Abstract
Cancer is the major health problem around the world. Research efforts in last few decades have been successful in providing better and effective treatments against both early stage and localized cancer but clinical options against advanced metastatic stage/s of cancer remain limited. The high morbidity and mortality in most of the cancers are attributed to their metastatic spread to distant organs. Due to its extreme clinical relevance, the metastasis has been extensively studied and is now understood as a highly complex biological event that involves multiple steps including acquisition of invasiveness by cancer cells, intravasation into circulatory system, survival in the circulation, arrest in microvasculature, extravasation and growth at distant organs. The increasing understanding of molecular underpinnings of these events has provided excellent opportunity to target metastasis especially through non-toxic and biologically effective nutraceuticals. Silibinin, a popular dietary supplement isolated from milk thistle seed extracts, is one such natural agent that has shown biological efficacy through pleiotropic mechanisms against variety of cancers and is currently in clinical trials. Recent pre-clinical studies have also shown strong efficacy of silibinin to target cancer cell’s migratory and invasive characteristics as well as their ability to metastasize to distant organs. Detailed mechanistic analyses revealed that silibinin targets signaling molecules involved in the regulation of epithelial-to-mesenchymal transition (EMT), proteases activation, adhesion, motility, invasiveness as well as the supportive tumor-microenvironment components, thereby inhibiting metastasis. Overall, the long history of human use, remarkable non-toxicity and pre-clinical efficacy strongly favor clinical use of silibinin against advanced metastatic cancers.
Keywords: Metastasis, Silibinin, EMT, Tumor microenvironment, Proteases
Cancer metastasis
Cancer is a major public health problem in the United States and other parts of the world. According to the American Cancer Society, a total of 1,479,350 new cancer cases and 562,340 deaths from cancer were estimated to occur in the United States in 2009 [1]. Although progress has been made towards reducing the cancer incidence and mortality rates as well as improving survival, cancer still accounts for more deaths than heart disease in persons younger than 85 years of age [1]. The morbidity and mortality due to cancer are primarily determined by the stage of its diagnosis. For example, in prostate cancer patients diagnosed at early stage of the disease with only localized growth of the cancer, 5 year survival rate is high and is near to 100% [2–4]. However, in late stage prostate cancer patients, where disease has spread to distant organs, the median survival of patients is reduced to only 12–15 months [2–4]. The tendency of cancer cells to spread to distant organs in the body is known as ‘metastasis’ and is considered responsible for more than 90% of cancer-associated deaths [5–7]. Most patients with metastatic disease respond only transitely to conventional treatments and ultimately succumb to this disease. Due to its extreme clinical relevance, the biological underpinnings of metastasis have been extensively studied [5,8–10].
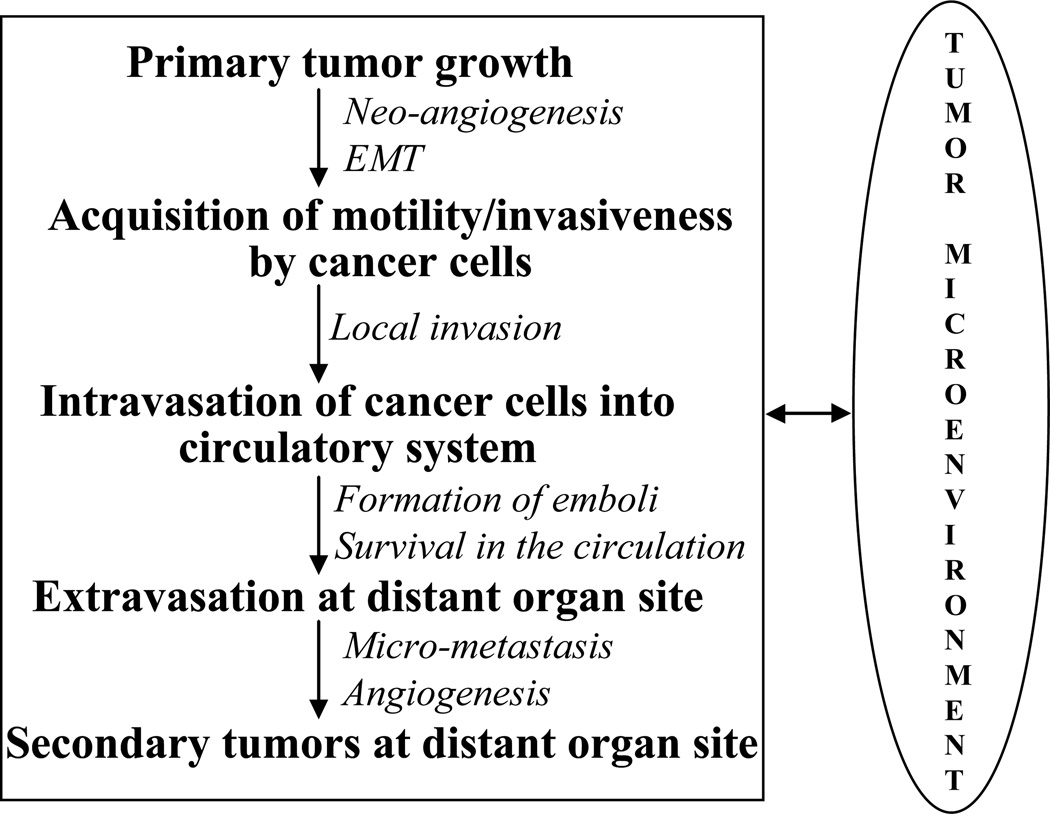
Metastasis is now known as an extremely complex, multi-step and multi-functional biological event that eventually leads to death of the cancer patients [5,6,11]. During metastasis, cancer cells acquire motility, invade locally and enter into the systemic blood circulation (intravasation), survive in the circulation, arrest in microvasculature and subsequently extravasate and grow at distant organs (Fig. 1) [2,9,12,13]. In this complex phenomenon of cancer cells dissemination, acquisition of motility and invasiveness are the first major events during which cancer cells shed many of their epithelial characteristics, undergo drastic cyotskeletal alterations and acquire highly motile and invasive mesenchymal phenotype [14,15]. These series of changes in cancer cells have been termed as epithelial-to-mesenchymal transition (EMT) and is reminiscent of the highly conserved and fundamental process of EMT that occurs during early embryonic development [10]. The role of EMT in cancer progression has been well established now, and presumed an indispensable component of metastasis by cancer cells [15]. Next, the motile and invasive cancer cells in the primary tumors detach, invade and intravasate into blood or lymphatic circulatory system (Fig. 1). In the circulation, cancer cells interact with platelets and leucocytes to create aggregates or emboli, which protect them from the shear stress as well as immune response of the body (Fig. 1) [13,16,17]. The formation of these aggregates by cancer cells also support their settling in the capillaries of distant organs, extravasation and initial growth at distant organ site (Fig. 1) [9,18]. Once established at the distant metastatic site, many cancer cells recapitulate the differentiated phenotype of the primary tumors through a process called mesenchymal-to-epithelial transition (MET) [8,15]. Therefore, the important steps that enable metastasis appear to be reversible indicating the existence of dynamic components in human tumor progression.
Fig. 1.
There are multiple steps involved in the metastasis of cancer cells, and cancer cells’ interaction with their immediate microenvironment is critical at each of these steps.
One of the peculiar features of cancer cells is their propensity to metastasize to specific organ/s [19]. For example, prostate cancer cells mainly metastasize to bones; advanced colon cancer cells frequently spread to liver; lung cancer cells settle in adrenal glands, liver, brain and bone; melanomas migrate to liver, brain and skin; and breast cancer cells preferentially metastasize to lung and bones. Over a century ago, Stephen Paget first reported a non-random pattern of metastasis of cancer cells to certain organs while analyzing autopsy records of 735 women who endured breast cancer [13,19]. He proposed “seed and soil hypothesis”, in which he compared the metastasis of cancer cells to the dispersal of the seeds by plants. He postulated that seeds (‘cancer cells’) can grow only in a congenial soil (‘specific distant organ microenvironment’). According to this theory, osteotropic cancer cells like breast, prostate or lung, possess certain intrinsic properties that enable them to grow in the bone; and the bone microenvironment provides a fertile soil for their growth. This theory, which placed main emphasis on the compatibility between metastatic cancer cells and their microenvironment, was contested by James Ewing, who alternatively proposed that metastatic propensity of cancer cells is mostly dictated by circulatory patterns, and that metastasizing cancer cells would settle in organ/s to which they have the maximum vascular access [19]. Subsequent analyses of patterns of metastatic spread showed that although regional recurrences are highly dependent on the efficiency of vascular perfusion, distant metastatic recurrence for most cancers is non-random phenomenon, with no correlation with anatomically defined patterns of lymphatic or hematogenous circulation [20,21]. For example, Batson and others showed that vertebral venous system enables cancer cells from the pelvic region and breast to by-pass the pulmonary circulation, and could be responsible for the high propensity of breast and prostate cancer cells to produce metastasis in the axial skeleton [22,23]. However, this explanation alone does not explain the high incidence of metastasis of these cancer cells to other bones and organs. Recent molecular studies have identified the unique genetic signatures in cancer cells that mediate their organ-specific pattern of metastatic colonization [11,24–26]. Thus, Paget’s “seed and soil hypothesis” has still prevailed, and currently the molecular determinants of both seed and soil (tumor and its microenvironment) are rigorously investigated.
Now it is well appreciated that the interaction between cancer cells and their local microenvironment is crucial towards each step of cancer progression including their metastasis [9,27]. The success of metastasis is considered to be dependent on the cumulative ability of cancer cells to appropriately respond to the distinct microenvironment at each step in the metastatic cascade starting from primary tumor growth to final metastatic site (Fig. 1). The tumor microenvironment comprises diverse cell populations including endothelial cells of the blood and lymphatic circulation, stromal fibroblasts and a variety of bone marrow-derived cells (BMDCs) including macrophages, myeloid-derived suppressor cells, monocytes and mesenchymal stem cells. These vast variety of cells play vital role in tumor growth, angiogenesis, invasion, intravasation, immune-suppression and distant metastatic growth [9]. Beside these cells, at distant site, cancer cells also interact with the local cellular components, invariably to its own advantage. For example, after extravasation into bone, breast cancer cells interact with osteoclasts and promote their osteoclastic activity towards greater bone resorption [13]. The local bone resorption debulks the bone, thereby providing space for cancer cells, and also releases survival factors and growth factors that promote breast cancer cells proliferation in their new home [13]. Recent studies suggest that interaction between cancer cells and their microenvironment need not be a local event, and the communication between cancer cells and their distant site microenvironment could occur even before actual metastasis [28,29]. Kaplan et al. showed that cancer cells at the primary site secrete factors which mobilize the bone-marrow derived hematopoietic progenitor cells (HPCs) to create favorable niche (termed as ‘premetastatic niche’) at the target distant organs before the arrival of metastatic cancer cells [28]. However, few other studies contradict this role of primary tumors in metastasis and point that the presence of primary tumor inhibits metastasis, while the removal of primary tumor promotes metastatic growth [30,31]. These disparate studies further suggest the complexity of cancer metastasis as well as the need for more studies to clearly understand the dynamic interaction between cancer cells and their microenvironment.
It’s now largely accepted that a sub-population of cancer cells called ‘cancer stem cells (CSCs)’ are primarily responsible for the initiation and growth of most of the cancers [32,33]. Recent studies suggest that stem cell population not only sustains the growth as well as heterogeneity of the primary tumors by deregulating the balance between ‘self-renewal’ and ‘differentiation’ but also their dissemination into surrounding tissues as well as distant organs [7,33,34]. It is believed that only cancer stem-like cells possess the necessary genetic plasticity to adapt to ever-changing microenvironment during the different steps of metastasis. This argument is supported by the fact that only a minute population of circulating cancer cells (probably less than 0.01%) has the ability to establish successful metastasis [9,35,36]. It has also been proposed that the EMT in cancer cells not only provides motility and invasiveness but also increases the stem cell-like characteristics of metastasizing cells [7,8,37]. The exact role of cancer stem cells or stem-like cells in metastasis is still being scrutinized but this concept further enhances the degree of complexity of this biological phenomenon.
