted response, consistent with freezing. This divergent pattern of response may be influenced by type (137) and timing (138) of maltreatment.
Psychosocial Correlates of Exposure
Simultaneous to disruptions in brain development that occur with exposure to mistreatment are alterations in the development of psychological structures. Alterations have been observed in the form of poor self-concept, worthlessness and negative views of the world. Further, victims of maltreatment show deficits in what is called deontic reasoning (reasoning about duties and obligations we owe one another) which puts victims at increased risk for future victimization (139). Victims of maltreatment are also more likely to show insecure attachment, associated with diminished expectations of support as well as poor emotion regulation capacities (140).
Treatment Implications
The first question is whether interventions exist that can reduce a child’s risk of abuse and neglect? The Nurse-Family Partnership has been shown in randomized control trials to reduce the incidence of abuse (particularly physical abuse) and neglect of firstborn children of high-risk mothers (141). There is also emerging evidence for the efficacy of other interventions against the emergence or reoccurrence of physical abuse. However, no interventions have been shown to be effective in reducing risk for sexual abuse, emotional abuse, witnessing domestic violence or recurrence of neglect (141).
The second question is whether preemptive interventions exist that can reduce long-term risk for psychiatric illness in maltreated children prior to the emergence of psychopathology? This is an important but largely unexplored area. Third, are there good acute treatments with long-term benefits for maltreated children with psychopathology? Trauma-focused cognitive-behavioral therapy for sexually abused children with symptoms of posttraumatic stress has the most evidence of efficacy (141), but long-term outcome studies are sparse. Assessing and treating parents may also be critical, as maltreatment is often associated with parental psychopathology and parenting problems (26). Recent efforts to develop neurobiologically-informed treatments provide preliminary evidence that lower post-treatment cortisol levels may be associated with reduced effects on hippocampal development (106).
Finally, what can be recommended for adults with ecophenotypic variants of major depression, anxiety disorders, substance abuse or posttraumatic stress? Results of a recent meta-analysis show that depressed subjects with maltreatment history respond more poorly to treatment (13), suggesting that standard first-line recommendations for depression may be inadequate for these individuals. The finding that the Cognitive Behavioral Analysis System of Psychotherapy was more effective than nefazodone (40) in maltreated individuals with chronic depression is intriguing, but research is needed to ascertain whether these findings apply to other medications, other systems of therapy, and to maltreated individuals with less chronic conditions. Integrative trauma-focused treatments have been developed for maltreated individuals with substance abuse that are more helpful than standard treatments, though results are far from ideal (83). Childhood maltreatment is often associated with development of insecure attachment patterns (24), and Mentalization Based Therapy appears to have beneficial effects in patients with insecure attachment patterns across a range of disorders including major depression, substance abuse and borderline personality disorder (142). Efforts to reduce allostatic load and inflammation (19) may also be of benefit to maltreated individuals.
Recent recommendations for adults with maltreatment-related posttraumatic stress and are to adopt a sequential approach that begins with safety, education, stabilization, skill-building, and development of the therapeutic alliance before endeavoring to revisit or rework the trauma, as this may be destabilizing (143). Overall, we suspect that unknowingly mixing maltreated and non-maltreated subtypes in treatment trials may have left us with an incomplete understanding of risks and benefits. Stratifying subjects by maltreatment histories may provide more definitive insights and delineate a clearer course of action for each subtype.
Conclusions
Childhood maltreatment is a complex etiological agent that appears to vary in impact based on the timing, type, and severity of exposure coupled with a number of susceptibility and resilience co-factors. We propose using the term ecophenotype to delineate these psychiatric conditions. We specifically recommend, as a first step, adding the specifier ‘With Maltreatment History’ or ‘With Early Life Stress’ to these Axis-I disorders so that these populations can be studied separately or stratified within a sample. This will lead to a richer understanding of differences in clinical presentation, genetic underpinnings, biological correlates, treatment response and outcomes. Doing so may also help resolve inconsistencies in the literature resulting from unassessed differences in the percent of maltreated subjects within a given study.
Supplementary Material
Acknowledgments
This work was supported in part by research grants RO1 MH-66222 and RO1 MH-091391 from the National Institute of Mental Health, RO1 grant DA-017846 from the National Institute on Drug Abuse and by support from the Brain and Behavior Research Foundation to MHT as a John W. Alden Trust Investigator.
