Abstract
Didansyl derivatives of amino acids and N-phenyl-1-naphthylamine were used to localize membrane hydrophobic sites in glycerol-extracted chicken skeletal muscle fibers. Epifluorescence microscopy revealed that such sites coincide with the distribution of mitochondria, the transverse tubular (T) system and the sarcoplasmic reticulum (SR). They are specifically associated with myofibril Z lines and occasionally extend from one Z plane to the next longitudinally along the muscle fiber. The hydrophobic probes interact noncovalently with the Z lines, and their induced fluorescence can be eliminated by exposure of the myofibrils to ionic detergents, nonionic detergents, or phospholipase C, before or after addition of the hydrophobic label.
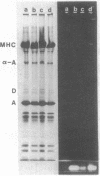
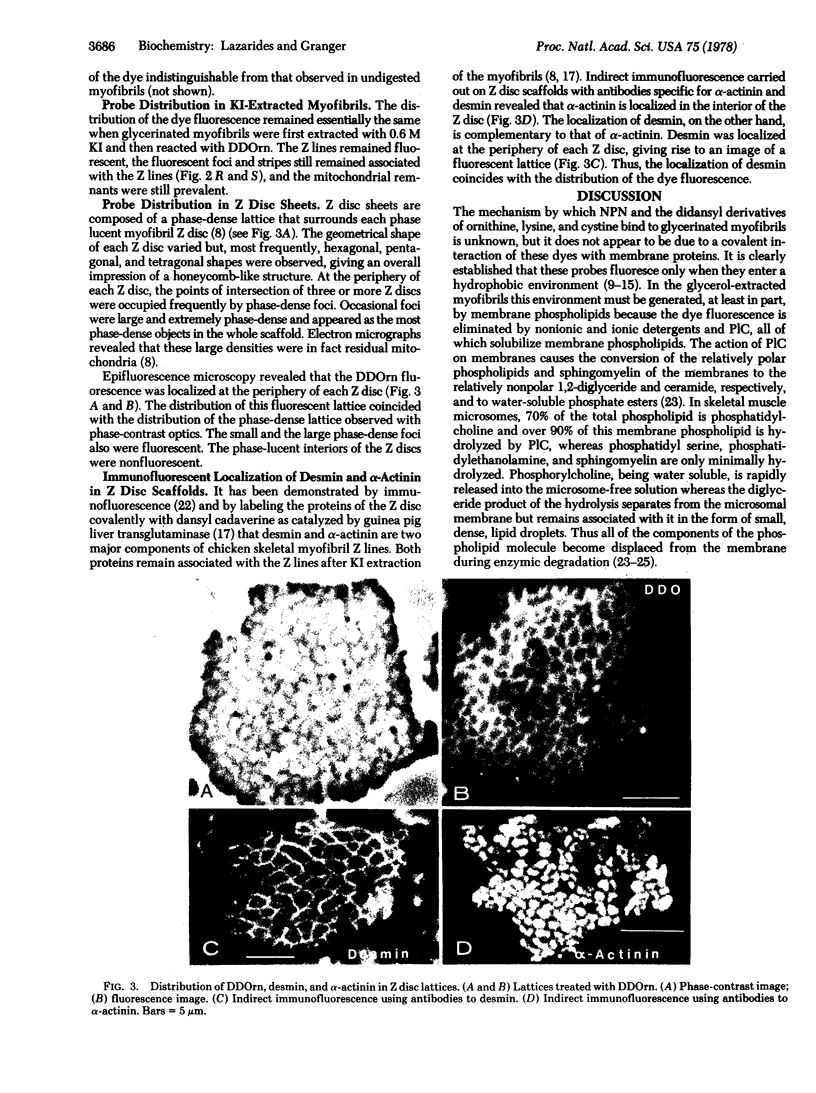
Extraction of glycerinated fibers with 0.6 M KI removes the majority of sarcomeric actin and myosin and leaves a scaffold of longitudinally interconnected Z planes. Membrane fluorescence remains tightly associated with these Z planes and with the remnant mitochondria. Shearing of such scaffolds results in the cleavage of the longitudinal connections and the production of large sheets of interconnected, close-packed Z discs in a honeycomb-like array. Comparison of the localization of two Z disc proteins, desmin and α-actinin, with that of the membrane material reveals that α-actinin is localized in the interior of each myofibril Z disc whereas both desmin and the membrane material surround each disc. Thus, glycerination and KI extraction of muscle fibers leaves remnants of T system and SR membranes tightly associated with the Z disc honeycomb lattice. Because the Z discs are connected at their peripheries through the T system appear to the plasma membrane, desmin and this membrane structure appear to be connected throughout the whole Z plane up to and including the plasma membrane. The congruent localization of desmin and the T system strongly suggests that this molecule mediates the adhesion of this membrane system around each Z disc.