As summarized above, metastasis is an extremely complex phenomenon and is critical in our efforts to lower the cancer-associated morbidity and mortality; however, treating patients with advance metastatic stage has remained a big challenge. Currently, chemotherapy, radiotherapy, hormone therapy, biological therapy, surgery or a combination of these therapies is employed to treat advance stage cancer patients. But in most cases, these therapies either alone or in combinations provide only a marginal or no survival benefit [38–40]. The clinical effectiveness of most of these therapies is constrained by the fact that during metastasis cancer cells spread to different organs, which incidentally also cause greater systemic toxicity. Further, current diagnostics have limited capability to identify the extent and spread of micro-metastasis in the body, thereby, making it practically impossible to fully wipe-out metastatic cells from the body. Furthermore, due to significant toxicity, the application of current therapeutic regimes is limited by the patient’s age or overall health, and in such situations patients have limited treatment options, if none. Not only current therapeutics have inadequate effectiveness, the cost of these treatments is exorbitant putting a great burden on the state exchequer. The finance side of treating the advance metastatic stage of the disease is relatively critical in developing economies with scant resources, and most of the treatments remain out of the reach of the patients [41,42]. Overall, the ineffectiveness, significant toxicity issues and exorbitant cost associated with current treatment regimes demand a fresh look at our approaches toward treating the advanced metastatic stage of cancer.
There are increasing evidences that nutraceutical agents are important in the prevention and intervention of cancer [43–48]. There are evidences that dietary/non-dietary agents and life style not only determine the cancer incidences but could also affect growth and progression or aggressiveness of the cancer cells [43,49–52]. The use of nutraceutical agents in prevention and intervention of metastasis is very attractive based upon the facts that these agents are already in human use and have limited or no-toxicity issues; target multiple events associated with metastasis through several mechanisms thereby limiting the chances of developing resistance by cancer cells; and are comparatively economical and could be used practically even in aged patients or in patients with compromised health. Overall, the list of nutraceuticals agents with efficacy against cancer metastasis is growing steadily and few of these agents have already entered the clinical phase [44,53–56]. The focus of present review is on the anti-metastatic efficacy of one such nutraceuticals agent, namely Silibinin (Fig. 2).
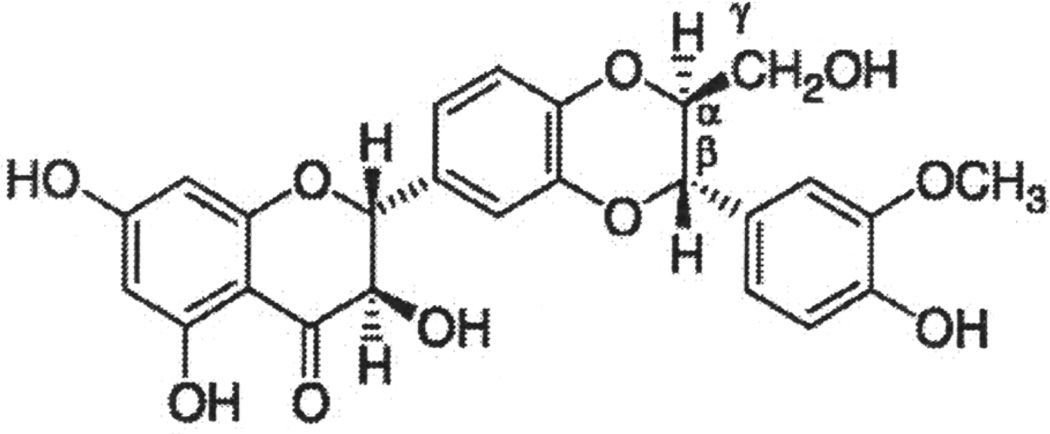
Fig. 2.
Chemical structure of silibinin.
Silibinin: Source, metabolism, bioavailability and anti-cancer efficacy
Silibinin (C25H22O10, molecular weight, 482.44) is isolated from the seeds of Silybum marianum (L.) Gaertn (Family Asteraceae), which is also known as milk thistle. Milk thistle extract has centuries-old history of use in folk medicine to treat variety of illnesses including jaundice, gallstones, hemorrhage, bronchitis, varicose vein, and for several other purposes. In recent times, it is more popular for the prevention and/or treatment of various liver disorders like viral hepatitis, fatty liver associated with long term alcohol use, liver damage from drugs & industrial toxins such as carbon tetrachloride [48,57]. It is also considered a powerful antidote against poisoning by death cap mushroom (Amanita phalloides) [57,58]. German commission E has recommended its use for dyspeptic complaints and liver conditions, including toxin-induced liver damage and hepatic cirrhosis, also as a supportive therapy for chronic inflammatory liver conditions [59]. Milk thistle extract or silibinin is currently not approved for any medical use in the United States, but sold as a dietary supplement; it is one of the most frequently sold herbal products in the United States [59].
The standardized milk thistle extract contains approximately 70–80% of the defined flavonoids and flavonolignans (together known as ‘silymarin’), and approximately 20–30% chemically undefined fractions, compromising mostly of polyphenols and aliphatic fatty acids (---). Silibinin is the main component of silymarin complex and constitutes about 50–60% of it depending upon the formulation [60]. Silymarin is considered to be primarily conjugated and excreted into bile and urine and appears to have minimal phase I metabolism; however, limited data exist suggesting the role for phase II metabolic pathways and transporters [61–63]. Pharmacokinetic analysis of silymarin (where silibinin is the major active constituent) given to healthy volunteers has revealed that flavonoids present therein are rapidly metabolized to their conjugates such as sulfates and glucuronides, and can be detected in the human plasma [61]. It is also observed that conjugated silibinin metabolites had slower elimination as compared to free silibinin [61]. Now it is confirmed that silibinin is composed of 1:1 mixture of two diastereoisomers silybin A and silybin B [60]. Recent metabolic studies illustrated that silybin B is more efficiently glucuronidated compared to silybin A suggesting a stereoselectivity in their metabolism [61].
Silibinin is known to have poor bioavailability because of two main reasons, namely it has multi-ring structure (Fig. 2) that is too large to be absorbed by simple diffusion and that silibinin has poor miscibility with oils and other lipids, severely limiting its ability to cross the lipid-rich outer membrane of the enterocytes of the small intestine [64]. Therefore, there have been several efforts to prepare silibinin formulations to increase its bioavailability [65–67]. In this regard, silibinin has been complexed with phosphatidylcholine, this formulation is known as ‘Silipide’ (trade name ‘Siliphos’). Pharmacokinetic studies in healthy subjects have clearly shown the higher absorption of silibinin in the plasma and liver from siliphos compare to conventional silibinin [65,68]. Siliphos tested in prostate cancer patients and colon cancer patients also revealed high plasma bioavailability [55,56,69]. Comparative analyses of two clinical studies revealed high bioavailability of silibinin in the colon tissue but a poor levels into prostate tissue [55,69], suggesting the organs specific differences in the silibinin bioavailability following oral administration. Recently, the bioavailability of silibinin was reported to be significantly enhanced when administered in beagle dogs as silibinin-nanosuspensions [67]. This study also suggests that uptake and bioavailability of silibinin could be further enhanced through altering the nanoparticle size [67]. Overall, recent efforts toward increasing the bioavailability of silibinin are promising and encouraging.
The anti-cancer efficacy of silibinin is clearly evident from the published reports against various cancers in last two decades, which were mainly through targeting proliferation, apoptosis, inflammation, angiogenesis and cancer cell metabolism (summarized in Table 1). Silibinin is known to activate cellular-check points and cyclin-dependent kinase inhibitors (CDKIs), decrease the levels of both cyclins and CDKs, and to induce strong cell cycle arrest in cancer cells [70–72]. Silibinin also targets CDK-CDKI interaction, CDK kinase activity, Rb phosphorylation, and E2Fs in cancer cells [70,71,73]. Silibinin treatment stimulates apoptotic machinery (both extrinsic and intrinsic) and induces apoptotic death in various cancer cells [74–78]. Silibinin has also been reported to possess remarkable anti-angiogenic efficacy through targeting VEGF, VEGF receptors and iNOS [79–81]. Additionally, silibinin is reported to target an array of cellular signaling pathways and molecules including EGFR, MAPKs, AP1, HIF-1α, STATs, PI3K/Akt, β-catenin, IGF-IGFBP3, NF-κB, COX2 etc; and these molecular alterations contribute toward most of silibinin’ biological effects [82–89]. Recent studies have clearly shown that silibinin also possesses strong anti-invasive and anti-metastatic efficacy against various cancers, and these studies along with mechanistic details are discussed next.
Table 1.
Summary of the anti-cancer efficacy of silibinin against various cancer types.
| Cancer Type | Model | References |
|---|---|---|
| Prostate | Cell culture studies | [60,70,72,73,88, 89, 91–93,132–146] |
| Animal studies (xenografts and TRAMP mice model) |
[80,82,86,90, 129,147– 150] |
|
| Human studies (phase I and II clinical trial) | [55,56] | |
| Skin | Cell culture studies | [124,151–156] |
| Animal studies (chemicals- and UVB- induced skin carcinogenesis models) |
[125,126,130,157–160] | |
| Lung | Cell culture studies | [71,102,114,131,161] |
| Animal studies (xenografts and urethane- induced lung carcinogenesis model) |
[81,127,162,163] | |
| Colon | Cell culture studies | [74,75,84,164–167] |
| Animal studies (xenografts, AOM-induced colon carcinogenesis model, Apcmin transgenic mice model) |
[74,84,128,150,164,168– 171] |
|
| Human studies | [69] | |
| Breast | Cell culture studies | [77,100,146,172–178] |
| Animal studies (HER-2/neu and C3(1) SV40 T,t antigen transgenic mice model) |
[99,179] | |
| Glioblastoma | Cell culture studies | [78,116,180,181] |
| Animal studies (xenograft model) | [181] | |
| Hepatic | Cell culture studies | [115,182–186] |
| Animal studies (xenograft model) | [187] | |
| Ovarian | Cell culture studies | [178,188,189] |
| Animal (xenograft model) | [189] | |
| Urinary Bladder |
Cell culture studies | [190–193] |
| Animal studies (nitrosamine-induced urinary bladder carcinogenesis) |
[191,194] | |
| Leukemia | Cell culture studies | [195–197] |
| Cervical | Cell culture studies | [146,184] |
| Kidney | Cell culture studies | [76,198] |
| Animal studies (xenograft model) | [199] | |
| Laryngeal | Cell culture studies | [200] |
| Osteosarcoma | Cell culture studies | [103] |
| Oral | Cell culture studies | [101] |
| Gastric | Cell culture studies | [201] |
Details of broad-spectrum anti-metastatic efficacy of silibinin
There are numerous studies suggesting the broad-spectrum efficacy of silibinin in inhibiting cancer metastasis. These studies are elaborated in detail below:
Prostate cancer
We first reported the anti-metastatic potential of silibinin in TRAMP (Transgenic Adenocarcinoma of the Mouse Prostate) mice, which is a most-frequently utilized pre-clinical model to evaluate the in vivo efficacy of cancer chemopreventive and therapeutic agents against prostate cancer. In this model, mice develop spontaneous progressive stages of prostate cancer that is driven by the expression of SV40 early genes (T/t; Tag) specifically in the prostatic epithelium. In these mice, all stages of human prostate cancer are recapitulated from early lesions of prostate intraepithelial neoplasia (PIN) to late stage metastatic adenocarcinoma. We reported that silibinin feeding (as siliphos) for 11 weeks (20–31 weeks of TRAMP male mice age) strongly inhibits the progression of PIN into the advanced stages of prostate cancer [90]. Importantly, in this study, silibinin feeding strongly inhibited the local invasion of prostate cancer cells to seminal vesicle [90]. Further, a phenomenal anti-metastatic effect of silibinin was reported with no secondary tumors in distant organs (liver and kidney) in silibinin-fed mice [90]. The strong anti-metastatic efficacy of silibinin in TRAMP mice model was also evident where silibinin was fed in a stage specific manner [80]. This study showed that TRAMP mice develop distant metastasis between 12–20 week age and number of metastatic lesion increases with the increasing age of the mice [80]. However, in this study, silibinin feeding during 12–20 weeks (age of TRAMP mice) significantly inhibited the lung metastasis; it also strongly decreased the metastasis to liver, lung and kidney when fed between 20–30 and 30–45 weeks of TRAMP mice age [80]. These two studies clearly established the strong anti-metastatic efficacy of silibinin in prostate cancer cells in vivo.