Footnotes
Disclosures
The authors have no conflicts to declare regarding this work. Dr. Teicher has developed and patented technology for the assessment of attention deficit hyperactivity disorder, which has been licensed by McLean Hospital to BioBehavioral Diagnostic Company (BioBDx). Dr. Teicher has received royalty payments, research support, travel and consulting fees from BioBDx. Dr. Teicher has also received, within the last 5 years, research support from CNS Response, Inc. and The Litebook Company, LTD. Dr. Samson has no conflicts to declare.
References
- 1.Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, et al. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv. 2002;53(8):1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- 2.Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Archives of general psychiatry. 2010;67(7):712–719. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- 3.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of general psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Widom CS, DuMont K, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Archives of general psychiatry. 2007;64(1):49–56. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Anda RF, Brown DW, Felitti VJ, Bremner JD, Dube SR, Giles WH. Adverse childhood experiences and prescribed psychotropic medications in adults. Am J Prev Med. 2007;32(5):389–394. doi: 10.1016/j.amepre.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cougle JR, Timpano KR, Sachs-Ericsson N, Keough ME, Riccardi CJ. Examining the unique relationships between anxiety disorders and childhood physical and sexual abuse in the National Comorbidity Survey-Replication. Psychiatry research. 2010;177(1–2):150–155. doi: 10.1016/j.psychres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Archives of general psychiatry. 2000;57(10):953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 9.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- 10.Herman JL, Perry JC, van der Kolk BA. Childhood trauma in borderline personality disorder. The American journal of psychiatry. 1989;146(4):490–495. doi: 10.1176/ajp.146.4.490. [DOI] [PubMed] [Google Scholar]
- 11.Zanarini MC, Williams AA, Lewis RE, Reich RB, Vera SC, Marino MF, et al. Reported pathological childhood experiences associated with the development of borderline personality disorder. The American journal of psychiatry. 1997;154(8):1101–1106. doi: 10.1176/ajp.154.8.1101. [DOI] [PubMed] [Google Scholar]
- 12.Cutajar MC, Mullen PE, Ogloff JR, Thomas SD, Wells DL, Spataro J. Schizophrenia and other psychotic disorders in a cohort of sexually abused children. Archives of general psychiatry. 2010;67(11):1114–1119. doi: 10.1001/archgenpsychiatry.2010.147. [DOI] [PubMed] [Google Scholar]
- 13.Nanni V, Uher R, Danese A. Childhood Maltreatment Predicts Unfavorable Course of Illness and Treatment Outcome in Depression: A Meta-Analysis. The American journal of psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11020335. appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez MJ, Roura P, Oses A, Foguet Q, Sola J, Arrufat FX. Prevalence and clinical impact of childhood trauma in patients with severe mental disorders. The Journal of nervous and mental disease. 2011;199(3):156–161. doi: 10.1097/NMD.0b013e31820c751c. [DOI] [PubMed] [Google Scholar]
- 15.Leverich GS, McElroy SL, Suppes T, Keck PE, Jr, Denicoff KD, Nolen WA, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological psychiatry. 2002;51(4):288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- 16.Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. The American journal of psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of psychiatric research. 2011;45(7):886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 19.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 20.De Bellis MD. Developmental traumatology: the psychobiological development of maltreated children and its implications for research, treatment, and policy. Dev Psychopathol. 2001;13(3):539–564. doi: 10.1017/s0954579401003078. [DOI] [PubMed] [Google Scholar]
- 21.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Miniati M, Rucci P, Benvenuti A, Frank E, Buttenfield J, Giorgi G, et al. Clinical characteristics and treatment outcome of depression in patients with and without a history of emotional and physical abuse. Journal of psychiatric research. 2010;44(5):302–309. doi: 10.1016/j.jpsychires.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan KR. Towards a scientific taxonomy of depression. Dialogues Clin Neurosci. 2008;10(3):301–308. doi: 10.31887/DCNS.2008.10.3/krrkrishnan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ford JD, Cloitre M. Best practices in psychotherapy for children and adolescents. In: Courtois CA, Ford JD, editors. Treating complex traumatic stress disorders. New York: Guilford Press; 2009. pp. 59–81. Treating complex traumatic stress disorders. [Google Scholar]