Keywords: actin, α-actinin, desmin, T system, sarcoplasmic reticulum
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzi A. The application of fluorescent probes in membrane studies. Q Rev Biophys. 1975 May;8(2):237–316. doi: 10.1017/s0033583500001803. [DOI] [PubMed] [Google Scholar]
- Eisenberg B., Eisenberg R. S. Selective disruption of the sarcotubular system in frog sartorius muscle. A quantitative study with exogenous peroxidase as a marker. J Cell Biol. 1968 Nov;39(2):451–467. doi: 10.1083/jcb.39.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. SARCOLEMMAL INVAGINATIONS CONSTITUTING THE T SYSTEM IN FISH MUSCLE FIBERS. J Cell Biol. 1964 Sep;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finean J. B., Martonosi A. The action of phospholipase C on muscle microsomes: a correlation of electron microscope and biochemical data. Biochim Biophys Acta. 1965 Jun 1;98(3):547–553. doi: 10.1016/0005-2760(65)90151-7. [DOI] [PubMed] [Google Scholar]
- Flanagan M. T., Hesketh T. R. Electrostatic interactions in the binding of fluorescent probes to lipid membranes. Biochim Biophys Acta. 1973 Mar 29;298(3):535–545. doi: 10.1016/0005-2736(73)90072-2. [DOI] [PubMed] [Google Scholar]
- Goli D. E., Suzuki A., Temple J., Holmes G. R. Studies on purified -actinin. I. Effect of temperature and tropomyosin on the -actinin-F-actin interaction. J Mol Biol. 1972 Jun 28;67(3):469–488. doi: 10.1016/0022-2836(72)90464-0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Simons K. The binding of detergents to lipophilic and hydrophilic proteins. J Biol Chem. 1972 Jun 10;247(11):3656–3661. [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Hubbard B. D. Immunological characterization of the subunit of the 100 A filaments from muscle cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4344–4348. doi: 10.1073/pnas.73.12.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Reynolds J. A., Tanford C. The binding of deoxycholate and Triton X-100 to proteins. J Biol Chem. 1973 Jul 25;248(14):4926–4932. [PubMed] [Google Scholar]
- Makino S., Woolford J. L., Jr, Tanford C., Webster R. E. Interaction of deoxycholate and of detergents with the coat protein of bacteriophage f1. J Biol Chem. 1975 Jun 10;250(11):4327–4332. [PubMed] [Google Scholar]
- Martonosi A., Donley J., Halpin R. A. Sarcoplasmic reticulum. 3. The role of phospholipids in the adenosine triphosphatase activity and Ca++ transport. J Biol Chem. 1968 Jan 10;243(1):61–70. [PubMed] [Google Scholar]
- Mendell J. R. Unusual features of the T-system of the pectoralis muscle of the chicken. J Ultrastruct Res. 1971 Nov;37(3):383–387. doi: 10.1016/s0022-5320(71)80132-6. [DOI] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D., Schild R. F. The distribution of the T-system along the sarcomeres of frog and toad sartorius muscles. J Physiol. 1968 Jan;194(1):249–258. doi: 10.1113/jphysiol.1968.sp008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlubnaya Z. A., Tskhovrebova L. A., Zaalishtsbvili M. M., Stefanenko G. A. Electron microscopic study of alpha-actinin. J Mol Biol. 1975 Feb 25;92(2):357–359. doi: 10.1016/0022-2836(75)90234-x. [DOI] [PubMed] [Google Scholar]
- Radda G. K. Enzyme and membrane conformation in biochemical control. Biochem J. 1971 May;122(4):385–396. doi: 10.1042/bj1220385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Shear C. R. Cross-sectional myofibre and myofibril growth in immobilized developing skeletal muscle. J Cell Sci. 1978 Feb;29:297–312. doi: 10.1242/jcs.29.1.297. [DOI] [PubMed] [Google Scholar]
- Stromer M. H., Goll D. E. Studies on purified -actinin. II. Electron microscopic studies on the competitive binding of -actinin and tropomyosin to Z-line extracted myofibrils. J Mol Biol. 1972 Jun 28;67(3):489–494. doi: 10.1016/0022-2836(72)90465-2. [DOI] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Stryer L. Proximity relationships in rhodopsin. Proc Natl Acad Sci U S A. 1972 May;69(5):1104–1108. doi: 10.1073/pnas.69.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikami S., Robinson W. E., Hagins W. A. Topology of the outer segment membranes of retinal rods and cones revealed by a fluorescent probe. Science. 1974 Sep 27;185(4157):1176–1179. doi: 10.1126/science.185.4157.1176. [DOI] [PubMed] [Google Scholar]