Anti-migratory and anti-invasive efficacy of silibinin has also been reported in numerous cell culture studies [91–94]. Mokhtari et al. reported that silibinin treatment strongly inhibits migratory potential of human prostate carcinoma PC3 cells [91]. In another study, Wu et al. reported the dose-and time-dependent inhibitory effects of silibinin on the migratory and invasive characteristics of highly bone metastatic ARCaP(M) cells [93]. We have also observed similar strong inhibitory effect of silibinin on the migratory and invasive potential of three human prostate cancer cell lines namely PC3, PC3MM2 and C4-2B cells in wound healing and invasion chamber assays, respectively [94]. Importantly, in these studies the anti-invasive and anti-migratory effect of silibinin was evident at concentrations where its cytotoxicity to cancer cells was minimal [91,93,94].
Cell-matrix interaction replaces cell-cell interaction during EMT in cancer cells, and plays important role in the growth, motility and invasiveness of cancer cells [14,15]. Silibinin treatment has been reported to significantly decrease the adhesion of PC3 cells with type I collagen [91]. It is important to mention here that bones are rich in type I collagen, therefore, interaction of prostate cancer cells with type I collagen is also important at distant metastatic bone site. Silibinin treatment also inhibited the adhesion of human prostate cancer PC3M cells with ECM proteins hyaluronan and fibronectin through targeting the expression of transmembrane protein CD44 and its variant form CD44v7-10 [95]. These studies suggest that silibinin treatment significantly attenuates the interaction of prostate cancer cells with their ECM components, which could adversely affect their motility and invasiveness.
As mentioned earlier, prostate cancer cells have high propensity to metastasize to bones [3]. During bone metastasis, prostate cancer cells even express genes like osteocalcin, bone sialoprotein, osteopontin, RANK ligand (RANKL), whose expression is normally restricted to bone cells [13,96]. This phenomenon is termed as ‘osteomimicry’ and is considered as an extreme effort by prostate cancer cells to adapt to their microenvironment. Our unpublished data has shown that silibinin could target many osteomimicry related proteins such as RANKL in prostate cancer cells. Once settled in bones, prostate cancer cells alter the delicate balance of bone remodeling orchestrated by two types of bone cells namely osetoclasts (involved in bone degradation) and osteoblasts (involved in bone formation) [12,13,96]. Prostate cancer cells secrete factors like RANKL, PTHrP (parathyroid hormone-related peptide) and uPA (urokinase-type plasminogen activator) that are involved in osetoclasts maturation and activation; thereby promote bone mineralization and liberation of various growth factors including TGF-β, IGFs, BMPs, PDGF etc [12,13,96]. Bone degradation provides prostate cancer cells the initial space to expand, and the released growth factors promote prostate cancer cell survival and proliferation. These growth factors secreted by bone degradation and those secreted by prostate cancer cells like endothelin-1 (ET-1), BMPs, Wnts, promote osteoblasts maturation and formation of new bone [12,13,96]. Mature osteoblasts also secrete growth factors which further promote prostate cancer cells growth in bone [13,96]. Overall, this vicious cycle involving prostate cancer cells, osteoclasts and osteoblasts promotes bone degradation as well as deposition of new ‘woven type bone’ (uneven/immature/embryonic), and thereby compromises bone health and leads to bone complications in prostate cancer patients. Since the initiation of osteoclastogenesis is the critical step in the establishment of prostate cancer growth in bone; targeting osteoclastogenesis has been considered a novel strategy against the bone metastasis by prostate cancer cells. Importantly, silibinin has been reported to significantly target osteoclastogenesis [97,98]. Kim et al. reported that silibinin treatment strongly inhibits RANKL-induced osteoclastogenesis in pre-osteoclastic RAW264.7 cells and in bone marrow-derived monocyte/macrophage cells [97]. This study also showed that silibinin significantly decreases TNF-α-induced osteoclastogenesis in osteoclast precursor cells from bone marrow [97]. Silibinin treatment also inhibited the osteoclastogenesis in bone marrow cells incubated directly with osteoblasts in the presence of 1,25(OH)2D3 [97]. In our ongoing studies, we observed that the exposure of RAW264.7 cells to the conditioned media from prostate cancer cells increases their osteoclastic activity as well as number of differentiated osteoclasts [98]. However, conditioned media collected from silibinin pre-treated prostate cancer cells (PC3, PC3MM2 and C4-2B) has significantly lesser potency to induce osteoclastic activity or osteoclastic differentiation in RAW264.7 cells [98]. These studies clearly suggest that silibinin treatment could alter the tumor microenvironment towards lowering the metastatic growth of prostate cancer cells.
Breast cancer
Breast cancer is one of the leading causes of cancer-related incidences and mortality among women [1]. In most breast cancer cases, death occurs due to metastatic spread of cancer cells to distant organs mainly to bone, liver, lung and brain. Provinciali et al. first studied the effect of silibinin treatment (in the form of silipide) on the development of mammary tumors in HER-2/neu transgenic mice [99]. In these mice, the overexpression of HER-2/neu is under the control of the mouse mammary tumor virus promoter, and these mice spontaneously develop tumors in their mammary glands at an early age. This study showed that silibinin treatment delays the development of spontaneous mammary tumors and reduces the number of mammary tumor masses [99]. Importantly, in this study, silibinin treatment significantly reduced the percentage of mice with lung metastasis as well as the mean size of lung metastasis [99]. This anti-cancer efficacy of silibinin was mainly attributed to the down-regulation of HER-2/neu expression, and the induction of senescent-like growth arrest and apoptosis through a p53-mediated pathway [99]. In another in vitro study, Lee et al. reported that silibinin treatment significantly inhibits the PMA (phorbol 12-myristate)-induced invasiveness of MCF-7 human breast carcinoma cells [100]. These in vivo and in vitro studies suggest that silibinin could effectively inhibit metastasis of breast cancer cells.
Lung Cancer
Lung cancer is the most common cause of death due to cancer in both men and women around the world. Lung cancer cells tend to metastasize very early after their primary growth, and that is one of the reasons that lung cancer is a very life-threatening cancer and one of the most difficult cancers to treat. While lung cancer can spread to any organ in the body, certain organs like adrenal glands, liver, brain and bone are the most common sites for lung cancer metastasis. Chen et al. analyzed the effect of silibinin feeding on the metastatic potential of Lewis lung carcinoma cells injected sub-cutaneously in C57BL/6 male mice [101]. This study showed that silibinin treatment significantly decreases the tumor mass, tumor volume as well as lung metastases [101]. In another study, Chu et al. showed that silibinin treatment exerts a dose- and time-dependent inhibitory effect on the migratory and invasive potential of human lung cancer A549 cells [102]. These studies suggest that silibinin could effectively target the invasive and metastatic potential of lung cancer cell.
Osteosarcoma
Osteosarcoma is the most common primary malignant tumor of the bone, and 80% of osteosarcoma patients develop pulmonary and hepatic metastases. Hsieh et al. showed that silibinin treatment significantly decreases the migratory and invasive potential of osteosarcoma MG-63 cells [103]. Silibinin treatment also inhibited the adhesive capability of MG-63 cells towards type IV collagen [103]. Further studies are awaited to confirm the in vivo anti-metastatic efficacy of silibinin against osteosarcoma cells.
Oral Cancer
Squamous cell carcinoma of the tongue is a common malignancy of the oral cavity and these cancer cells usually metastasize to lymph nodes of the neck. Study by Chen et al. showed the inhibitory effect of silibinin on the migratory and invasive potential of human tongue squamous cell carcinoma cells in vitro [101]. Silibinin treatment also affected the interaction of these cancer cells with type I collagen [101]. These in vitro studies still need to be confirmed in in vivo model of oral cancer metastasis.
Mechanistic details of silibinin anti-metastatic efficacy
Silibinin targets most of the metastatic events shown in Fig. 1, and in this section we have detailed possible mechanisms that contribute to strong anti-metastatic efficacy of silibinin.
Silibinin targets EMT in cancer cells
EMT is a dynamic, multistep and highly coordinated process that includes the loss of inter-cellular junctions, disruption of the tumor basement membrane, activation and rearrangement of the cytoskeleton resulting in increased motility, and the release of cells from parent epithelial tissue [8,15]. The molecular bases of EMT is very complex and involve several interconnected pathways and signaling molecules including growth factors, receptor tyrosine kinases, Notch, NF-κB, Ras superfamily small GTPases, catenins and integrins [8,14,15]. However, most of these pathways converge together to down-regulate the expression of epithelial molecule E-cadherin [104]. E-cadherin is a transmembrane glycoprotein with an extracellular domain that interacts with the E-cadherin molecule on adjacent cells, and an intracellular domain associated with a multi-protein complex comprising three catenins (α, β and p-120) [104]. β-catenin binds tightly to the cytoplasmic domain of E-cadherin and through α-catenin to the actin microfilament network of the cytoskeleton [104]. In general, this complex is important in regulating the cell-cell adhesion, cell polarity and cell shape. The loss of E-cadherin frees β-catenin from the membranous pool, thus making it available for nuclear signaling, which is known to promote cancer cell proliferation, invasiveness and EMT [105]. Therefore, downregulation of E-cadherin expression represents the determinant step in the destabilization of epithelial architecture, and is regulated through a combination of genetic, epigenetic, transcriptional and post-transcriptional mechanisms [14]. Major transcriptional repressors of E-cadherin are zinc finger family members Snail and Slug, the basic helix-loop-helix factors E47 and Twist, and two-handed zinc factors ZEB1 and SIP1 [14]. These transcriptional factors are considered inducers of EMT and are important in cancer cell metastasis [14]. The loss of epithelial characteristics during EMT is also accompanied by increased expression of mesenchymal markers such as cyotskeletal proteins vimentin, smooth muscle actin, γ-actin, β-filamin, and extracellular matrix components such as fibronectin and collagen precursors [15]. The upregulation of these proteins facilitate pseudopod formation and cyotskeletal remodeling for cancer cell motility and invasion [15].