- 25.Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children's emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. The American journal of psychiatry. 2011;168(1):65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufman J, Birmaher B, Brent D, Dahl R, Bridge J, Ryan ND. Psychopathology in the relatives of depressed-abused children. Child abuse & neglect. 1998;22(3):171–181. doi: 10.1016/s0145-2134(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13(3):451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- 28.Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child abuse & neglect. 2004;28(7):771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Kluft RP. An update on multiple personality disorder. Hosp Community psychiatry. 1987;38(4):363–373. doi: 10.1176/ps.38.4.363. [DOI] [PubMed] [Google Scholar]
- 30.Ross CA, Miller SD, Bjornson L, Reagor P, Fraser GA, Anderson G. Abuse histories in 102 cases of multiple personality disorder. Can J psychiatry. 1991;36(2):97–101. doi: 10.1177/070674379103600204. [DOI] [PubMed] [Google Scholar]
- 31.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 32.Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. Jama. 2001;286(24):3089–3096. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- 33.Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, et al. A registry-based twin study of depression in men. Archives of general psychiatry. 1998;55(5):468–472. doi: 10.1001/archpsyc.55.5.468. [DOI] [PubMed] [Google Scholar]
- 34.MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, et al. Childhood abuse and lifetime psychopathology in a community sample. The American journal of psychiatry. 2001;158(11):1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 35.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9(11):e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fergusson DM, Swain-Campbell NR, Horwood LJ. Does sexual violence contribute to elevated rates of anxiety and depression in females? Psychological medicine. 2002;32(6):991–996. doi: 10.1017/s0033291702005986. [DOI] [PubMed] [Google Scholar]
- 37.Hovens JG, Giltay EJ, Wiersma JE, Spinhoven P, Penninx BW, Zitman FG. Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatr Scand. 2012;126(3):198–207. doi: 10.1111/j.1600-0447.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- 38.Harkness KL, Wildes JE. Childhood adversity and anxiety versus dysthymia co-morbidity in major depression. Psychological medicine. 2002;32(7):1239–1249. doi: 10.1017/s0033291702006177. [DOI] [PubMed] [Google Scholar]
- 39.Matza LS, Revicki DA, Davidson JR, Stewart JW. Depression with atypical features in the National Comorbidity Survey: classification, description, and consequences. Archives of general psychiatry. 2003;60(8):817–826. doi: 10.1001/archpsyc.60.8.817. [DOI] [PubMed] [Google Scholar]
- 40.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harkness KL, Bagby RM, Kennedy SH. Childhood maltreatment and differential treatment response and recurrence in adult major depressive disorder. J Consult Clin Psychol. 2012;80(3):342–353. doi: 10.1037/a0027665. [DOI] [PubMed] [Google Scholar]
- 42.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(9):E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 44.Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biological psychiatry. 2010;67(4):357–364. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data. The American journal of psychiatry. 2012 doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PubMed] [Google Scholar]
- 46.van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of general psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glessner JT, Wang K, Sleiman PM, Zhang H, Kim CE, Flory JH, et al. Duplication of the SLIT3 locus on 5q35.1 predisposes to major depressive disorder. PloS one. 2010;5(12):e15463. doi: 10.1371/journal.pone.0015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of general psychiatry. 2009;66(11):1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrion VG, Weems CF, Ray R, Reiss AL. Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. Journal of the American Academy of Child and Adolescent psychiatry. 2002;41(2):166–173. doi: 10.1097/00004583-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 52.van der Kolk BA. Developmental trauma disorder: Toward a rational diagnosis for children with complex trauma histories. Psychiatric Annals. 2005;35:401–408. [Google Scholar]
- 53.Schoedl AF, Costa MC, Mari JJ, Mello MF, Tyrka AR, Carpenter LL, et al. The clinical correlates of reported childhood sexual abuse: an association between age at trauma onset and severity of depression and PTSD in adults. J Child Sex Abus. 2010;19(2):156–170. doi: 10.1080/10538711003615038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cloitre M, Stolbach BC, Herman JL, van der Kolk B, Pynoos R, Wang J, et al. A developmental approach to complex PTSD: childhood and adult cumulative trauma as predictors of symptom complexity. J Trauma Stress. 2009;22(5):399–408. doi: 10.1002/jts.20444. [DOI] [PubMed] [Google Scholar]
- 55.Sher L. The concept of post-traumatic mood disorder and its implications for adolescent suicidal behavior. Minerva Pediatr. 2008;60(6):1393–1399. [PubMed] [Google Scholar]