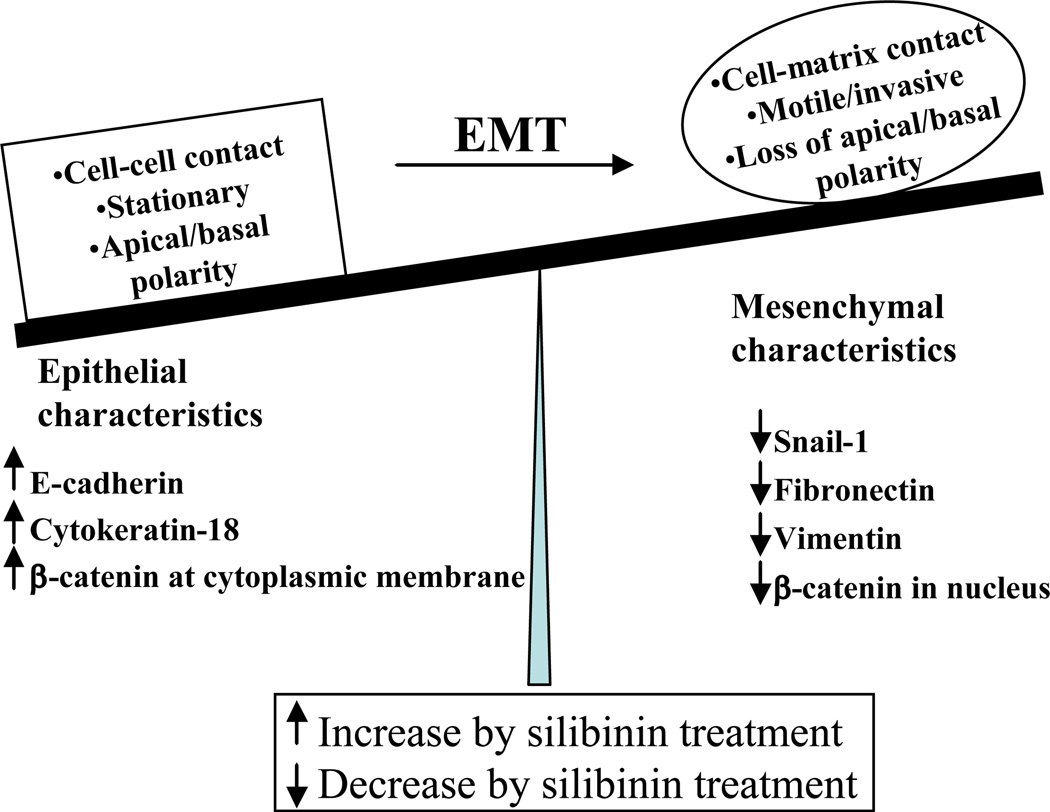
Due to critical importance of EMT in cancer metastasis, targeting EMT has been considered an exciting opportunity towards preventing cancer cells from acquiring motility and invasiveness. Silibinin has been widely reported to target EMT through promoting epithelial characteristics while significantly inhibiting the expression of mesenchymal markers (Fig. 3) [80,90,92,93]. We reported that in TRAMP mice with increasing age and disease stage prostate cancer cells increasingly express mesenchymal markers/regulators while loosing the expression of epithelial markers [80]. For example, at PIN stage, E-cadherin expression was high and it was localized on the intercellular junctions, while the expression of its transcriptional repressor Snail-1 was low; however, with the disease progression to poorly-differentiated stage, E-cadherin expression was lost, while nuclear expression of Snail-1 was increased, suggesting EMT during prostate tumor progression in this model [80]. Importantly, the expression of E-cadherin was significantly higher in tumor tissues from the mice fed with 1% silibinin in diet and that corresponded to low-pathological grade of the disease in these mice [80]. Furthermore, silibinin treatment significantly decreased Snail-1 and fibronectin expression in the prostate tumor tissues [80]. In another study, where silibinin was fed for 11 weeks to TRAMP mice, we observed an increase in E-cadherin expression, and a strong decrease in Snail-1 and vimentin expression [90]. These studies clearly suggested the strong in vivo potential of silibinin to inhibit EMT and disease progression, which might contribute to its strong anti-metastatic efficacy.
Fig. 3.
Silibinin promotes epithelial characteristics and inhibits mesenchymal characteristics, thereby strongly inhibits epithelial-to-mesenchymal transition (EMT) in cancer cells.
There are several cell culture studies that also support strong anti-EMT potential of silibinin [92,93]. Wu et al. reported that the anti-invasive and anti-migratory properties of silibinin are due to its strong inhibitory effect on vimentin expression in ARCaP(M) cells [93]. In a recent study, the same group showed that silibinin treatment up-regulates cytokeratin-18 (an epithelial marker) and down-regulates vimentin expression in prostate cancer cells; and these molecular events were associated with morphological reversal of EMT phenotype [92]. This study also suggested that the inhibitory effect of silibinin on EMT could be through targeting various transcriptional factors (NF-κB, ZEB1 and Slug) [92]. In our recently completed in vitro study, we observed that silibinin targets EMT in PCA cells through enhancing E-cadherin expression at cellular membranes [94]. Further, silibinin treatment inhibited the levels of cytoplasmic and nuclear β-catenin without affecting the β-catenin present at cellular membranes [94]. These in vitro studies clearly suggest that silibinin targets EMT in cancer cells through multiple mechanisms (Fig. 3).
Silibinin targets proteases
Proteases have been implicated in many cancer-related biological activities, mainly because of their ability to break down components of the extracellular matrix, allowing cancer and endothelial cells to spread. Among various types of proteases, family of zinc-dependent endopetidases known as ‘matrix metalloproteinases (MMPs)’ is considered most important as biomarker for cancer prognosis and therapeutic [106,107]. There are direct evidences suggesting the role of MMPs in tumor growth and invasion. For example, MMP-9 deficient mice show reduced formation of melanoma metastasis; MMP-2 knockout mice show reduced melanoma tumor progression [108,109]. The expression of MMPs has also been used to predict the risk of metastasis in many cancers [107]. The expression of MMP-2 and MMP-9 in papillary thyroid carcinoma is well correlated with their invasive capacity and lymph node metastasis [107]. High serum level of MMP-2 has been correlated with the presence of metastases in lung cancer [110]. MMPs like MMP-3 and MMP-7 cleave extracellular domain of E-cadherin, and the ratio of MMPs and E-cadherin has been used to predict metastasis and tumor recurrence in different cancers [107,111]. Therefore, inhibition of MMP activity is considered an exciting approach to target growth and invasiveness of neoplastic cells, and currently several MMP inhibitors are in clinical trial [107,112]. Another attractive approach to target MMPs is through promoting the expression of endogenous inhibitors of MMPs known as tissue inhibitors of metalloproteinases (TIMPs) [113]. In this regard, silibinin has been extensively studied for its efficacy against MMPs. We reported that in TRAMP mice, silibinin feeding significantly decreases expression of MMPs (MMP-2, MMP-3 and MMP-9) but increases TIMP-2 expression in prostate tumor tissue [80,90]. Silibinin treatment has also been shown to significantly decrease expression of MMPs and to increase expression of TIMP-2 in vitro in wide variety of cancer cells [93,100–102,114]. Silibinin treatment decreased MMP-2 expression and increased TIMP-2 protein expression in highly metastatic lung cancer A549 cells and tongue cancer SCC-4 cells [101,102,114]. Further, silibinin treatment strongly inhibited MMP-2 expression in prostate cancer ARCaP(M) cells, osteosarcoma MG-63 cells and human hepatocellular carcinoma HepG-2 cells [93,103,115]. Further, silibinin treatment decreased the activity-, gene- and proteinexpression of PMA-induced MMP-9 expression in MCF-7 breast carcinoma cells [100]. Momeny et al. reported the inhibitory effect of silibinin on MMP-9 expression in human glioblastoma U87MG cells [116]. These studies clearly suggest that silibinin inhibits MMPs while increases TIMP-2 expression in a wide variety of cancer cells.
The serine protease urokinase-type plasminogen activator (uPA) and its receptor (uPAR) have been implicated in several tumor processes including adhesion, migration, proliferation and angiogenesis through their interactions with integrins and vitronectin, and through activation of intracellular signaling pathways [117–119]. uPA is known to catalyze the conversion of plasminogen to plasmin, which in turn, exerts strong proteolytic effects including the activation of metalloproteinases and growth factors [117]. The high expression of uPA/uPAR has been correlated with tumor progression and, in some cases, poor patient post-operative survival [119]. We have reported that uPAR level is up-regulated during prostate tumor progression, but silibinin significantly inhibits uPAR expression [80]. Further, silibinin treatment has been reported to inhibit uPA and uPAR expression in several cancer cell lines in vitro [101–103,116]. Cathepsin B is another important cysteine protease whose expression is reported to be higher in cancer cells and is known to mediate cancer cells dissemination through ECM degradation and activation of other proteinases [117]. Silibinin treatment has been reported to decrease cathepsin B expression in highly invasive human glioma cells [116]. Overall, these studies suggest that anti-migratory/anti-invasive efficacy of silibinin could be through its inhibitory effects on a wide-range of proteases.
Silibinin targets MAPK signaling
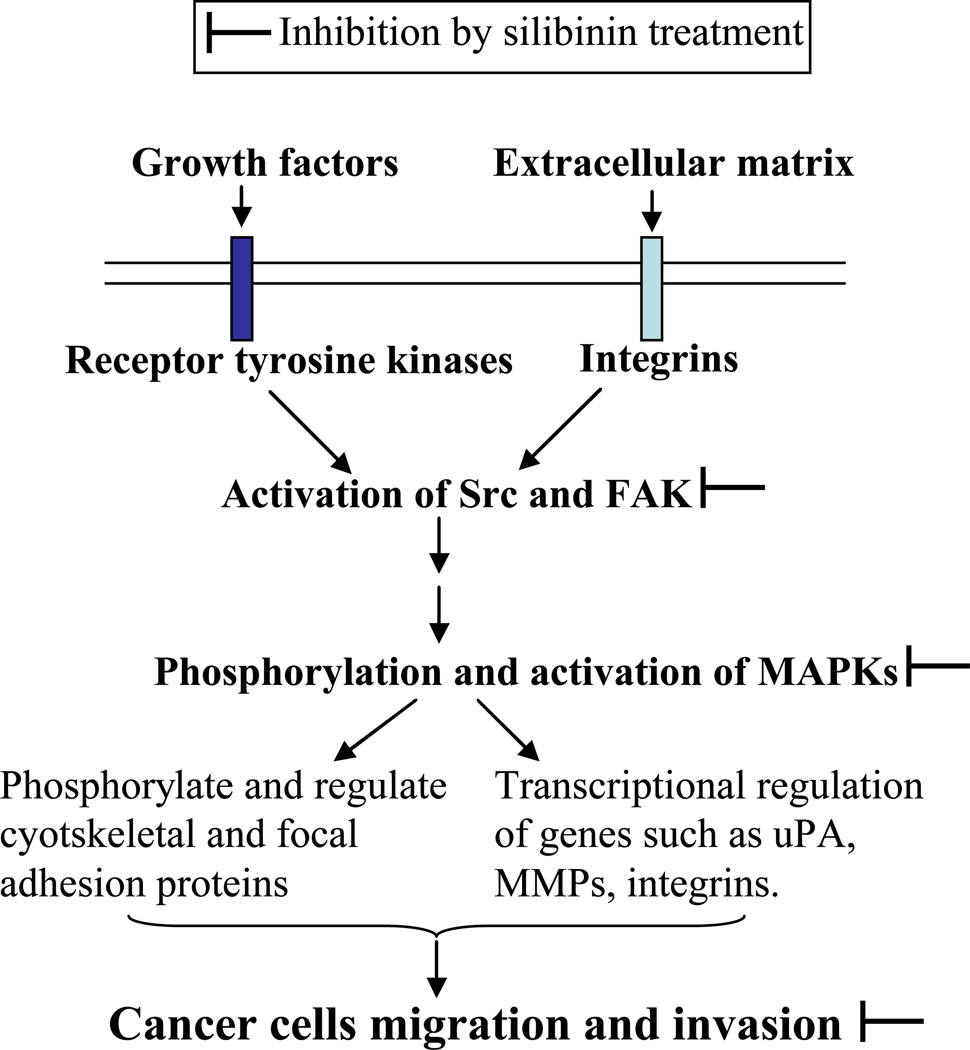
MAPKs (ERK1/2, JNK1/2 and p38) belong to widely conserved family of serine/threonine kinases that are activated through reversible phosphorylation and mediate signal transduction of a wide variety of extracellular stimuli (cellmatrix adhesion, growth factors etc.) into intracellular cascades. Recent studies have implicated their role in the regulation of cancer cell motility and invasiveness [120,121]. The sustained activation of MAPK signaling relies mainly on the cross talks between integrins and receptor tyrosine kinases (RTKs) [122]. These signals converge on to activate non-receptor kinases Src and FAK, which eventually leads to activation of MAPKs (Fig. 4) [122]. MAPKs are reported to regulate several transcriptional factors such as AP-1, NF-κB as well as expression of migration-related genes such as uPA, MMPs, β(3)-integrin, cathepsin (Fig. 4) [103,122,123]. ERK has also been reported to regulate the dynamics of focal adhesion and cyotskeletal reorganization through phosphorylating specific cyotskeletal and focal adhesion proteins such as paxillin, focal adhesion kinase (FAK), myosin light chain kinase (MLCK) and microtubule associated protein (MAP) [122]. Overall, inhibition of MAPK signaling might have the potential to prevent cancer cell proliferation, migration, invasion and metastasis.
Fig. 4.
Silibinin treatment decreases activation of MAPKs, and as a result inhibits cancer cells migration and invasion.
The efficacy of silibinin to target MAPK-mediated mitogenic signaling has been extensively reported [86,124–126]. There are now reports suggesting that silibinin also targets MAPK signaling towards inhibiting the migration and invasion of cancer cells (Fig. 4) [101,103,114,115]. Chen et al. showed that silibinin-mediated decrease in MMP-2, uPA and invasiveness of A549 cells is mainly through inhibition of ERK1/2 [114]. Lee et al. reported in MCF-7 cells that specific inhibition of MAPK signaling is directly involved in the regulation of PMA-induced MMP-9 expression [100]. Study by Hsieh et al. showed that silibinin targets FAK and ERK1/2 expression, suppresses c-Jun levels and AP-1-binding activity and strongly decreases MMP-2 and uPA expression in MG-63 cells [103]. Importantly, inhibitory effect of silibinin on the invasiveness of MG-63 cells was compromised when these cells were transfected to over-express active MEK1 [103]. In our unpublished in vitro studies, we have also observed that silibinin strongly inhibits Src activation in prostate cancer cells. These results clearly support a critical role of MAPK inhibition in silibinin anti-invasive efficacy (Fig. 4).
Silibinin targets tumor microenvironment
As mentioned earlier, tumor microenvironment is an intrinsic part of tumor at all stages of tumor development including metastasis. Therefore, in addition to existing preventive/therapeutic efforts directed at tumor cells, it is essential to develop new measures targeted toward tumor microenvironment. Tumor microenvironment is also considered an attractive target for cytostatic drugs as it is (relatively) genetically stable and chances of it developing chemoresistance are remote. Till date, detailed effect of silibinin has been studied only on one tumor microenvironment component i.e. tumor angiogenesis.
Neo-angiogenesis refers to formation of new blood vessels within tumors and is considered important constituent of tumor microenvironment. Formation of these vessels is necessary to provide nutrients and oxygen to the growing tumor cells and also to remove waste products. Furthermore, malignant cells exit from primary tumors into blood circulation only after tumor become neo-vascularized. Furthermore, at distant sites, metastatic cancer cells must again induce angiogenesis to grow beyond micro-metastasis. Therefore, targeting angiogenesis is an important strategy to target cancer cells growth and metastasis. There is plethora of reports suggesting the strong anti-angiogenic efficacy of silibinin in various cancer models [80,81,90,127,128]. Silibinin treatment has been reported to inhibit tube formation and invasive capability of endothelial cells in in vitro assays [79]. Further, silibinin treatment has been reported to significantly decrease microvessels (established and newly formed) density in animal models for variety of cancers [81,127,129]. Silibinin treatment is reported to modulate expression of signaling molecules involved in angiogenesis regulation such as VEGF, VEGF receptors, FGF, HIF-1α, iNOS, COX2, NF-κB, STATs, interleukins, angiopoietin-2, Angreceptor tyrosine kinase [80,81,89,127,128,130,131]. Recently, we also showed that silibinin treatment decreases the population of tumor associated macrophages (TAMs) towards targeting the angiogenic microenvironment in lung tumors [127]. These studies suggest that silibinin might be effective in altering the immediate tumor microenvironment, but further studies are required to understand its effects on other components of tumor microenvironment involved in cancer metastasis.
Preventive and Therapeutic implications of silibinin anti-metastatic efficacy
Silibinin has a long history of human use and is devoid of side effects even when acute or chronic doses of silibinin are administered in animals and humans [56,68,69]. Various traditional toxicological tests have confirmed the non-toxic nature of silibinin and its safety profile [56,68]. In fact, rodent LD50 values for silibinin have never been achieved. Currently, close to dozen clinical trials are in progress examining the liver protective as well as other therapeutic benefits of silibinin. Now there are enough pre-clinical evidences that establish silibinin as an ‘antimetastatic agent’. These studies have shown that silibinin targets migratory/invasive and metastatic behavior of a wide-variety of cancer cells. Silibinin is reported to act through pleiotropic mechanisms including targeting of EMT-related events, inhibiting proteases expression and MAPK signaling, as well as adversely affecting the tumor microenvironment. These studies advocate greater use of silibinin in the clinical management of advanced metastatic stage of the disease. Silibinin could be potentially used as a preventing agent in patients diagnosed at early localized stage of the disease. Based upon its efficacy in pre-clinical studies, it is reasonable to expect that silibinin treatment would inhibit EMT in localized cancer cells, thereby prevent the metastatic progression of the disease. Since silibinin also possesses strong anti-angiogenic characteristics, it is expected to inhibit cancer growth but should also prevent the progression of micro-metastases that remain undiagnosed or under-diagnosed; thereby silibinin could prevent or delay the growth of metastatic lesions. Our completed pre-clinical studies have shown that silibinin is also effective against metastatic progression of cancer even when used as therapeutic agent; therefore silibinin should also be tested in clinic to treat advance stage of the cancer. In preventive studies, silibinin should be administered for a reasonable period of time before expecting a clinical outcome; however, in therapeutic settings, it should be administered through intravenous route to achieve a high systemic dose with the expectation that clinical outcomes could be measured in reasonably shorter duration. In this regard, it is important to emphasize here that the bioavailability of oral silibinin has been reasonably enhanced through its formulation with phosphatidylcholine and lately with nanoparticles. Intravenous injectable silibinin formulation (silibinin-Legalon) is already available and used recently against mushroom poisoning-related liver toxicity with successful outcomes, further suggesting that clinical benefits of silibinin against cancer metastasis should be exploited in near future.
Conclusions and Future directions
Metastasis is a multistep and multifunctional biological event and is considered the final and most-life threatening stage of cancer progression. The high morbidity and mortality related to cancer metastasis are due to lack of effectiveness of current therapeutic regimes; alternatively, the use of nutraceutical agents has been suggested for the prevention and treatment of advance cancers. Nutraceutical agent silibinin has shown remarkable anti-metastatic efficacy against many cancers in various pre-clinical models. The anti-metastatic efficacy of silibinin has been reported through pleiotropic mechanisms including the inhibition of EMT in caner cells. However, more studies are still needed to further confirm the anti-EMT and anti-metastatic effect of silibinin in wide variety of human cancer cells under in vivo conditions. Furthermore, silibinin should be tested in the metastatic models, which more closely represent human metastatic disease condition. In future, there should also be greater emphasis on to understand the role of tumor microenvironment in metastasis initiation and progression as well as how silibinin affects these interactions. Overall, it is reasonable to anticipate that in the near future silibinin would be an important non-toxic nutraceutical agent in the management of metastatic cancers.
Acknowledgement
This work was supported by NCI RO1 grant CA102514,.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 3.Tantivejkul K, Kalikin LM, Pienta KJ. Dynamic process of prostate cancer metastasis to bone. J Cell Biochem. 2004;91(4):706–717. doi: 10.1002/jcb.10664. [DOI] [PubMed] [Google Scholar]
- 4.Hadaschik BA, Gleave ME. Therapeutic options for hormone-refractory prostate cancer in 2007. Urol Oncol. 2007;25(5):413–419. doi: 10.1016/j.urolonc.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat Rev Cancer. 2006;6(6):449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro J, Fodde R. Cancer stemness and metastasis: Therapeutic consequences and perspectives. Eur J Cancer. 46(7):1198–1203. doi: 10.1016/j.ejca.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Weinberg RA. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Gupta GP, Minn AJ, Kang Y, Siegel PM, Serganova I, Cordon-Cardo C, et al. Identifying site-specific metastasis genes and functions. Cold Spring Harb Symp Quant Biol. 2005;70:149–158. doi: 10.1101/sqb.2005.70.018. [DOI] [PubMed] [Google Scholar]
- 12.Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11(1):57–67. doi: 10.1038/aja.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buijs JT, van der Pluijm G. Osteotropic cancers: From primary tumor to bone. Cancer Lett. 2009;273(2):177–193. doi: 10.1016/j.canlet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 16.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59(6):1295–1300. [PubMed] [Google Scholar]
- 17.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110(1):133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karpatkin S, Pearlstein E, Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastases. Ann N Y Acad Sci. 1981;370:101–118. doi: 10.1111/j.1749-6632.1981.tb29726.x. [DOI] [PubMed] [Google Scholar]
- 19.Fidler IJ. The pathogenesis of cancer metastasis: The 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 20.Weiss L. Metastasis of cancer: A conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000;19(3–4):I–XI. 193–383. [PubMed] [Google Scholar]
- 21.Sugarbaker EV. Cancer metastasis: A product of tumor-host interactions. Curr Probl Cancer. 1979;3(7):1–59. doi: 10.1016/s0147-0272(79)80008-2. [DOI] [PubMed] [Google Scholar]
- 22.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coman DR, de LR. The role of the vertebral venous system in the metastasis of cancer to the spinal column; experiments with tumor-cell suspensions in rats and rabbits. Cancer. 1951;4(3):610–618. doi: 10.1002/1097-0142(195105)4:3<610::aid-cncr2820040312>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 24.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin Exp Metastasis. 2009;26(5):433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. Cd44+ cd24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98(4):756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 27.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28(3):297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. Vegfr1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demicheli R, Retsky MW, Hrushesky WJ, Baum M. Tumor dormancy and surgery-driven interruption of dormancy in breast cancer: Learning from failures. Nat Clin Pract Oncol. 2007;4(12):699–710. doi: 10.1038/ncponc0999. [DOI] [PubMed] [Google Scholar]
- 31.Demicheli R, Retsky MW, Swartzendruber DE, Bonadonna G. Proposal for a new model of breast cancer metastatic development. Ann Oncol. 1997;8(11):1075–1080. doi: 10.1023/a:1008263116022. [DOI] [PubMed] [Google Scholar]
- 32.Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci U S A. 107(6):2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 34.Hurt EM, Farrar WL. Cancer stem cells: The seeds of metastasis? Mol Interv. 2008;8(3):140–142. doi: 10.1124/mi.8.3.7. [DOI] [PubMed] [Google Scholar]
- 35.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 36.Fidler IJ. Metastasis: Guantitative analysis of distribution and fate of tumor embolilabeled with 125 i-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773–782. [PubMed] [Google Scholar]
- 37.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elvin P, Garner AP. Tumour invasion and metastasis: Challenges facing drug discovery. Curr Opin Pharmacol. 2005;5(4):374–381. doi: 10.1016/j.coph.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen NP, Bishop M, Borok TJ, Welsh J, Hamilton R, Cohen D, et al. Pattern of failure following chemoradiation for locally advanced non-small cell lung cancer: Potential role for stereotactic body radiotherapy. Anticancer Res. 30(3):953–961. [PubMed] [Google Scholar]