- 56.Najavits LM, Walsh M. Dissociation, PTSD, and substance abuse: an empirical study. J Trauma Dissociation. 2012;13(1):115–126. doi: 10.1080/15299732.2011.608781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamen C, Bergstrom J, Koopman C, Lee S, Gore-Felton C. Relationships among childhood trauma, posttraumatic stress disorder, and dissociation in men living with HIV/AIDS. J Trauma Dissociation. 2012;13(1):102–114. doi: 10.1080/15299732.2011.608629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frewen PA, Pain C, Dozois DJ, Lanius RA. Alexithymia in PTSD: psychometric and FMRI studies. Ann N Y Acad Sci. 2006;1071:397–400. doi: 10.1196/annals.1364.029. [DOI] [PubMed] [Google Scholar]
- 59.van der Kolk BA, Roth S, Pelcovitz D, Sunday S, Spinazzola J. Disorders of extreme stress: The empirical foundation of a complex adaptation to trauma. J Trauma Stress. 2005;18(5):389–399. doi: 10.1002/jts.20047. [DOI] [PubMed] [Google Scholar]
- 60.Roth S, Newman E, Pelcovitz D, van der Kolk B, Mandel FS. Complex PTSD in victims exposed to sexual and physical abuse: results from the DSM-IV Field Trial for Posttraumatic Stress Disorder. J Trauma Stress. 1997;10(4):539–555. doi: 10.1023/a:1024837617768. [DOI] [PubMed] [Google Scholar]
- 61.Nutt DJ, Malizia AL. Structural and functional brain changes in posttraumatic stress disorder. J Clin psychiatry. 2004;65(Suppl 1):11–17. [PubMed] [Google Scholar]
- 62.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5(11):1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. The American journal of psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 64.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancini C, Van Ameringen M, MacMillan H. Relationship of childhood sexual and physical abuse to anxiety disorders. The Journal of nervous and mental disease. 1995;183(5):309–314. doi: 10.1097/00005053-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Simon NM, Herlands NN, Marks EH, Mancini C, Letamendi A, Li Z, et al. Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depress Anxiety. 2009;26(11):1027–1032. doi: 10.1002/da.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bruce LC, Heimberg RG, Blanco C, Schneier FR, Liebowitz MR. Childhood maltreatment and social anxiety disorder: implications for symptom severity and response to pharmacotherapy. Depress Anxiety. 2012;29(2):131–138. doi: 10.1002/da.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berkowitz RL, Coplan JD, Reddy DP, Gorman JM. The human dimension: how the prefrontal cortex modulates the subcortical fear response. Rev Neurosci. 2007;18(3–4):191–207. doi: 10.1515/revneuro.2007.18.3-4.191. [DOI] [PubMed] [Google Scholar]
- 70.Pietrini F, Godini L, Lazzeretti L, Benni L, Pracucci C, Talamba GA, et al. [Neuroimaging and neurobiology of social anxiety] Riv Psichiatr. 2010;45(6):349–360. [PubMed] [Google Scholar]
- 71.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 73.Huang S, Trapido E, Fleming L, Arheart K, Crandall L, French M, et al. The long-term effects of childhood maltreatment experiences on subsequent illicit drug use and drug-related problems in young adulthood. Addict Behav. 2011;36(1–2):95–102. doi: 10.1016/j.addbeh.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lo CC, Cheng TC. The impact of childhood maltreatment on young adults' substance abuse. Am J Drug Alcohol Abuse. 2007;33(1):139–146. doi: 10.1080/00952990601091119. [DOI] [PubMed] [Google Scholar]
- 75.Oshri A, Tubman JG, Burnette ML. Childhood maltreatment histories, alcohol and other drug use symptoms, and sexual risk behavior in a treatment sample of adolescents. Am J Public Health. 2012;102(Suppl 2):S250–S257. doi: 10.2105/AJPH.2011.300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walton G, Co SJ, Milloy MJ, Qi J, Kerr T, Wood E. High prevalence of childhood emotional, physical and sexual trauma among a Canadian cohort of HIV-seropositive illicit drug users. AIDS Care. 2011;23(6):714–721. doi: 10.1080/09540121.2010.525618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medrano MA, Hatch JP, Zule WA, Desmond DP. Psychological distress in childhood trauma survivors who abuse drugs. Am J Drug Alcohol Abuse. 2002;28(1):1–13. doi: 10.1081/ada-120001278. [DOI] [PubMed] [Google Scholar]
- 78.Bernstein DP, Stein JA, Handelsman L. Predicting personality pathology among adult patients with substance use disorders: effects of childhood maltreatment. Addict Behav. 1998;23(6):855–868. doi: 10.1016/s0306-4603(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 79.Kerr T, Stoltz JA, Marshall BD, Lai C, Strathdee SA, Wood E. Childhood trauma and injection drug use among high-risk youth. J Adolesc Health. 2009;45(3):300–302. doi: 10.1016/j.jadohealth.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams JK, Smith DC, An H, Hall JA. Clinical outcomes of traumatized youth in adolescent substance abuse treatment: a longitudinal multisite study. J Psychoactive Drugs. 2008;40(1):77–84. doi: 10.1080/02791072.2008.10399763. [DOI] [PubMed] [Google Scholar]