- 40.Gimbel MI, Paty PB. A current perspective on local excision of rectal cancer. Clin Colorectal Cancer. 2004;4(1):26–35. doi: 10.3816/ccc.2004.n.007. discussion 36-27. [DOI] [PubMed] [Google Scholar]
- 41.Wilson CM, Tobin S, Young RC. The exploding worldwide cancer burden: The impact of cancer on women. Int J Gynecol Cancer. 2004;14(1):1–11. doi: 10.1111/j.1048-891x.2004.14178.x. [DOI] [PubMed] [Google Scholar]
- 42.Magrath I, Litvak J. Cancer in developing countries: Opportunity and challenge. J Natl Cancer Inst. 1993;85(11):862–874. doi: 10.1093/jnci/85.11.862. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: Potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234(8):825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trottier G, Bostrom PJ, Lawrentschuk N, Fleshner NE. Nutraceuticals and prostate cancer prevention: A current review. Nat Rev Urol. 7(1):21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal R, Deep G. Kava, a tonic for relieving the irrational development of natural preventive agents. Cancer Prev Res (Phila Pa) 2008;1(6):409–412. doi: 10.1158/1940-6207.CAPR-08-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennis T, Fanous M, Mousa S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr Cancer. 2009;61(5):587–597. doi: 10.1080/01635580902825530. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Tharakan ST, Sung B, Anand P. Potential of spice-derived phytochemicals for cancer prevention. Planta Med. 2008;74(13):1560–1569. doi: 10.1055/s-2008-1074578. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2006;26(6B):4457–4498. [PubMed] [Google Scholar]
- 49.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: An "Old-age" Disease with an "Age-old" Solution. Cancer Lett. 2008;267(1):133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 50.Basu A, Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: Conclusions from clinical trials. Eur J Clin Nutr. 2007;61(3):295–303. doi: 10.1038/sj.ejcn.1602510. [DOI] [PubMed] [Google Scholar]
- 51.Longo VD, Fontana L. Calorie restriction and cancer prevention: Metabolic and molecular mechanisms. Trends Pharmacol Sci. 31(2):89–98. doi: 10.1016/j.tips.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bemis DL, Katz AE, Buttyan R. Clinical trials of natural products as chemopreventive agents for prostate cancer. Expert Opin Investig Drugs. 2006;15(10):1191–1200. doi: 10.1517/13543784.15.10.1191. [DOI] [PubMed] [Google Scholar]
- 53.Chambers AF. Influence of diet on metastasis and tumor dormancy. Clin Exp Metastasis. 2009;26(1):61–66. doi: 10.1007/s10585-008-9164-4. [DOI] [PubMed] [Google Scholar]
- 54.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 55.Flaig TW, Glode M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 70(8):848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 56.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, et al. A phase i and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25(2):139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 57.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124(5):491–504. [PubMed] [Google Scholar]
- 58.Rainone F. Milk thistle. Am Fam Physician. 2005;72(7):1285–1288. [PubMed] [Google Scholar]
- 59.Post-White J, Ladas EJ, Kelly KM. Advances in the use of milk thistle (silybum marianum) Integr Cancer Ther. 2007;6(2):104–109. doi: 10.1177/1534735407301632. [DOI] [PubMed] [Google Scholar]
- 60.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, et al. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65(10):4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 61.Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos. 2008;36(1):65–72. doi: 10.1124/dmd.107.017566. [DOI] [PubMed] [Google Scholar]
- 62.Venkataramanan R, Komoroski B, Strom S. In vitro and in vivo assessment of herb drug interactions. Life Sci. 2006;78(18):2105–2115. doi: 10.1016/j.lfs.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Flora K, Hahn M, Rosen H, Benner K. Milk thistle (silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139–143. doi: 10.1111/j.1572-0241.1998.00139.x. [DOI] [PubMed] [Google Scholar]
- 64.Silybin-phosphatidylcholine complex. Monograph. Altern Med Rev. 2009;14(4):385–390. [PubMed] [Google Scholar]
- 65.Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: The silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 2009;14(3):226–246. [PubMed] [Google Scholar]
- 66.Yanyu X, Yunmei S, Zhipeng C, Qineng P. The preparation of silybin-phospholipid complex and the study on its pharmacokinetics in rats. Int J Pharm. 2006;307(1):77–82. doi: 10.1016/j.ijpharm.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Zhang D, Liu Z, Liu G, Duan C, Jia L, et al. In vitro and in vivo evaluation of silybin nanosuspensions for oral and intravenous delivery. Nanotechnology. 21(15):155104. doi: 10.1088/0957-4484/21/15/155104. [DOI] [PubMed] [Google Scholar]
- 68.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (siliphos) Altern Med Rev. 2005;10(3):193–203. [PubMed] [Google Scholar]
- 69.Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: Silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12(9):2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 70.Deep G, Singh RP, Agarwal C, Kroll DJ, Agarwal R. Silymarin and silibinin cause g1 and g2-m cell cycle arrest via distinct circuitries in human prostate cancer pc3 cells: A comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25(7):1053–1069. doi: 10.1038/sj.onc.1209146. [DOI] [PubMed] [Google Scholar]
- 71.Mateen S, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin inhibits human nonsmall cell lung cancer cell growth through cell-cycle arrest by modulating expression and function of key cell-cycle regulators. Mol Carcinog. 49(3):247–258. doi: 10.1002/mc.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zi X, Agarwal R. Silibinin decreases prostate-specific antigen with cell growth inhibition via g1 arrest, leading to differentiation of prostate carcinoma cells: Implications for prostate cancer intervention. Proc Natl Acad Sci U S A. 1999;96(13):7490–7495. doi: 10.1073/pnas.96.13.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tyagi A, Agarwal C, Agarwal R. The cancer preventive flavonoid silibinin causes hypophosphorylation of rb/p107 and rb2/p130 via modulation of cell cycle regulators in human prostate carcinoma du145 cells. Cell Cycle. 2002;1(2):137–142. [PubMed] [Google Scholar]
- 74.Kaur M, Velmurugan B, Tyagi A, Deep G, Katiyar S, Agarwal C, et al. Silibinin suppresses growth and induces apoptotic death of human colorectal carcinoma lovo cells in culture and tumor xenograft. Mol Cancer Ther. 2009;8(8):2366–2374. doi: 10.1158/1535-7163.MCT-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma ht-29 cells. Oncogene. 2003;22(51):8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 76.Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y. Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking egfr-erk activation in renal cell carcinoma. Cancer Lett. 2008;272(1):61–69. doi: 10.1016/j.canlet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 77.Wang HJ, Tashiro S, Onodera S, Ikejima T. Inhibition of insulin-like growth factor 1 receptor signaling enhanced silibinin-induced activation of death receptor and mitochondrial apoptotic pathways in human breast cancer mcf-7 cells. J Pharmacol Sci. 2008;107(3):260–269. doi: 10.1254/jphs.08054fp. [DOI] [PubMed] [Google Scholar]
- 78.Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, et al. Silibinin sensitizes human glioma cells to trail-mediated apoptosis via dr5 up-regulation and down-regulation of c-flip and survivin. Cancer Res. 2007;67(17):8274–8284. doi: 10.1158/0008-5472.CAN-07-0407. [DOI] [PubMed] [Google Scholar]
- 79.Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, akt and nf-kappab: Implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24(7):1188–1202. doi: 10.1038/sj.onc.1208276. [DOI] [PubMed] [Google Scholar]
- 80.Raina K, Rajamanickam S, Singh RP, Deep G, Chittezhath M, Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68(16):6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98(12):846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 82.Singh RP, Sharma G, Dhanalakshmi S, Agarwal C, Agarwal R. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomarkers Prev. 2003;12(9):933–939. [PubMed] [Google Scholar]
- 83.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: Bench to bedside. Mol Carcinog. 2006;45(6):436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 84.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Chemoprevention of intestinal tumorigenesis in apcmin/+ mice by silibinin. Cancer Res. 70(6):2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh RP, Agarwal R. Prostate cancer prevention by silibinin. Curr Cancer Drug Targets. 2004;4(1):1–11. doi: 10.2174/1568009043481605. [DOI] [PubMed] [Google Scholar]
- 86.Singh RP, Raina K, Deep G, Chan D, Agarwal R. Silibinin suppresses growth of human prostate carcinoma pc-3 orthotopic xenograft via activation of extracellular signal-regulated kinase 1/2 and inhibition of signal transducers and activators of transcription signaling. Clin Cancer Res. 2009;15(2):613–621. doi: 10.1158/1078-0432.CCR-08-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li L, Zeng J, Gao Y, He D. Targeting silibinin in the antiproliferative pathway. Expert Opin Investig Drugs. 19(2):243–255. doi: 10.1517/13543780903533631. [DOI] [PubMed] [Google Scholar]
- 88.Tyagi A, Sharma Y, Agarwal C, Agarwal R. Silibinin impairs constitutively active tgfalpha-egfr autocrine loop in advanced human prostate carcinoma cells. Pharm Res. 2008;25(9):2143–50. doi: 10.1007/s11095-008-9545-z. [DOI] [PubMed] [Google Scholar]
- 89.Jung HJ, Park JW, Lee JS, Lee SR, Jang BC, Suh SI, et al. Silibinin inhibits expression of hif-1alpha through suppression of protein translation in prostate cancer cells. Biochem Biophys Res Commun. 2009;390(1):71–76. doi: 10.1016/j.bbrc.2009.09.068. [DOI] [PubMed] [Google Scholar]
- 90.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14(23):7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (pc-3) cell line. Cell Biol Int. 2008;32(8):888–92. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Wu K, Zeng J, Li L, Fan J, Zhang D, Xue Y, et al. Silibinin reverses epithelial-to-mesenchymal transition in metastatic prostate cancer cells by targeting transcription factors. Oncol Rep. 2010;23(6):1545–1552. [PubMed] [Google Scholar]
- 93.Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and mmp-2 expression. Acta Pharmacol Sin. 2009;30(8):1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deep G, Gangar SC, Agarwal R. Silibinin inhibits epithelial to mesenchymal transition in prostate cancer cells: Role of e-cadherin and beyond. Proc. 101th AACR Annual Meeting, Washington DC, April 2010. 2010 Abstract number 5650. [Google Scholar]
- 95.Handorean AM, Yang K, Robbins EW, Flaig TW, Iczkowski KA. Silibinin suppresses cd44 expression in prostate cancer cells. Am J Transl Res. 2009;1(1):80–86. [PMC free article] [PubMed] [Google Scholar]
- 96.Msaouel P, Pissimissis N, Halapas A, Koutsilieris M. Mechanisms of bone metastasis in prostate cancer: Clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22(2):341–355. doi: 10.1016/j.beem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Kim JH, Kim K, Jin HM, Song I, Youn BU, Lee J, et al. Silibinin inhibits osteoclast differentiation mediated by tnf family members. Mol Cells. 2009 doi: 10.1007/s10059-009-0123-y. [DOI] [PubMed] [Google Scholar]
- 98.Gangar SC, Deep G, Agarwal R. Silibinin inhibits advanced human prostate carcinoma-induced osteoclastogenesis. Proc. 101th AACR Annual Meeting, Washington DC, April 2010. 2010 Abstract number 5661. [Google Scholar]
- 99.Provinciali M, Papalini F, Orlando F, Pierpaoli S, Donnini A, Morazzoni P, et al. Effect of the silybin-phosphatidylcholine complex (idb 1016) on the development of mammary tumors in her-2/neu transgenic mice. Cancer Res. 2007;67(5):2022–2029. doi: 10.1158/0008-5472.CAN-06-2601. [DOI] [PubMed] [Google Scholar]
- 100.Lee SO, Jeong YJ, Im HG, Kim CH, Chang YC, Lee IS. Silibinin suppresses pma-induced mmp-9 expression by blocking the ap-1 activation via mapk signaling pathways in mcf-7 human breast carcinoma cells. Biochem Biophys Res Commun. 2007;354(1):165–171. doi: 10.1016/j.bbrc.2006.12.181. [DOI] [PubMed] [Google Scholar]
- 101.Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF, Chu SC. Silibinin inhibits invasion of oral cancer cells by suppressing the mapk pathway. J Dent Res. 2006;85(3):220–225. doi: 10.1177/154405910608500303. [DOI] [PubMed] [Google Scholar]
- 102.Chu SC, Chiou HL, Chen PN, Yang SF, Hsieh YS. Silibinin inhibits the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Mol Carcinog. 2004;40(3):143–149. doi: 10.1002/mc.20018. [DOI] [PubMed] [Google Scholar]
- 103.Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC, Lu KH. Silibinin suppresses human osteosarcoma mg-63 cell invasion by inhibiting the erk-dependent c-jun/ap-1 induction of mmp-2. Carcinogenesis. 2007;28(5):977–987. doi: 10.1093/carcin/bgl221. [DOI] [PubMed] [Google Scholar]
- 104.Baranwal S, Alahari SK. Molecular mechanisms controlling e-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384(1):6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, et al. Role of wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol. 2007;14(11):1034–1039. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 106.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vihinen P, Kahari VM. Matrix metalloproteinases in cancer: Prognostic markers and therapeutic targets. Int J Cancer. 2002;99(2):157–166. doi: 10.1002/ijc.10329. [DOI] [PubMed] [Google Scholar]
- 108.Itoh T, Tanioka M, Matsuda H, Nishimoto H, Yoshioka T, Suzuki R, et al. Experimental metastasis is suppressed in mmp-9-deficient mice. Clin Exp Metastasis. 1999;17(2):177–181. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 109.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase a-deficient mice. Cancer Res. 1998;58(5):1048–1051. [PubMed] [Google Scholar]
- 110.Garbisa S, Scagliotti G, Masiero L, Di Francesco C, Caenazzo C, Onisto M, et al. Correlation of serum metalloproteinase levels with lung cancer metastasis and response to therapy. Cancer Res. 1992;52(16):4548–4549. [PubMed] [Google Scholar]
- 111.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter e-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114(Pt 1):111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 112.Li X, Wu JF. Recent developments in patent anti-cancer agents targeting the matrix metalloproteinases (mmps) Recent Pat Anticancer Drug Discov. 5(2):109–141. doi: 10.2174/157489210790936234. [DOI] [PubMed] [Google Scholar]
- 113.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur J Cell Biol. 1997;74(2):111–122. [PubMed] [Google Scholar]
- 114.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both pi3k-akt and mapk signaling pathways. Chem Biol Interact. 2005;156(2–3):141–150. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 115.Momeny M, Khorramizadeh MR, Ghaffari SH, Yousefi M, Yekaninejad MS, Esmaeili R, et al. Effects of silibinin on cell growth and invasive properties of a human hepatocellular carcinoma cell line, hepg-2, through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur J Pharmacol. 2008;591(1–3):13–20. doi: 10.1016/j.ejphar.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 116.Momeny M, Malehmir M, Zakidizaji M, Ghasemi R, Ghadimi H, Shokrgozar MA, et al. Silibinin inhibits invasive properties of human glioblastoma u87mg cells through suppression of cathepsin b and nuclear factor kappa b-mediated induction of matrix metalloproteinase 9. Anticancer Drugs. 21(3):252–260. doi: 10.1097/cad.0b013e3283340cd7. [DOI] [PubMed] [Google Scholar]
- 117.Rao JS. Molecular mechanisms of glioma invasiveness: The role of proteases. Nat Rev Cancer. 2003;3(7):489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 118.Mohanam S, Sawaya RE, Yamamoto M, Bruner JM, Nicholson GL, Rao JS. Proteolysis and invasiveness of brain tumors: Role of urokinase-type plasminogen activator receptor. J Neurooncol. 1994;22(2):153–160. doi: 10.1007/BF01052890. [DOI] [PubMed] [Google Scholar]
- 119.Mohanam S, Gladson CL, Rao CN, Rao JS. Biological significance of the expression of urokinase-type plasminogen activator receptors (upars) in brain tumors. Front Biosci. 1999;4:D178–D187. doi: 10.2741/mohanam. [DOI] [PubMed] [Google Scholar]
- 120.Huang C, Jacobson K, Schaller MD. Map kinases and cell migration. J Cell Sci. 2004;117(Pt 20):4619–4628. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 121.Huang C, Jacobson K, Schaller MD. A role for jnk-paxillin signaling in cell migration. Cell Cycle. 2004;3(1):4–6. [PubMed] [Google Scholar]
- 122.Wu WS, Wu JR, Hu CT. Signal cross talks for sustained mapk activation and cell migration: The potential role of reactive oxygen species. Cancer Metastasis Rev. 2008;27(2):303–314. doi: 10.1007/s10555-008-9112-4. [DOI] [PubMed] [Google Scholar]
- 123.Silletti S, Yebra M, Perez B, Cirulli V, McMahon M, Montgomery AM. Extracellular signal-regulated kinase (erk)-dependent gene expression contributes to l1 cell adhesion molecule-dependent motility and invasion. J Biol Chem. 2004;279(28):28880–28888. doi: 10.1074/jbc.M404075200. [DOI] [PubMed] [Google Scholar]
- 124.Singh RP, Dhanalakshmi S, Mohan S, Agarwal C, Agarwal R. Silibinin inhibits uvb- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappab in mouse epidermal jb6 cells. Mol Cancer Ther. 2006;5(5):1145–1153. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 125.Gu M, Dhanalakshmi S, Mohan S, Singh RP, Agarwal R. Silibinin inhibits ultraviolet b radiation-induced mitogenic and survival signaling, and associated biological responses in skh-1 mouse skin. Carcinogenesis. 2005;26(8):1404–1413. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 126.Mallikarjuna G, Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and akt signaling. Cancer Res. 2004;64(17):6349–6356. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 127.Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD, et al. Growth inhibition and regression of lung tumors by silibinin: Modulation of angiogenesis by macrophage-associated cytokines and nuclear factor-kappab and signal transducers and activators of transcription 3. Cancer Prev Res (Phila Pa) 2009;2(1):74–83. doi: 10.1158/1940-6207.CAPR-08-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68(6):2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 129.Singh RP, Deep G, Blouin MJ, Pollak MN, Agarwal R. Silibinin suppresses in vivo growth of human prostate carcinoma pc-3 tumor xenograft. Carcinogenesis. 2007;28(12):2567–2574. doi: 10.1093/carcin/bgm218. [DOI] [PubMed] [Google Scholar]
- 130.Gu M, Singh RP, Dhanalakshmi S, Agarwal C, Agarwal R. Silibinin inhibits inflammatory and angiogenic attributes in photocarcinogenesis in skh-1 hairless mice. Cancer Res. 2007;67(7):3483–3491. doi: 10.1158/0008-5472.CAN-06-3955. [DOI] [PubMed] [Google Scholar]
- 131.Chittezhath M, Deep G, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits cytokine-induced signaling cascades and down-regulates inducible nitric oxide synthase in human lung carcinoma a549 cells. Mol Cancer Ther. 2008;7(7):1817–1826. doi: 10.1158/1535-7163.MCT-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171(5):1934–1938. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- 133.Sharma Y, Agarwal C, Singh AK, Agarwal R. Inhibitory effect of silibinin on ligand binding to erbb1 and associated mitogenic signaling, growth, and DNA synthesis in advanced human prostate carcinoma cells. Mol Carcinog. 2001;30(4):224–236. doi: 10.1002/mc.1032. [DOI] [PubMed] [Google Scholar]
- 134.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Identifying the differential effects of silymarin constituents on cell growth and cell cycle regulatory molecules in human prostate cancer cells. Int J Cancer. 2008;123(1):41–50. doi: 10.1002/ijc.23485. [DOI] [PubMed] [Google Scholar]
- 135.Flaig TW, Su LJ, Harrison G, Agarwal R, Glode LM. Silibinin synergizes with mitoxantrone to inhibit cell growth and induce apoptosis in human prostate cancer cells. Int J Cancer. 2007;120(9):2028–2033. doi: 10.1002/ijc.22465. [DOI] [PubMed] [Google Scholar]
- 136.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and tnfalpha-induced activation of nf-kappab and sensitizes human prostate carcinoma du145 cells to tnfalpha-induced apoptosis. Oncogene. 2002;21(11):1759–1767. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 137.Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma du145 cells to doxorubicin-induced growth inhibition, g2-m arrest, and apoptosis. Clin Cancer Res. 2002;8(11):3512–3519. [PubMed] [Google Scholar]
- 138.Agarwal C, Tyagi A, Kaur M, Agarwal R. Silibinin inhibits constitutive activation of stat3, and causes caspase activation and apoptotic death of human prostate carcinoma du145 cells. Carcinogenesis. 2007;28(7):1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 139.Dhanalakshmi S, Agarwal P, Glode LM, Agarwal R. Silibinin sensitizes human prostate carcinoma du145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. Int J Cancer. 2003;106(5):699–705. doi: 10.1002/ijc.11299. [DOI] [PubMed] [Google Scholar]
- 140.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. P21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6(10):2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 141.Tyagi A, Agarwal C, Agarwal R. Inhibition of retinoblastoma protein (rb) phosphorylation at serine sites and an increase in rb-e2f complex formation by silibinin in androgen-dependent human prostate carcinoma lncap cells: Role in prostate cancer prevention. Mol Cancer Ther. 2002;1(7):525–532. [PubMed] [Google Scholar]
- 142.Zi X, Zhang J, Agarwal R, Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60(20):5617–5620. [PubMed] [Google Scholar]
- 143.Thelen P, Jarry H, Ringert RH, Wuttke W. Silibinin down-regulates prostate epithelium-derived ets transcription factor in lncap prostate cancer cells. Planta Med. 2004;70(5):397–400. doi: 10.1055/s-2004-818965. [DOI] [PubMed] [Google Scholar]
- 144.Tyagi A, Bhatia N, Condon MS, Bosland MC, Agarwal C, Agarwal R. Antiproliferative and apoptotic effects of silibinin in rat prostate cancer cells. Prostate. 2002;53(3):211–217. doi: 10.1002/pros.10146. [DOI] [PubMed] [Google Scholar]
- 145.Zhu W, Zhang JS, Young CY. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line lncap. Carcinogenesis. 2001;22(9):1399–1403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- 146.Bhatia N, Zhao J, Wolf DM, Agarwal R. Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: Comparison with silymarin. Cancer Lett. 1999;147(1–2):77–84. doi: 10.1016/s0304-3835(99)00276-1. [DOI] [PubMed] [Google Scholar]
- 147.Deep G, Raina K, Singh RP, Oberlies NH, Kroll DJ, Agarwal R. Isosilibinin inhibits advanced human prostate cancer growth in athymic nude mice: Comparison with silymarin and silibinin. Int J Cancer. 2008 doi: 10.1002/ijc.23879. [DOI] [PubMed] [Google Scholar]
- 148.Singh RP, Dhanalakshmi S, Tyagi AK, Chan DC, Agarwal C, Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62(11):3063–3069. [PubMed] [Google Scholar]
- 149.Raina K, Serkova NJ, Agarwal R. Silibinin feeding alters the metabolic profile in tramp prostatic tumors: 1h-nmrs-based metabolomics study. Cancer Res. 2009;69(9):3731–3735. doi: 10.1158/0008-5472.CAN-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verschoyle RD, Greaves P, Patel K, Marsden DA, Brown K, Steward WP, et al. Evaluation of the cancer chemopreventive efficacy of silibinin in genetic mouse models of prostate and intestinal carcinogenesis: Relationship with silibinin levels. Eur J Cancer. 2008;44(6):898–906. doi: 10.1016/j.ejca.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 151.Dhanalakshmi S, Agarwal C, Singh RP, Agarwal R. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance uvb-induced apoptosis in mouse epithelial jb6 cells. J Biol Chem. 2005;280(21):20375–20383. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 152.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultraviolet b radiation-caused apoptosis in hacat human immortalized keratinocytes. Carcinogenesis. 2004;25(1):99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 153.Mohan S, Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin modulates uvb-induced apoptosis via mitochondrial proteins, caspases activation, and mitogen-activated protein kinase signaling in human epidermoid carcinoma a431 cells. Biochem Biophys Res Commun. 2004;320(1):183–189. doi: 10.1016/j.bbrc.2004.05.153. [DOI] [PubMed] [Google Scholar]