- 81.Sacks JY, McKendrick K, Banks S. The impact of early trauma and abuse on residential substance abuse treatment outcomes for women. J Subst Abuse Treat. 2008;34(1):90–100. doi: 10.1016/j.jsat.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Shane P, Diamond GS, Mensinger JL, Shera D, Wintersteen MB. Impact of victimization on substance abuse treatment outcomes for adolescents in outpatient and residential substance abuse treatment. Am J Addict. 2006;15(Suppl 1):34–42. doi: 10.1080/10550490601003714. [DOI] [PubMed] [Google Scholar]
- 83.Sullivan JM, Evans K. Integrated treatment for the survivor of childhood trauma who is chemically dependent. J Psychoactive Drugs. 1994;26(4):369–378. doi: 10.1080/02791072.1994.10472457. [DOI] [PubMed] [Google Scholar]
- 84.Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, et al. Risk taking and the adolescent reward system: a potential common link to substance abuse. The American journal of psychiatry. 2012;169(1):39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- 85.Asensio S, Romero MJ, Palau C, Sanchez A, Senabre I, Morales JL, et al. Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol. 2010;15(4):504–516. doi: 10.1111/j.1369-1600.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 86.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological psychiatry. 2009;66(3):206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sheu YS, Polcari A, Anderson CM, Teicher MH. Harsh corporal punishment is associated with increased T2 relaxation time in dopamine-rich regions. NeuroImage. 2010;53(2):412–419. doi: 10.1016/j.neuroimage.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, et al. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30(22):7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- 91.De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, et al. Developmental traumatology. Part II: Brain development. Biological psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 92.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 93.Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biological psychiatry. 2004;56(2):80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 95.Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, et al. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry research. 2008;162(3):256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. The American journal of psychiatry. 2010;167(12):1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, et al. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Frodl T, Reinhold E, Koutsouleris N, Reiser M, Meisenzahl EM. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. Journal of psychiatric research. 2010;44(13):799–807. doi: 10.1016/j.jpsychires.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 99.Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological medicine. 1997;27(4):951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- 100.Vermetten E, Schmahl C, Lindner S, Loewenstein RJ, Bremner JD. Hippocampal and amygdalar volumes in dissociative identity disorder. The American journal of psychiatry. 2006;163(4):630–636. doi: 10.1176/appi.ajp.163.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in trauma-exposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34(5):383–388. [PMC free article] [PubMed] [Google Scholar]
- 102.Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry research. 2004;131(2):125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 103.Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of general psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- 104.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research-Neuroimaging. 2003;122(3):193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- 105.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in hippocampal subfields CA3, dentate gyrus and subiculum. Proceedings of the National Academy of Sciences of the United States of America. doi: 10.1073/pnas.1115396109. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119(3):509–516. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- 107.Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biological psychiatry. 2006;59(6):523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 108.Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological psychiatry. 2001;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- 109.De Bellis MD, Keshavan MS, Spencer S, Hall J. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. The American journal of psychiatry. 2000;157(7):1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 110.Ito Y, Teicher MH, Glod CA, Ackerman E. Preliminary evidence for aberrant cortical development in abused children: a quantitative EEG study. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10:298–307. doi: 10.1176/jnp.10.3.298. [DOI] [PubMed] [Google Scholar]
- 111.Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biological psychiatry. 2009;66(7):642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tomoda A, Sheu YS, Rabi K, Suzuki H, Navalta CP, Polcari A, et al. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. NeuroImage. 2011;54(Suppl 1):S280–S286. doi: 10.1016/j.neuroimage.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological psychiatry. 2002;52(11):1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 114.Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological psychiatry. 2006;59(10):975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 115.Tomoda A, Suzuki H, Rabi K, Sheu YS, Polcari A, Teicher MH. Reduced prefrontal cortical gray matter volume in young adults exposed to harsh corporal punishment. NeuroImage. 2009;47(Suppl 2):T66–T71. doi: 10.1016/j.neuroimage.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological psychiatry. 2003;53(10):879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 117.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. The American journal of psychiatry. 1999;156(4):575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 118.Anderson CM, Teicher MH, Polcari A, Renshaw PF. Abnormal T2 relaxation time in the cerebellar vermis of adults sexually abused in childhood: potential role of the vermis in stress-enhanced risk for drug abuse. Psychoneuroendocrinology. 2002;27(1–2):231–244. doi: 10.1016/s0306-4530(01)00047-6. [DOI] [PubMed] [Google Scholar]
- 119.De Bellis MD, Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological psychiatry. 2006;60(7):697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 120.Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of child psychology and psychiatry, and allied disciplines. 2009;50(8):943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 121.Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lupien SJ, Parent S, Evans AC, Tremblay RE, Zelazo PD, Corbo V, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14324–14329. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 124.Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–1993. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- 125.Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage. 2010;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teicher MH, Samson JA, Polcari A, Andersen SL. Length of time between onset of childhood sexual abuse and emergence of depression in a young adult sample: a retrospective clinical report. J Clin psychiatry. 2009;70(5):684–691. doi: 10.4088/jcp.08m04235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65(3):227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tomoda A, Polcari A, Anderson CM, Teicher MH. Reduced Visual Cortex Gray Matter Volume and Thickness in Young Adults Who Witnessed Domestic Violence during Childhood. PloS one. 2012;7(12):e52528. doi: 10.1371/journal.pone.0052528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi J, Jeong B, Polcari A, Rohan ML, Teicher MH. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage. 2012;59(2):1071–1079. doi: 10.1016/j.neuroimage.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LeDoux JE. Harmondsworth, England: Viking Penguin; 2002. Synaptic Self: how our brains become who we are. [Google Scholar]
- 131.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behavioural brain research. 2006;174(2):215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 132.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 133.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl) 2011;214(1):367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, et al. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological psychiatry. 2009;66(1):62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ali N, Pruessner JC. The salivary alpha amylase over cortisol ratio as a marker to assess dysregulations of the stress systems. Physiol Behav. 2012;106(1):65–72. doi: 10.1016/j.physbeh.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 137.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13(3):677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 138.Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, et al. Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents' cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37(9):1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 139.DePrince AP, Chu AT, Combs MD. Trauma-related predictors of deontic reasoning: a pilot study in a community sample of children. Child abuse & neglect. 2008;32(7):732–737. doi: 10.1016/j.chiabu.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 140.Cloitre M, Stovall-McClough C, Zorbas P, Charuvastra A. Attachment organization, emotion regulation, and expectations of support in a clinical sample of women with childhood abuse histories. J Trauma Stress. 2008;21(3):282–289. doi: 10.1002/jts.20339. [DOI] [PubMed] [Google Scholar]
- 141.Macmillan HL, Wathen CN, Barlow J, Fergusson DM, Leventhal JM, Taussig HN. Interventions to prevent child maltreatment and associated impairment. Lancet. 2009;373(9659):250–266. doi: 10.1016/S0140-6736(08)61708-0. [DOI] [PubMed] [Google Scholar]
- 142.Fonangy P, Gergely G, Jurist E, Target M. Affect Regulation, Mentalization, and the Development of the Self. New York: Other Press LLC; 2002. [Google Scholar]
- 143.Courtois CA, Ford JD, Cloitre M. Treating complex traumatic stress disorders: An evidence-based guide. In: Courtois CA, Ford JD, editors. Best practices in psychotherapy for adults. New York: The Guilford Press; 2009. pp. 82–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.