- 154.Bhatia N, Agarwal C, Agarwal R. Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma a431 cells. Nutr Cancer. 2001;39(2):292–299. doi: 10.1207/S15327914nc392_20. [DOI] [PubMed] [Google Scholar]
- 155.Svobodova A, Zdarilova A, Walterova D, Vostalova J. Flavonolignans from silybum marianum moderate uva-induced oxidative damage to hacat keratinocytes. J Dermatol Sci. 2007;48(3):213–224. doi: 10.1016/j.jdermsci.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 156.Singh RP, Tyagi AK, Zhao J, Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in sencar mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. 2002;23(3):499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 157.Gu M, Dhanalakshmi S, Singh RP, Agarwal R. Dietary feeding of silibinin prevents early biomarkers of uvb radiation-induced carcinogenesis in skh-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1344–1349. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 158.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in skh-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/cip1 in epidermis. Carcinogenesis. 2004;25(8):1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 159.Gu M, Singh RP, Dhanalakshmi S, Mohan S, Agarwal R. Differential effect of silibinin on e2f transcription factors and associated biological events in chronically uvb-exposed skin versus tumors in skh-1 hairless mice. Mol Cancer Ther. 2006;5(8):2121–2129. doi: 10.1158/1535-7163.MCT-06-0052. [DOI] [PubMed] [Google Scholar]
- 160.Zhao J, Agarwal R. Tissue distribution of silibinin, the major active constituent of silymarin, in mice and its association with enhancement of phase ii enzymes: Implications in cancer chemoprevention. Carcinogenesis. 1999;20(11):2101–2108. doi: 10.1093/carcin/20.11.2101. [DOI] [PubMed] [Google Scholar]
- 161.Sharma G, Singh RP, Chan DC, Agarwal R. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–2655. [PubMed] [Google Scholar]
- 162.Singh RP, Mallikarjuna GU, Sharma G, Dhanalakshmi S, Tyagi AK, Chan DC, et al. Oral silibinin inhibits lung tumor growth in athymic nude mice and forms a novel chemocombination with doxorubicin targeting nuclear factor kappab-mediated inducible chemoresistance. Clin Cancer Res. 2004;10(24):8641–8647. doi: 10.1158/1078-0432.CCR-04-1435. [DOI] [PubMed] [Google Scholar]
- 163.Yan Y, Wang Y, Tan Q, Lubet RA, You M. Efficacy of deguelin and silibinin on benzo(a)pyrene-induced lung tumorigenesis in a/j mice. Neoplasia. 2005;7(12):1053–1057. doi: 10.1593/neo.05532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Silibinin suppresses growth of human colorectal carcinoma sw480 cells in culture and xenograft through down-regulation of beta-catenin-dependent signaling. Neoplasia. 12(5):415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143(1):58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 166.Yang SH, Lin JK, Huang CJ, Chen WS, Li SY, Chiu JH. Silibinin inhibits angiogenesis via flt-1, but not kdr, receptor up-regulation. J Surg Res. 2005;128(1):140–146. doi: 10.1016/j.jss.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 167.Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer lovo cell line. J Surg Res. 2003;113(1):133–138. doi: 10.1016/s0022-4804(03)00229-4. [DOI] [PubMed] [Google Scholar]
- 168.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Silibinin suppresses spontaneous tumorigenesis in apc min/+ mouse model by modulating beta-catenin pathway. Pharm Res. 2009;26(12):2558–2567. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sangeetha N, Felix AJ, Nalini N. Silibinin modulates biotransforming microbial enzymes and prevents 1,2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer. Eur J Cancer Prev. 2009;18(5):385–394. doi: 10.1097/CEJ.0b013e32832d1b4f. [DOI] [PubMed] [Google Scholar]
- 170.Sangeetha N, Aranganathan S, Nalini N. Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine induced rat colon cancer. Invest New Drugs. 28(3):225–233. doi: 10.1007/s10637-009-9237-5. [DOI] [PubMed] [Google Scholar]
- 171.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male fisher 344 rats. Cancer Prev Res (Phila Pa) 2008;1(5):376–384. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tyagi AK, Agarwal C, Chan DC, Agarwal R. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma mcf-7 and mda-mb468 cells. Oncol Rep. 2004;11(2):493–499. [PubMed] [Google Scholar]
- 173.Wang HJ, Wei XF, Jiang YY, Huang H, Yang Y, Fan SM, et al. Silibinin induces the generation of nitric oxide in human breast cancer mcf-7 cells. Free Radic Res. 44(5):577–584. doi: 10.3109/10715761003692495. [DOI] [PubMed] [Google Scholar]
- 174.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells mda-mb 468: Induction of g1 arrest through an increase in cip1/p21 concomitant with a decrease in kinase activity of cyclindependent kinases and associated cyclins. Clin Cancer Res. 1998;4(4):1055–1064. [PubMed] [Google Scholar]
- 175.Kim S, Kim SH, Hur SM, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents tpa-induced mmp-9 expression by down-regulation of cox-2 in human breast cancer cells. J Ethnopharmacol. 2009;126(2):252–257. doi: 10.1016/j.jep.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 176.Lin CJ, Sukarieh R, Pelletier J. Silibinin inhibits translation initiation: Implications for anticancer therapy. Mol Cancer Ther. 2009;8(6):1606–1612. doi: 10.1158/1535-7163.MCT-08-1152. [DOI] [PubMed] [Google Scholar]
- 177.Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Kim JS, et al. Silibinin prevents tpa-induced mmp-9 expression and vegf secretion by inactivation of the raf/mek/erk pathway in mcf-7 human breast cancer cells. Phytomedicine. 2009;16(6–7):573–580. doi: 10.1016/j.phymed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 178.Scambia G, De Vincenzo R, Ranelletti FO, Panici PB, Ferrandina G, D'Agostino G, et al. Antiproliferative effect of silybin on gynaecological malignancies: Synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32A(5):877–882. doi: 10.1016/0959-8049(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 179.Verschoyle RD, Brown K, Steward WP, Gescher AJ. Consumption of silibinin, a flavonolignan from milk thistle, and mammary cancer development in the c3(1) sv40 t,t antigen transgenic multiple mammary adenocarcinoma (tag) mouse. Cancer Chemother Pharmacol. 2007 doi: 10.1007/s00280-007-0611-8. [DOI] [PubMed] [Google Scholar]
- 180.Qi L, Singh RP, Lu Y, Agarwal R, Harrison GS, Franzusoff A, et al. Epidermal growth factor receptor mediates silibinin-induced cytotoxicity in a rat glioma cell line. Cancer Biol Ther. 2003;2(5):526–531. doi: 10.4161/cbt.2.5.452. [DOI] [PubMed] [Google Scholar]
- 181.Kim KW, Choi CH, Kim TH, Kwon CH, Woo JS, Kim YK. Silibinin inhibits glioma cell proliferation via ca2+/ros/mapk-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res. 2009;34(8):1479–1490. doi: 10.1007/s11064-009-9935-6. [DOI] [PubMed] [Google Scholar]
- 182.Varghese L, Agarwal C, Tyagi A, Singh RP, Agarwal R. Silibinin efficacy against human hepatocellular carcinoma. Clin Cancer Res. 2005;11(23):8441–8448. doi: 10.1158/1078-0432.CCR-05-1646. [DOI] [PubMed] [Google Scholar]
- 183.Brandon-Warner E, Sugg JA, Schrum LW, McKillop IH. Silibinin inhibits ethanol metabolism and ethanol-dependent cell proliferation in an in vitro model of hepatocellular carcinoma. Cancer Lett. 291(1):120–129. doi: 10.1016/j.canlet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Garcia-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mtor/p70s6k/4e-bp1 signalling pathway in human cervical and hepatoma cancer cells: Implications for anticancer therapy. Oncogene. 2009;28(3):313–324. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 185.Huber A, Thongphasuk P, Erben G, Lehmann WD, Tuma S, Stremmel W, et al. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. Biochim Biophys Acta. 2008;1780(5):837–847. doi: 10.1016/j.bbagen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 186.Lah JJ, Cui W, Hu KQ. Effects and mechanisms of silibinin on human hepatoma cell lines. World J Gastroenterol. 2007;13(40):5299–5305. doi: 10.3748/wjg.v13.i40.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15(16):1943–1950. doi: 10.3748/wjg.15.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhou L, Liu P, Chen B, Wang Y, Wang X, Internati MC, et al. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28(2A):1119–1127. [PubMed] [Google Scholar]
- 189.Gallo D, Giacomelli S, Ferlini C, Raspaglio G, Apollonio P, Prislei S, et al. Antitumour activity of the silybin-phosphatidylcholine complex, idb 1016, against human ovarian cancer. Eur J Cancer. 2003;39(16):2403–2410. doi: 10.1016/s0959-8049(03)00624-5. [DOI] [PubMed] [Google Scholar]
- 190.Tyagi A, Singh RP, Agarwal C, Agarwal R. Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of cip1/p21 in apoptosis induction in bladder transitional-cell papilloma rt4 cells: Evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis. 2006;27(11):2269–2280. doi: 10.1093/carcin/bgl098. [DOI] [PubMed] [Google Scholar]
- 191.Singh RP, Tyagi A, Sharma G, Mohan S, Agarwal R. Oral silibinin inhibits in vivo human bladder tumor xenograft growth involving down-regulation of survivin. Clin Cancer Res. 2008;14(1):300–308. doi: 10.1158/1078-0432.CCR-07-1565. [DOI] [PubMed] [Google Scholar]
- 192.Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating cdki-cdk-cyclin cascade, and caspase 3 and parp cleavages. Carcinogenesis. 2004;25(9):1711–1720. doi: 10.1093/carcin/bgh180. [DOI] [PubMed] [Google Scholar]
- 193.Tyagi AK, Agarwal C, Singh RP, Shroyer KR, Glode LM, Agarwal R. Silibinin down-regulates survivin protein and mrna expression and causes caspases activation and apoptosis in human bladder transitional-cell papilloma rt4 cells. Biochem Biophys Res Commun. 2003;312(4):1178–1184. doi: 10.1016/j.bbrc.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 194.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, et al. Chemopreventive effects of silymarin and silibinin on n-butyl-n-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male icr mice. Mol Cancer Ther. 2007;6(12 Pt 1):3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 195.Gharagozloo M, Khoshdel Z, Amirghofran Z. The effect of an iron (iii) chelator, silybin, on the proliferation and cell cycle of jurkat cells: A comparison with desferrioxamine. Eur J Pharmacol. 2008;589(1–3):1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 196.Danilenko M, Wang Q, Wang X, Levy J, Sharoni Y, Studzinski GP. Carnosic acid potentiates the antioxidant and prodifferentiation effects of 1alpha,25-dihydroxyvitamin d3 in leukemia cells but does not promote elevation of basal levels of intracellular calcium. Cancer Res. 2003;63(6):1325–1332. [PubMed] [Google Scholar]
- 197.Kang SN, Lee MH, Kim KM, Cho D, Kim TS. Induction of human promyelocytic leukemia hl-60 cell differentiation into monocytes by silibinin: Involvement of protein kinase c. Biochem Pharmacol. 2001;61(12):1487–1495. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 198.Cheung CW, Vesey DA, Nicol DL, Johnson DW. Silibinin inhibits renal cell carcinoma via mechanisms that are independent of insulin-like growth factor-binding protein 3. BJU Int. 2007;99(2):454–460. doi: 10.1111/j.1464-410X.2007.06571.x. [DOI] [PubMed] [Google Scholar]
- 199.Cheung CW, Taylor PJ, Kirkpatrick CM, Vesey DA, Gobe GC, Winterford C, et al. Therapeutic value of orally administered silibinin in renal cell carcinoma: Manipulation of insulin-like growth factor binding protein-3 levels. BJU Int. 2007;100(2):438–444. doi: 10.1111/j.1464-410X.2007.07012.x. [DOI] [PubMed] [Google Scholar]
- 200.Bang CI, Paik SY, Sun DI, Joo YH, Kim MS. Cell growth inhibition and down-regulation of survivin by silibinin in a laryngeal squamous cell carcinoma cell line. Ann Otol Rhinol Laryngol. 2008;117(10):781–785. doi: 10.1177/000348940811701014. [DOI] [PubMed] [Google Scholar]
- 201.Kim S, Choi MG, Lee HS, Lee SK, Kim SH, Kim WW, et al. Silibinin suppresses tnf-alpha-induced mmp-9 expression in gastric cancer cells through inhibition of the mapk pathway. Molecules. 2009;14(11):4300–4311. doi: 10.3390/molecules14114300. [DOI] [PMC free article] [PubMed] [Google Scholar]